Global Journal of Medical and Clinical Short Communications
Complete response of merkel cell carcinoma with brain metastases to pembrolizumab and radiotherapy: A case report
Ansgar Koechel1*, Franz-Joachim Hilke2, Bernhard Klumpp3, Christopher Schroeder4, Thomas Eigentler1, Diana Lomberg1, Claus Garbe1 and Ulrike Leiter1
2Department for Dermatology, University of Berlin, Germany
3Department for Radiology, Rems-Murr-Kliniken Winnenden, Germany
4Institute of Medical Genetics and Applied Genomics, University Tübingen, Germany
Cite this as
: Koechel A, Hilke FJ, Klumpp B, Schroeder C, Eigentler T, et al. (2021) Complete response of merkel cell carcinoma with brain metastases to pembrolizumab and radiotherapy: A case report. Glob J Medical Clin Case Rep 8(2): 059-062. DOI: 10.17352/2455-5282.000129Introduction
Merkel cell carcinoma is a rare, but highly malignant and fast growing skin-cancer with high recurrence-rates, deriving from endothelial and neuroendocrine tissues. For advanced Merkel cell carcinoma prognosis is poor [1] and even under cytotoxic chemotherapy there is only a median progression-free survival of only 3 months [2,3]. Even though Merkel cell carcinoma frequently shows lymphatic metastases, it almost never shows cerebral involvement [1]. To our knowledge there are only twelve reported cases of cerebral metastases. Furthermore this is the first reported case of complete response in a patient with advanced Merkel cell carcinoma Stage IV with brain metastases under combined treatment with pembrolizumab and radiotherapy.
Etiologic factors are advancing age, fair skin, ultraviolet light exposure, immunocompromise, and the Merkel-cell polyomavirus (MCPyV), which is associated with approximately 80% of Merkel cell carcinomas. In MCPyV-positive Merkel cell carcinomas tumor-cells express large T-antigen, which inactivates p53 and Rb [2]. Affected patients were found to produce MCPyV T-antigen–specific T cells and antibodies in relation to disease severity [4-6]. Virus-associated Merkel cell carcinoma showed significant lower mutation rates than MCPyV-negative Merkel cell carcinoma [7]. The detected low mutational rate and the absence of typical driver mutations in the presented patient are in line with a MCPyV infection positive Merkel cell carcinoma. Positive expression of PD-L1 on tumor cells and PD-1 on tumor-infiltrating lymphocytes suggests an endogenous tumor reactive immune response that might be unleashed by anti–PD-1 or anti–PD-L1 drugs [7-9]. Recently immunotherapy has been shown to be a highly effective and well tolerated treatment. A phase 2 – study with 26 patients with advanced Merkel cell carcinoma undergoing anti-PD1 treatment, showed a response rate of 56% [10]. Two clinical studies demonstrated a response rate as high or even higher as in melanoma [9,11]. Corresponding cases with advanced courses, especially with cerebral metastases, and therapeutic options have not been reported so far.
Case presentation
A 60-year-old man was referred to our outpatient clinic in March 2015 with slowly progressive, skin-colored nodule on the dorsum of his right hand. The nodule grew slowly over a period of about two years. Until first presentation three similar nodules developed on his right forearm. The patient had no further complaints. Personal and family history was negative for cutaneous or any other malignancy. The patient was obese (BMI 41 kg/m²) and had an history of arterial hypertension, chronic obstructive pulmonary disease, tachyarrhythmia accompanying atrial fibrillation with consecutive oral anticoagulation, peripheral arterial occlusive disease Fontaine stage IV, secondary polycythemia, substituted hypothyroidism, and testicle atresia. Chronic recurrent polycythemia, known for several years, was classified as secondary to known COPD on the basis of non-pathological haemato-oncological and genetic investigations.
