Global Journal of Medical and Clinical Short Communications
Atypical Pyoderma Gangrenosum with Ulcerative Colitis treated successfully with prednisolone and mesalazine: A case report
Doaa AE Abou-Taleb1*, Mahmoud F Sherif2 and Ahmed S Mohammed3
2Department of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt
3Department of General Surgery, Faculty of Medicine, Assiut University, Assiut, Egypt
Cite this as
: Abou-Taleb DAE, Sherif MF, Mohammed AS (2021) Atypical Pyoderma Gangrenosum with Ulcerative Colitis treated successfully with prednisolone and mesalazine: A case report. Glob J Medical Clin Case Rep 8(2): 065-068. DOI: 10.17352/2455-5282.000131Introduction: Pyoderma Gangrenosum (PG) manifests as recurrent deep ulceration of the skin and PG is often associated with a variety of systemic diseases, such as Inflammatory Bowel Diseases (IBD), inflammatory arthropathies, hematologic malignancies and hepatitis. There has been neither laboratory finding nor histological feature diagnostic of PG, and diagnosis of PG is mainly made based on the exclusion criteria.
Patient and methods: A male patient 21 years old, complained of rectal bleeding of 3 weeks duration. He presented with a large, painful and rapidly progressive cutaneous ulcer in the right flank. Laboratory and microbiological investigations, colonoscopic biopsy and a skin biopsy from the ulcer were performed.
Results: An atypical presentation of PG with Ulcerative Colitis (UC) was diagnosed. The PG rapidly resolved after starting treatment with prednisolone and mesalazine and the ulcer healed with scar. After successful treatment the patient suddenly stopped all his treatment and he came back again with rectal bleeding and a rounded, painful and rapidly progressive cutaneous ulcer in each cheek. One month after starting prednisolone and mesalazine treatment, complete healing of both ulcers has occurred.
Conclusion: A rare atypical presentation of PG with risk of misdiagnosis and the rapid healing of PG with combination of prednisolone and mesalazine therapy were concluded.
Introduction
Pyoderma Gangrenosum (PG) is one of the neutrophilic dermatoses, it is a rare inflammatory, ulcerative skin disease which occurs in association with different systemic diseases such as Inflammatory Bowel Diseases (IBD), inflammatory arthropathies, haematological malignancies, paraproteinaemia, and hepatitis [1-4]. The exact pathogenesis is not well known, but different studies have suggested an abnormal immune response in patients with genetic predisposition. No laboratory or histological findings for diagnosis of PG; its diagnosis is mainly done by exclusion criteria [5,6].
We reported an adult male patient with Ulcerative Colitis (UC) who presented with atypical presentation of PG in the right flank and both cheeks. A rapid healing of the ulcers was achieved by prednisolone and mesalazine therapy.
Case report
A male patient 21 years old was referred and admitted to the department of dermatology, Venereology and Andrology of Assiut University Hospital, Assiut, Egypt. He was presented with a large, painful and rapidly progressive cutaneous ulcer in the right flank. The condition was associated with rectal bleeding of 3 weeks duration. Two weeks before a lesion appeared in the same skin area presenting as a small red plaque surrounded by erythema. The lesion rapidly progressed to a large and painful cutaneous ulceration. Antibiotic treatment with amoxicillin and ciprofloxacin was ineffective and the patient received paracetamol every 6 hours for pain relief.
