Global Journal of Medical and Clinical Case Reports
Will the sulphur polypore (laetiporus sulphureus) become a new functional food?
Jiri Patocka1,2*
2Biomedical Research Centre, University Hospital, Hradec Kralove, Czech Republic
Cite this as
Patocka J (2019) Will the sulphur polypore (laetiporus sulphureus) become a new functional food?. Glob J Medical Clin Case Rep 6(1): 006-009. DOI: 10.17352/2455-5282.000068Mushrooms are a rich source of chemical compounds. Such a mushroom is also polypore laetiporus sulphureus, in which a large number of bioactive substances with cytotoxic, antimicrobial, anticancer, anti-inflammatory, hypoglycemic, and antioxidant activity have been found. This short review summarizes the results of the most important chemical and biological studies of the fruiting bodies and the mycelial cultures of L. sulphureus. Since the ingredients of this edible mushroom have beneficial effects on human health, it could become a functional food.
Introduction
The sulphur shelf (laetiporus sulphureus Bull.:Fr.) Murrill.), also known as crab-of-the-woods or chicken-of-the-woods, is a saprophyte mushroom from the family Polyporaceae that grow on trees in Europe, Asia and North America. Its fruit bodies have a striking golden-yellow color and grow on tree trunks and branches (Figure 1). Old fruits slowly fade into pale beige or pale gray. L. sulphureus was first described by French mycologist Pierre Bulliard in 1789 as Boletus sulphureus. The current name comes from American mycologist William Murill. The mushroom is used as a food or as a folk medicine. It contains a large number of biologically active substances that have a beneficial effect on human health. It can therefore be assumed that it could be a new functional food and help with some diseases.
Food and/or medicine?
Over the generations, this mushroom has become an integral part of some national cuisines particularly for its taste. Besides, it is used in folk medicine for treatment of coughs, pyretic diseases, gastric cancer and rheumatism. Young fruit bodies are edible and their taste is described like crab or lobster. It is highly regarded in Great Britain, Germany, and North America and it is considered as potential source of natural antioxidants [1]. Some people have severe gastrointestinal adverse reactions after eating [2], including vomiting and fever, but this is now thought to be the result of confusion with morphologically similar species such as L. huroniensis and L. gilbertsonii [3].
Nutritioal values are 360 kcal/100 g of fresh fruiting bodies, total carbohydrates content was 64.9, proteins 11.9 and fats 5.9 g/100g of the dry mass of fruiting body [4]. Fats are represented by long chain fatty acids with 16 to 20 carbons and ethyl esters of fatty acids with 16 to 24 carbons, as well as sterols (ergosterol, ergosta-7,22-dien-3β-ol, ergosta-7-en-3β-ol and 24 ethylcholestan-3β-ol) [5].
The mushroom is remarkable in many respects and is valued as a significant source of numerous biologically active substances with potential use in many fields of human activity, but especially in medicine [6]. The presence of substances with favorable effect on human health makes the L. sulphureus a new functional food [7]. Experiments with its cultivation on artificial substrates [8], have already begun to be produced in large quantities outside the forest, like, for example button mushroom (Agaricus bisporus) or the oyster mushroom (Pleurotus ostreatus).
Bioactive substances
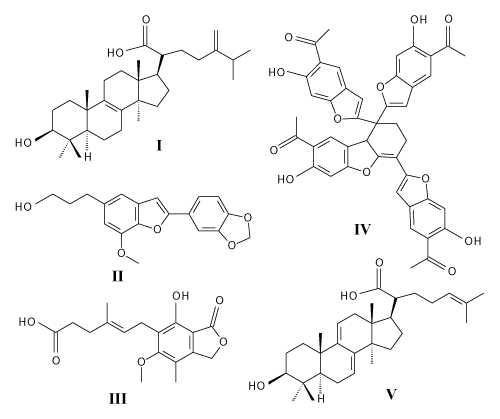
The most important bioactive compounds of this mushroom are lectins Tateno et al., [9-11] polysaccharides [12-18], phenols Olennikov et al., [1,19,20]m terpenoids León et al., [21-23], enzymes [24,25], polyene pigments [26], and polyunsaturated fatty acids [27]. The chemical formulas of some of the major bioactive substances L. sulphureus are shown in figure 2.