Physical examination revealed a round, skin-coloured, hemispherical, coarser, about 54 x 43 mm wide nodule. Peripheral blood testing indicated mild lymphopenia (0.88 10³/μl, norm: 1.1 - 3.2 10³/μl), prolonged International normalized Ratio (2.3, norm: < 1)) most likely due to oral anticoagulation with phenprocomon, thrombocytopenia (114 10³/μl, norm: 150 – 450 10³/μl), conjugated hyperbilirubinemia (0.5 mg/dl, norm: < 0.3 mg/dl), unconjugated hyperbilirubinemia (1.3 mg/dl, norm: < 1.1 mg/dl), and increased lactate dehydrogenase (312 U/l, norm: < 250 U/l).
Ultrasound revealed three similar, homogeneous, localized, about 10 to 20 mm wide, irregular, hypo-echogenic nodules on his right, lateral forearm.
The primary tumor and three in-transit metastases were excised with 20 mm excision margin. Histology showed tumor cell nests with atypia, mitotic activity and tumor necrosis as well as infiltration of lymphatic tissue. Immunostainings found negative findings for melanocytic markers and positive findings for keratinocyte markers as well as for Cytokeratin-20 which confirmed the diagnosis of Merkel cell carcinoma. Whole-body multi detector computed tomography did not give evidence for metastatic disease.
Advanced Merkel cell carcinoma, Stage IIIB (AJCC classification: pT2 pN2 cM0) [12,13], was diagnosed and subsequent radiotherapy with 50 GY of right forearm and hand was performed.
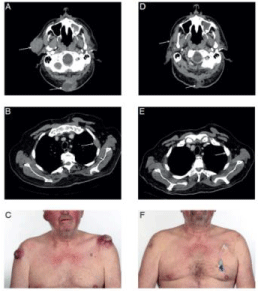
Six months after initial presentation the patient reported new nodules on the right volar hand similar to the pre-diagnosed subcutaneous metastases. New loco-regional, as well as distant subcutaneous metastases on both shoulders and the right cheek, lung metastases in the right caudal upper lobe, and bone metastases of the dorsal neck, left humerus and right os ileum were detected by positron emission tomography–computed tomography (Figure 1). Merkel cell carcinoma Stage IV was diagnosed.
A systemic therapy with carboplatin (AUC 4 IV) plus etoposide (80mg/m 2 IV) was started. During the therapy the patient had clinically massive progress with deterioration of the general condition and newly appeared cutaneous metastases up to 10 cm in size. Re-staging after four cycles of chemotherapy confirmed progressive metastases as well as new tumor manifestations in soft tissue, lung, and bones (Figure 1). With progressive diffuse metastatic infiltration of the long bones, patient developed pancytopenia, which required repeated blood transfusion. Due to progressive disease chemotherapy was stopped. Due to bone marrow infiltration, pancytopenia worsened despite the impairment of chemotherapy.
The interdisciplinary tumor board recommended off-label treatment with an anti-PD1 antibody [4-6], as there was no official approval at the time of the treatment in March 2017.
15 months after initial diagnosis, treatment with fixed dosed, body weight-based pembrolizumab 2mg/kg every three weeks was started, in combination with radiotherapy of multiple subcutaneous metastases.
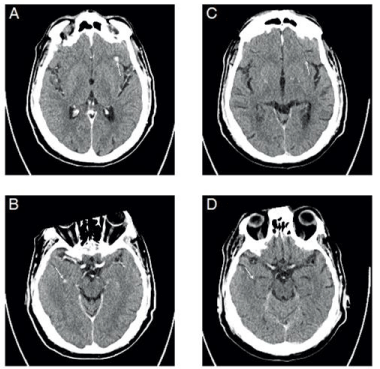
The first follow-up examination already indicated partial response with decreasing diameter of all metastases. Two months later next follow up computer tomography-examination gave evidence of further regression, and finally, 23 month after initial diagnosis, complete response could be achieved with residual fibrotic tissue nuchal and sclerosis of the former bone metastases (Figure 1). After 14 cycles of pembrolizumab had been administered, the next follow up examination indicated new occurrence of cerebral metastases (Figure 2). These were located bi-hemispheric, fronto-basal, parietal, supra-tentorial, and occipital, mostly round shaped, ranging from 5 mm (fronto-basal) to 3 mm (parietal). An additional cranial magnetic resonance imaging confirmed the presence of cerebral metastases.