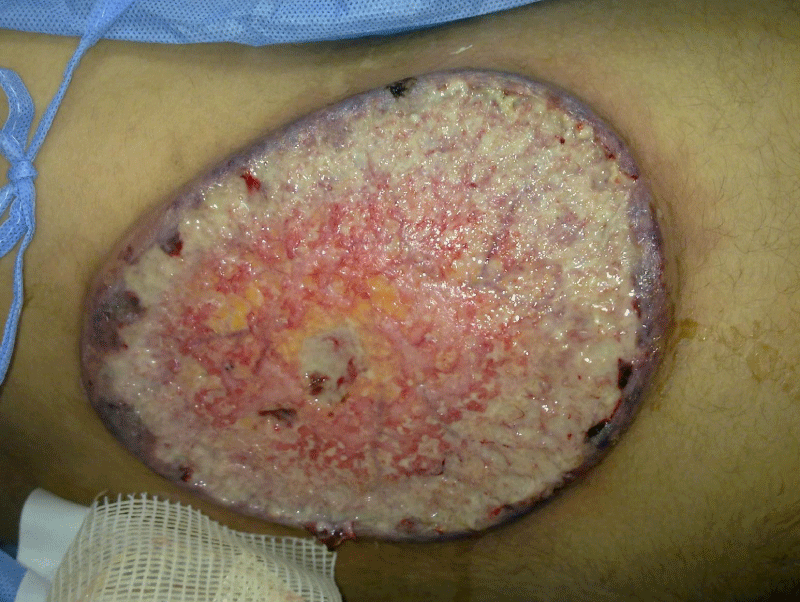
On examination, the patient had pallor and mild hyperthermia (37.8℃). He complained of 3-4 daily episodes of diarrhoea with rectal bleeding. No lymphadenopathy was observed. A large, oval, painful and rapidly progressive cutaneous ulcer of 15×12cm in diameters was over the right flank. The edges were well-defined, undermined and presented with granulated tissues, crusts, and purulent exudates (Figure 1). The lesion was very tender. A swab and microbiological examination of specimen from the ulcer was negative for bacteria and fungi. Laboratory investigations were done, complete blood count revealed white cell count of 15×109/L leucocytosis with neutrophilia, and microcytic hypochromic anaemia his haemoglobin was 7.6 g/dl. He received blood transfusion and his haemoglobin became 9 g/dl. The Erythrocyte Sedimentation Rate (ESR) was 32 mm/h. Stool analysis was reddish in colour and showed positive blood and mucus with pus cells 60/HPF, RBC over 80/HPF. Liver and kidney function tests were normal. Venereal Disease Research Laboratory (VDRL) test, HIV test, antinuclear and anti-DNA antibodies, rheumatoid factor and LE test were all negative. Venous and arterial functional studies, and chest X-ray, were normal. The findings of the MRI of the posterior abdominal wall were suggestive of malignant cutaneous ulcer. No detected abdominal findings inside the abdominal cavity.
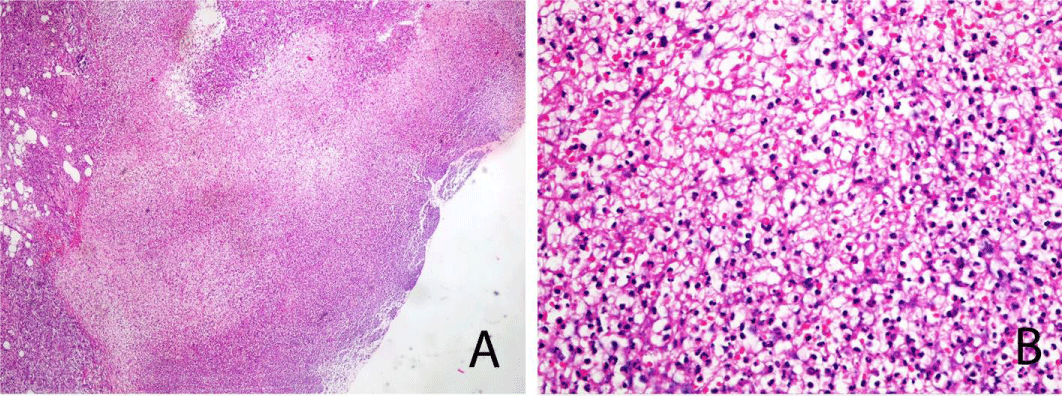
A Skin biopsy from the ulcer was performed under local anaesthesia and stained with H&E. Histopathological analysis showed central necrotizing suppurative inflammation with ulceration (Figure 2 A), and heavy neutrophilic suppurative inflammatory reaction with fibrin deposition (Figure 2B). The histopathological changes were consistent with pyoderma gangrenosum.