Pharmacology effects
The study of the pharmacological effects of L. sulphureus bioactive substances stems from the fact that this mushroom has been used for many centuries in the traditional folk medicine of many countries. The identified pharmacological effects confirm well-known, traditional uses and also reveal new possibilities. Cytotoxic, anticancer, antimicrobial, anti-inflammatory, hypoglycemic, and antioxidant effects were observed. A number of biologically active substances found in L. sulphureus are involved in the pharmacological effects. Structures of some are shown in figure 2.
Cytotoxic and anticancer efefects
Triterpenoids of lanostan-type isolated from the fruiting bodies demonstrate cytotoxic activity. Eburicoic acid (I, EA) is one of main cytotoxic components isolated from L. sulphureus. This substance suppresses the activation of macrophages, thereby alleviating the progression of inflammatory diseases [6]. EA does not cause any apparent cytotoxicity but significantly inhibits the release of inflammatory mediators, suppressed levels of mRNA expression, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 COX-2, and proinflammatory cytokines TNF-α, IL-6 and IL-1β. EA also reduces levels of phosphorylated PI3K, Akt, mTOR and NF-κBp65 in LPS-induced RAW264.7 cells [6]. Anti-inflammatory effects have also been demonstrated for acetyl-EA acid found in L. sulphureus var. miniature [28]. Similarly to the lanostan-type tetracyclic triterpenoids which are potential anticancer compounds [29], also illudin-type sesquiterpenoids from the L. sulphureus are responsible for cytotoxicity (He et al., 2015).
Cytotoxic activities were observed also in further compouds isolated from L. sulphureus: phenolic compounds of the benzofuran lignans type egonol (II), demethoxyegonol and egonol glucoside [30], mycophenolic acid (III) and its derivatives [20], laetirobin (IV) [31] which is capable of blocking tumor cell division (mitosis) and invoking apoptosis, and carboxymethyl derivatives of α-(1→3)-D-glucans which have a significant activity to inhibit tumor cell lines metabolism without significantly inhibiting normal cells metabolism [18]. Also, cyclodepsipeptide beauvericin was found in this mushroom [32].
Antimicrobial effect
The first mention of the antibacterial effect of L. sulphureus can be found in the study of Suay et al., [33], who investigated antimicrobial activity of 204 basidiomycetes. Antimicrobial activity of L. sulphureus against a wide spectrum of gram-positive and gram-negative bacteria including methicillin-resistant strain of Staphylococcus aureus (MRSA) and glycopeptide-resistant strain of Leuconostoc mesenteroides was recorded by Ershova et al., [34]. Different fruiting bodies extracts demonstrated an antimicrobial activity against the following strains: Bacillus cereus and B. subtilis, Micrococcus flavus and M. luteus [35], Enterococcus faecium and Proteus vulgaris [36], Bacillus cereus, Enterobacter cloacae, Escherichia coli, Listeria monocytogenes, Micrococcus flavus, Pseudomonas aeruginosa, Salmonella typhimurium and Staphylococcus aureus [37]. Antifungal activity of extracts was described against: Candida albicans [35], Alternaria alternata, Aspergillus wentii, Fusarium tricinctum, Microsporum gypseum, Penicillium gladioli and P. griseofulvum (Sakeyan 2006), Aspergillus niger, Botrytis cinerea, Fusarium oxysporum, and Sclerotinia sclerotiorum [38].