After extra-cerebral complete response it was decided to continue anti-PD 1 treatment, combined with whole brain radiotherapy to treat the newly occurred cerebral metastases. Over a period of two months the patient received an overall radiation dose of 35 GY (lateral, isocentric, opposing fields in mask technique, 6 MV photons, fractionation: 5 x 2.5 GY / week). After initial partial response with small remnants of the irradiated cerebral metastases on magnetic resonance imaging, the next regular follow up whole body and cerebral computer tomography in July 2017 did not give evidence for any remaining metastases thus achieving complete response (Figure 2).
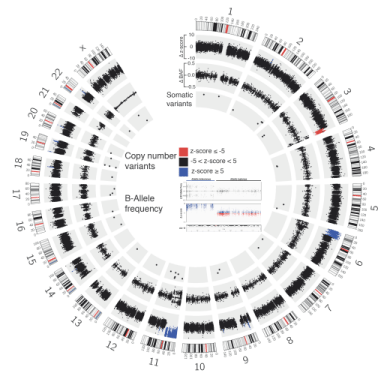
Whole exome sequencing of tumor tissue was performed to identify possible treatable mutations and identified 30 somatic alterations (Figure 3). The overall mutation rate was low (0.64 var/Mb). The nonsense variant in the E3 ubiquitin-protein ligase TRIP12 (c.3982G>C, p.E1328*) was predicted to be a driver variant by the Cancer Genome Interpreter [14]. Additionally, multiple CNVs were found, including a terminal deletion of the long arm of chromosome 3, a terminal amplification of the short arm of chromosome 6 and an amplification of the short arm of chromosome 11. The copy number loss on chromosome 3 involved the tumor suppressor gene TP63 and the copy number gains on chromosomes 6 and 11 involved the known oncogene HRAS. A smaller interstitial amplification was found on chromosome 22. Additionally we found a cluster amplification of a short region on chromosome X, which is present in the germline as well as the tumor. Unfortunately there were no further therapy options revealed.
Six months later the patient developed severe autoimmune pneumonitis and increasing neurological deficits. Cerebral MRI raised suspicion of carcinomatous meningitis which was confirmed by cerebrospinal fluid puncture. Within a few days the patient's condition deteriorated drastically and he died of respiratory insufficiency.
Discussion
Immune checkpoint inhibition is a promising new therapy for metastatic Merkelcell carcinoma, which has good response rates and an improved long-term response to therapy. In our case of a refractory Merkelcell carcinoma under chemotherapy, a complete response was achieved after switching to immune checkpoint inhibition. The novelty of the case presented here is due to several respects: it is one of the very few cases of Merkel cell carcinoma with cerebral metastases that regressed under therapy with checkpoint inhibition and whole brain radiotherapy and even showed complete response.
Possible mechanisms leading to treatment resistance in brain metastases could be hyperactivation of AKT (p-AKT) and loss of PTEN expression [15]. In our particular case, brain metastases derived from combined radiotherapy and continuing immunotherapy. Effectivness is as well described for brain metastases in advanced melanoma [16]. It was shown that radiotherapy has the ability to prime an endogenous antigen-specific immune response by increasing the percentage of antigen-experienced T cells and effector memory T cells in mouse model using the small animal radiation research platform [17].
Due to improved treatment options and prolonged survival, serial therapies are gaining importance. Whole exom sequencing could be recommended to evaluate further therapy options, even if in our case no therapeutic consequences resulted from the analysis.
Conclusion
The here presented case is of special interest since it impressively illustrates the possible therapeutic effects of combined treatment with check point inhibition and radiotherapy in advanced Merkel cell carcinoma with brain metastases.