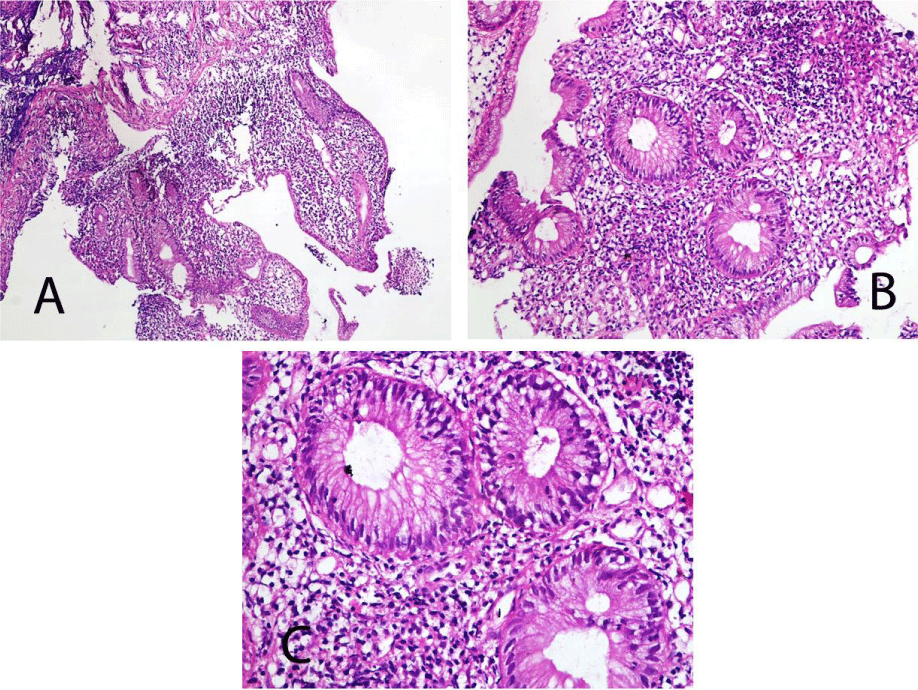
Colonoscopic biopsy was performed under general anaesthesia and stained with H&E. Histopathological analysis revealed marked mucosal architectural distortion (chronic colitis), shallow ulceration and pseudopolyps (Figure 3A), with neutrophilic infiltrate of crypts (cryptitis) and lamina propria (Figure 3B & 3C). The histopathological changes were consistent with ulcerative colitis.
Therefore, the patient was treated with oral prednisolone (40 mg/day) and mesalazine therapy (4 gm/day). His bowel symptoms and skin lesion both gradually improved 2 weeks after starting treatment (Figure 4A). The prednisolone was tapered gradually with clinical improvement. Mesalazine administration was continued. The PG completely resolved 30 days after beginning of treatment, although the ulcer healed with scar (Figure 4B).
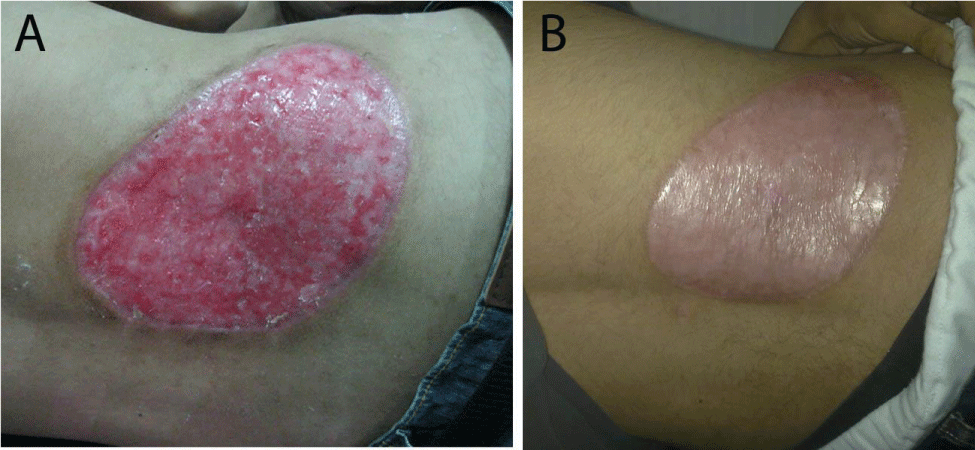
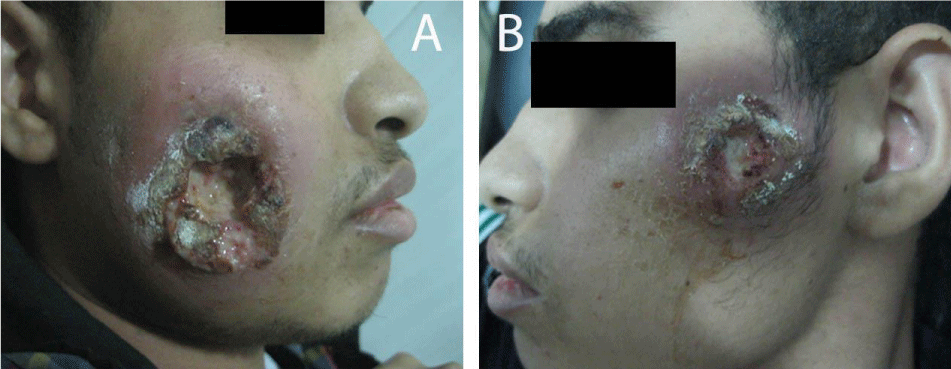
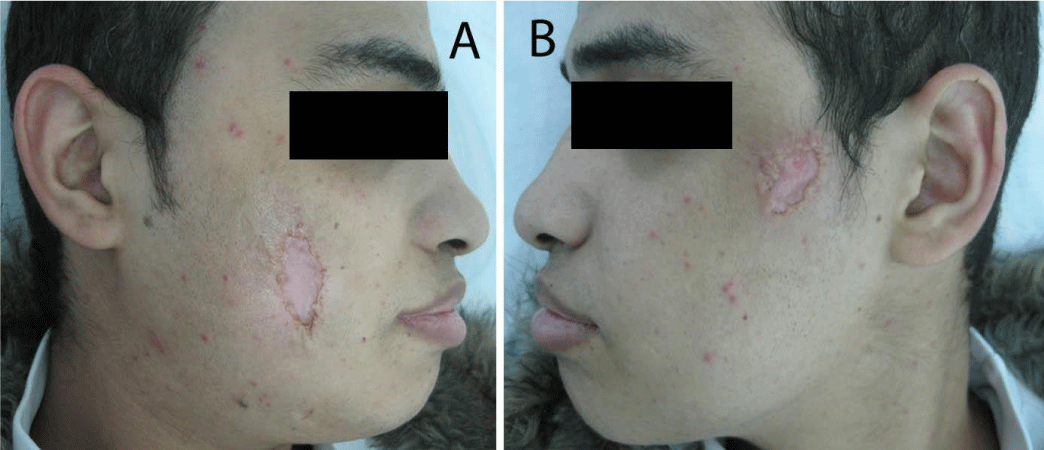
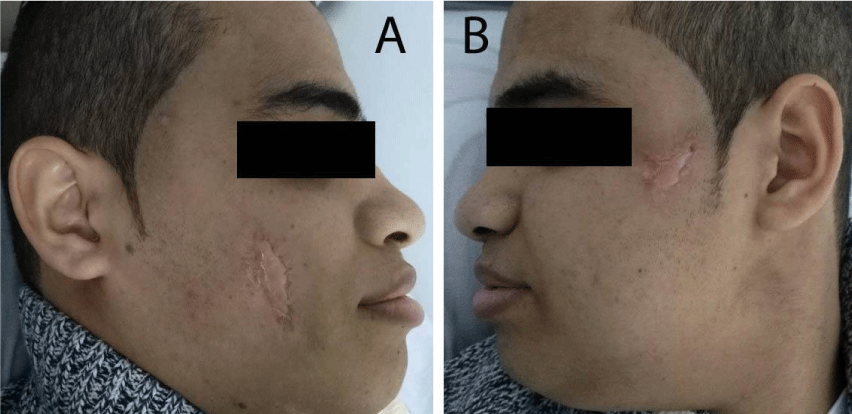
Two months after successful treatment with prednisolone and mesalazine, the patient felt that he is completely cured, he suddenly stopped all his treatment, and then he came back again with rectal bleeding. He presented with 2 rounded, painful and rapidly progressive cutaneous ulcerations, the first one in his right cheek and the second one in his left cheek of 5 days duration. The ulcers started as small red plaques with surrounding erythema, and then rapidly progressed to painful cutaneous ulcerations. On examination, the patient revealed 2 rounded, painful and rapidly progressive cutaneous ulcers, the first one central in his right cheek about 5x5 cm in diameter (Figure 5A), and the second one in the upper part of his left cheek about 3x3 cm in diameter (Figure 5B). The edges were undermined and presented with granulated tissues, crusts, and purulent exudates. The lesions were very tender. A skin biopsy from the ulcer of the right cheek was performed under local anaesthesia, to confirm again the diagnosis of pyoderma gangrenosum. Immediately we started the treatment with prednisolone and mesalazine. 2 weeks later, the rectal bleeding gradually stopped and his skin ulcers on both cheeks were improved (Figure 6 A&B). Complete healing of both ulcers with scars has occurred after one month of treatment (Figure 7 A&B). We advised the patient that he should be carefully followed up, and gradually tapered his treatment according to the clinical improvement.