Anti-inflammatory effect
The anti-inflammatory effect of L. sulphureus is explained by the presence of exopolysaccharide (EPS) which protects cells from apoptosis by significantly inducing inhibition of pro-inflammatory mediators in cells such as nitric oxide (NO), prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) without significant cytotoxicity [16] and also by presence lanostane triterpenoids which were identified as eburicoic acid derivatives [28]. These triterepenoids inhibited the NO production and supressed the production of pro-inflammatory cytokines, mainly inducible nitric oxide synthase, cyclooxygenase-2, interleukin (IL)-1β, IL-6 and TNF-α. Eburicoic acid is the main bioactive metabolite in the L. sulphureus against gastric ulcers in mice model [39].
Hypoglycemic effect
EPS also demonstrated the hypoglycemic effect in rats with single dose streptozotocin induced diabetes and caused an increased proliferation and regeneration of pancreatic islet β cells [13]. Other compound with anti-diabetic potential is dehydrotrametenolic acid (V), also isolated from fruiting bodies. It induces the differentiation of adipocytes in vitro, and reduces hyperglycemia in mice with non-insulin-dependent diabetes mellitus (Sato et al., 2002). Also EA (I) has antidiabetic and antihyperlipidemic effect and therapeutic potential in the treatment of type 2 diabetes and hyperlipidemia [40-42].
Conclusion
In recent years, mushrooms are increasingly being searched for new biologically active substances. Since mushrooms are an important part of the daily diet of people in many countries, their analysis is valuable in terms of natural chemoprevention. This is also the case with L. sulphureus, which contains biologically significant substances and could be considered a functional food. Several secondary metabolites have been discovered in the mushrooms of this fungus and have been shown to be cytotoxic, antitumor, antimicrobial, anti-inflammatory, and antidiabetic.
Competing interest
I declare that I have no competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
The research was funded by the Institutional program of the University Hospital Hradec Králové, Czech Republic.
- Klaus A, Kozarski M, Niksic M, Jakovljevic D, Todorovic N, et al. (2013) The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int J Food Sci Nutr 64: 599-610. Link: https://bit.ly/2Rf41Rf
- Miller KK, Miller HH, Miller OE (1981) Mushrooms in Color. South China Printing Co 286.
- Volk TJ (2001) Laetiporus cincinnatus, the white-pored chicken of the woods, Tom Volk's Fungus of the Month for July 2001. Tom Volk's Fungi. Link: https://bit.ly/2WI8FNR
- Ayaz FA, Torun H, Ozel A, Col M, Duran C, et al. (2011) Nutritional value of some wild edible mushrooms from Black Sea Region (Turkey). Turk J Biochem 36: 385-393. Link: https://bit.ly/2WEeOup
- Ericsson DCB, Ivonne JNR (2009) Sterol composition of the macromycete fungus Laetiporus sulphureus. Chem Nat Compd 45: 193-196. Link: https://bit.ly/2wTkUaR
- Wang J, Zhang P, He H, Se X, Sun W, et al. (2017) Eburicoic acid from Laetiporus sulphureus (Bull.:Fr.) Murrill attenuates inflammatory responses through inhibiting LPS-induced activation of PI3K/Akt/mTOR/NF-κB pathways in RAW264.7 cells. Naunyn Schmiedebergs Arch Pharmacol 390: 845-856. Link: https://bit.ly/2Wzm0mM