Dr. Koechel has nothing to disclose. Dr. Hilke has nothing to disclose. Dr. Klumpp has nothing to disclose. Dr. Schroeder reports grants from Novartis, grants from BMS Stiftung Immunonkologie, outside the submitted work. Dr. Eigentler reports personal fees from Bristol Myers Squibb, personal fees from Roche Pharma AG, personal fees from MSD, personal fees from Pierre Fabre, personal fees from LEO Pharma, personal fees from Sanofi/Regeneron, personal fees from Novartis, outside the submitted work. Dr. Lomberg has nothing to disclose. Dr. Garbe reports personal fees from Amgen, personal fees from MSD, grants and personal fees from Novartis, grants and personal fees from NeraCare, grants and personal fees from BMS, personal fees from Pierre Fabre, personal fees from Philogen, grants and personal fees from Roche, grants and personal fees from Sanofi, outside the submitted work. Dr. Leiter has nothing to disclose. The authors declare that there is no conflict of interest regarding the publication of this article. Ethical approval is not required at our institution to publish an anonymous case report. There were no grants supporting this work. Informed consent was obtained.
- Albores‐Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, et al. (2010) Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37: 20-27. Link: https://bit.ly/3wTiRRf
- Iyer JG, Blom A, Doumani R, Lewis C, Tarabadkar ES, et al. (2014) Response rate and durability of chemotherapy for metastatic Merkel cell carcinoma among 62 patients. American Society of Clinical Oncology 32. Link: https://bit.ly/2RjUSeU
- Tai PT, Yu E, Winquist E, Hammond A, Stitt L, et al. (2000) Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol 18: 2493-2499. Link: https://bit.ly/3pe9FUM
- Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, et al. (2011) Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res 17: 6671-6680. Link: https://bit.ly/3uMKPMQ
- Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, et al. (2013) Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res 19: 5351-5360. Link: https://bit.ly/3vQ9fXa
- Paulson KG, Carter JJ, Johnson LG , Cahill KW , Iyer JG ,et al. (2010) Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res 70: 388-8397. Link: https://bit.ly/2RhSqpf
- Goh G, Walradt T, Markarov V, Blom A, Riaz N, et al. (2016) Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 7: 3403. Link: https://bit.ly/3phwirB
- Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, et al. (2013) PD-L1 expression in the merkel cell carcinoma microenvironment: association with inflammation, merkel cell polyomavirus, and overall survival. Cancer Immunol Res 1: 54-63. Link: https://bit.ly/3ikZ7Si
- Terheyden P, Becker JC (2017) New developments in the biology and the treatment of metastatic Merkel cell carcinoma. Curr Opin Oncol 29: 221-226. Link: https://bit.ly/3pe7NLZ
- Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, et al. (2016) PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 374: 2542-2552. Link: https://bit.ly/3cduD14
- Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, et al. (2016) Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncology 17: 1374-1385. Link: https://bit.ly/3pi35fX
- Lebbe C, Becker JC, Grob JJ, Malvehy J, Del Marmol V, et al. (2015) Diagnosis and treatment of Merkel cell carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer 51: 2396-2403. Link: https://bit.ly/3iih7wL
- Lemos BD, Storer BE, Iyer JG , Phillips JL, Bichakjian CK, et al. (2010) Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 63: 751-761. Link: https://bit.ly/34HaWub
- What does Cancer Genome Interpreter do?. Link: https://bit.ly/3wU3aJG
- Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, et al. (2013) Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med 2: 76-85. Link: https://bit.ly/3wShSAD
- Ajithkumar T, Parkinson C, Fife K, Corrie P, Jefferies S, et al. (2015) Evolving treatment options for melanoma brain metastases. Lancet Oncol 16: e486-e497. Link: https://bit.ly/3g9MzLb
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, et al. (2015) Stereotactic Radiation Therapy Augments Antigen-Specific PD-1–Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 3: 345-355. Link: https://bit.ly/2RXIykZ

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