Discussion
Brunsting and Underwood [7] in 1949 described five patients with UC associated with rapidly progressive and painful suppurative skin ulcers with necrotic and undermined borders that were called PG. This lesion is a neutrophilic dermatosis associated with a variety of systemic diseases, such as IBD, paraproteinemia, arthritis and myeloproliferative diseases [1,4,8].
The relationship between the clinical course of PG and UC remains controversial. To clarify the uncertain relationship between PG and UC, an understanding the pathogenic relationship is important. There are some previous case reports suggesting that UC and PG may share a common pathogenic immune-mechanism. For example, one report proposed that the skin may reflect the primary pathogenic process in the colon as a Shwartzman phenomenon. Another report suggested that immune complexes from inflamed intestinal mucosa caused skin lesions [9]. Furthermore, other reports suggest that IL-15 and IL-8 play an important role in the relationship of PG and UC [10,11].
PG can occur at different sites but it is more common on the legs, in perineal, vulvar and penile regions. Atypical presentations may occur at other sites such as the face, the neck, the arms or the trunk [12]. Therefore, PG is an excluded diagnosis on the basis of laboratory findings and histopathology, associated with a high rate of clinical suspicion. The good clinical response to systemic corticosteroids associated with other immunosuppressive therapy such as cyclosporine and azathioprine is also important criteria [6,13].
Topical agents can be used for ulcers that are less than 2 cm, including corticosteroids, tacrolimus, sodium cromoglycate, dapsone, nicotine, and 5-aminosalicylic acid. Systemic corticosteroids are first line for severe disease as either oral prednisone (0.5-1 mg/kg/day) or pulse intravenous methylprednisone 1000 mg/day [13]. Other systemic agents that can be added with steroid-sparing benefits include cyclosporine, tacrolimus, thalidomide, colchicine, dapsone, sulfasalazine, azathioprine, methotrexate, mycophenolate, cyclophosphamide, chlorambucil, minocycline, intravenous immunoglobulin (IVIG), anti-TNFs, anti-interleukin-1, anti-interleukin-12 and anti-interleukin-23 agents [13].
In our patient, the bowel symptoms and the skin lesion both gradually improved 2 weeks after oral treatment with prednisolone and mesalazine. The PG resolved after 30 days of therapy, and the ulcers healed with scar. The rapid healing of such rapidly progressive, painful skin ulcers is unusual. In previous studies, Some patients refractory to steroid treatment improved from the combination of systemic corticosteroid with cyclosporine [14,15].
Mesalazine is the first line treatment for mild to moderate UC, and is thought be anti-inflammatory through induction of peroxisome Proliferator-Activated Receptor-Gamma(PPAR-γ) gene expression and Nuclear Factor kappa-B (NFκB) activation, as well as inhibiting prostaglandin and interleukin-1 synthesis [16]. However, no studies have yet been published on PPAR-γ gene expression, NFκB activation or prostaglandin and interleukin-1 synthesis in PG. Moreover, in consideration of possible association between PG and UC, the improvement of PG might be secondary to the improvement of UC [17]. A previous study reported a case of successfully treated PG with topical mesalazine cream [18], and the authors suggested that leukocytes’ motility and cytotoxicity were suppressed by mesalazine in PG. Another study suggested that mesalazine play a direct role for the treatment of PG [17].
Conclusion
A rare atypical presentation of PG with risk of misdiagnosis. The Combination of systemic corticosteroid and mesalazine therapy can be an effective treatment option of PG, especially when associated with UC, and can lead to rapid healing.