- Zhao H, Lan Y, Liu H, Zhu Y, Liu W, et al. (2017) Antioxidant and Hepatoprotective Activities of Polysaccharides from Spent Mushroom Substrates (Laetiporus sulphureus) in Acute Alcohol-Induced Mice. Oxid Med Cell Longev 2017: 5863523. Link: https://bit.ly/2KjsbJZ
- Pleszczyńska M, Wiater A, Siwulski M, Szczodrak J (2013) Successful large-scale production of fruiting bodies of Laetiporus sulphureus (Bull.: Fr.) Murrill on an artificial substrate. World J Microbiol Biotechnol 29: 753-758. Link: https://bit.ly/2WHzMIF
- Mancheño JM, Tateno H, Goldstein IJ, Hermoso JA (2004) Crystallization and preliminary crystallographic analysis of a novel haemolytic lectin from the mushroom Laetiporus sulphureus. Acta Crystallogr D Biol Crystallogr 60: 1139-1141. Link: https://bit.ly/2F82F6b
- Mancheño JM, Tateno H, Goldstein IJ, Martínez-Ripoll M, Hermoso JA (2005) Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J Biol Chem 280: 17251-17259. Link: https://bit.ly/2WC15iT
- Mancheño JM, Tateno H, Sher D, Goldstein IJ (2010) Laetiporus sulphureus lectin and aerolysin protein family. Adv Exp Med Biol 677: 67-80. Link: https://bit.ly/2F7Bx7n
- Angulo I, Acebrón I, de las Rivas B, Muñoz R, Rodríguez-Crespo I, et al. (2011) High-resolution structural insights on the sugar-recognition and fusion tag properties of a versatile β-trefoil lectin domain from the mushroom Laetiporus sulphureus. Glycobiology 21: 1349-1361. Link: https://bit.ly/2F9GdJM
- Hwang HS, Lee SH, Baek YM, Kim SW, Jeong YK, et al. (2008) Production of extracellular polysaccharides by submerged mycelial culture of Laetiporus sulphureus var. miniatus and their insulinotropic properties. Appl Microbiol Biotechnol 78: 419-429. Link: https://bit.ly/2XI5dz3
- Olennikov DN, Agafonova SV, Borovskiĭ GB, Penzina TA, Rokhin AV (2009a) Alkali-soluble polysaccharides of Laetiporus sulphureus (Bull.: Fr.) Murr fruit bodies. Prikl Biokhim Mikrobiol 45: 693-697. Link: https://bit.ly/2XJgLSC
- Olennikov DN, Agafonova SV, Borovskiĭ GB, Penzina TA, Rokhin AV (2009b) Water-soluble endopolysaccharides from the fruiting bodies of Laetiporus sulphureus (Bull.: Fr.) Murr. Prikl Biokhim Mikrobiol 45: 597-605. Link: https://bit.ly/2ZmLeXe
- Jayasooriya RG, Kang CH, Seo MJ, Choi YH, Jeong YK, et al. (2011) Exopolysaccharide of Laetiporus sulphureus var. miniatus downregulates LPS-induced production of NO, PGE₂, and TNF-α in BV2 microglia cells via suppression of the NF-κB pathway. Food Chem Toxicol 49: 2758-2764. Link: https://bit.ly/2IeQerf
- Seo MJ, Kang BW, Park JU, Kim MJ, Lee HH, et al. (2011) Biochemical characterization of the exopolysaccharide purified from Laetiporus sulphureus mycelia. J Microbiol Biotechnol 21: 1287-1293. Link: https://bit.ly/2XEyGKs
- Wiater A, Paduch R, Pleszczyńska M, Próchniak K, Choma A, et al. (2011) α-(1 → 3)-D-glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol Lett 33: 787-795. Link: https://bit.ly/2IcWRtS
- Sulkowska-Ziaja K, Muszynska B, Motyl P, Pasko P, Ekiert H (2012) Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int J Med Mushrooms 14: 385-393. Link: https://bit.ly/2RgAqXN
- Fan QY, Yin X, Li ZH, Li Y, Liu JK, et al. (2014) Mycophenolic acid derivatives from cultures of the mushroom Laetiporus sulphureu. Chin J Nat Med 12: 685-688. Link: https://bit.ly/2ZnRbmC