- Vacas AS, Torre AC, Bollea-Garlatti ML, Warley F, Galimberti RL (2017) Pyoderma gangrenosum: clinical characteristics, associated diseases, and responses to treatment in a retrospective cohort study of 31 patients. Int J Dermatol 56: 386-391. Link: https://bit.ly/3eGNbI5
- Pereira N, Brites MM, Goncalo M, Tellechea O, Figueiredo A (2013) Pyoderma gangrenosum - a review of 24 cases observed over 10 years. Int J Dermatol 52: 938-945. Link: https://bit.ly/3eBme8v
- Marzano AV, Ishak RS, Saibeni S, Crosti C, Meroni PL, et al. (2013) Autoinflammatory skin disorders in inflammatory bowel diseases, pyoderma gangrenosum and Sweet's syndrome: a comprehensive review and disease classification criteria. Clin Rev Allergy Immunol 45: 202-210. Link: https://bit.ly/3iu99Pz
- Yasin F, Assad S, Zahid M, Malik SA (2017) Extensive pyoderma gangrenosum: a challenging diagnosis and literature review of management. Cureus 9: e1486. Link: https://bit.ly/36NaMCr
- Marzano AV, Borghi A, Wallach D, Cugno M (2018) A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol 54: 114-130. Link: https://bit.ly/3iu9dih
- Ahn C, Negus D, Huang W (2018) Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol 14: 225-233. Link: https://bit.ly/36RR8oU
- Brunsting LA, Underwood LJ (1949) Pyoderma vegetans in association with chronic ulcerative colitis. Arch Derm Syphilol 60: 161-172. Link: https://bit.ly/3kFyQzy
- Ahronowitz I, Harp J, Shinkai K (2012) Etiology and management of pyoderma gangrenosum. Am J Clin Dermatol 13: 191-211. Link: https://bit.ly/3hT4CXV
- TripodiCutrì F, Salerno R, Lo Schiavo A, Gravina AG, Romano M, et al. (2009) Ulcerative colitis associated with leukocytoclastic vasculitis of the skin. Dig Liver Dis 41: e42- e44. Link: https://bit.ly/3hVi7GK
- Nishiwaki T, Ina K, Goto H, Watanabe O, Tsuzuki T, et al. (2005) Possible involvement of the interleukin-15 and interleukin-15 receptor system in a heightened state of lamina propria B cell activation and differentiation in patients with inflammatory bowel disease. J Gastroenteral 40:128-136. Link: https://bit.ly/3xWCFnA
- Oka M (2007) Pyoderma gangrenosum and interleukin 8. Br J Dermatol 157: 1279-1281. Link: https://bit.ly/3zlZDoK
- Ashchyan HJ, Butler DC, Nelson CA, Noe MH, Tsiaras WG, et al. (2018) The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol 154: 409-413. Link: https://bit.ly/3eBXT2I
- Alavi A, French LE, Davis MD, Brassard A, Kirsner RS (2017) Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol 18: 355-372. Link: https://bit.ly/3BzPTcm
- Futami H, KodairaM, Furuta T, Hanai H, Kaneko E (1998) Pyoderma gangrenosum complicating ulcerative colitis: Successful treatment with methylprednisolone pulse therapy and cyclosporine. J Gastroenteral 33: 408-411. Link: https://bit.ly/3rskzYq
- Reichrath J, Bens G, Bonowitz A, Tilgen W (2005) Treatment recommendations for pyoderma gangrenosum: an evidence based review of the literature based on more than 350 patients. J Am Acad Dermatol 53: 273-283. Link: https://bit.ly/3eEvedo
- Sandborn WJ (2008) Oral 5-ASA therapy in ulcerative colitis: what are the implications of the new formulations? J Clin Gastroenterol 42: 338-344. Link: https://bit.ly/3hTeqkP
- Lee JI, Park HJ, Lee JY, Cho BK (2010) A Case of Pyoderma Gangrenosum with Ulcerative Colitis Treated with Mesalazine. Ann Dermatol 22: 422-425. Link: https://bit.ly/36QJLy4
- Sanders CJ, Hulsmans RF (1993) Successful treatment of pyoderma gangrenosum with topical 5-aminosalicylic acid. Cutis 51: 262-264. Link: https://bit.ly/2W5cJIz

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