- He JB, Tao J, Miao XS, Bu W, Zhang S, et al. (2015a) Seven new drimane-type sesquiterpenoids from cultures of fungus Laetiporus sulphureus. Fitoterapia 102: 1-6. Link: https://bit.ly/2wOAmoK
- Yin X, Li ZH, Li Y, Feng T, Liu JK (2015) Four lanostane-type triterpenes from the fruiting bodies of mushroom Laetiporus sulphureus var. miniatus. J Asian Nat Prod Res 17: 793-799. Link: https://bit.ly/2KjrCzR
- He JB, Tao J, Miao XS, Feng YP, Bu W, et al. (2015b) Two new illudin type sesquiterpenoids from cultures of Phellinus tuberculosus and Laetiporus sulphureus. J Asian Nat Prod Res 17: 1054-1058. Link: https://bit.ly/2WJRmvV
- Lee JW, Park JY, Kwon M, Choi IG (2009) Purification and characterization of a thermostable xylanase from the brown-rot fungus Laetiporus sulphureus. J Biosci Bioeng 107: 33-37. Link: https://bit.ly/2MM2wf7
- Hong MR, Kim YS, Joo AR, Lee JK, Kim YS, et al. (2009) Purification and characterization of a thermostable beta-1,3-1,4-glucanase from Laetiporus sulphureus var. miniatus. J Microbiol Biotechnol 19: 818-822. Link: https://bit.ly/2KNX9tc
- Davoli P, Mucci A, Schenetti L, Weber RW (2005) Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 66: 817-823. Link: https://bit.ly/2XEYiXs
- Sinanoglou VJ, Zoumpoulakis P, Heropoulos G, Proestos C, Ćirić A, et al. (2015) Lipid and fatty acid profile of the edible fungus Laetiporus sulphurous. Antifungal and antibacterial properties. J Food Sci Technol 52: 3264-3272. Link: https://bit.ly/2ZqvYIV
- Saba E, Son Y, Jeon BR, Kim SE, Lee IK, et al. (2015) Acetyl Eburicoic Acid from Laetiporus sulphureus var. miniatus Suppresses Inflammation in Murine Macrophage RAW 264.7 Cells. Mycobiology 43: 131-136. Link: https://bit.ly/2IeLx0I
- Ríos JL, Andújar I, Recio MC, Giner RM (2012) Lanostanoids from fungi: a group of potential anticancer compounds. J Nat Prod 75: 2016-2044. Link: https://bit.ly/2MNMmSp
- Yoshikawa K, Bando S, Arihara S, Matsumura E, Katayama S (2001) A benzofuran glycoside and an acetylenic acid from the fungus Laetiporus sulphureus var. miniatus. Chem Pharm Bull (Tokyo) 49: 327-329. Link: https://bit.ly/2wTmrh7
- Lear MJ, Simon O, Foley TL, Burkart MD, Baiga TJ, et al. (2009) Laetirobin from the parasitic growth of Laetiporus sulphureus on Robinia pseudoacacia. J Nat Prod 72: 1980-1987. Link: https://bit.ly/2RcpEld
- Deol BS, Ridley DD, Singh P (1978) Isolation of cyclodepsipeptides from plant pathogenic fungi. Aust J Chem 31: 1397-1399. Link: https://bit.ly/2wQrdvV
- Suay I, Arenal F, Asensio FJ, Basilio A, Cabello MA, et al. (2000) Screening of basidiomycetes for antimicrobial activities. Antonie van Leeuwenhoek 78: 129-140. Link: https://bit.ly/2WBZIRp
- Ershova EI, Tikhonova OV, Lur'e LM, Efremenkova OV, Kamzolkina OV, et al. (2003) Antimicrobial activity of Laetiporus sulphureus strains grown in submerged culture. Antibiot Khimioter 48: 18-22. Link: https://bit.ly/2MKR8jn
- Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K (2006) Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem 101: 267-273. Link: https://bit.ly/2Idm8nX
- Demir MS, Yamaç M (2008) Antimicrobial activities of Basidiocarp, submerged mycelium ande xopolysaccharide of some native Basidiomycetes strains. JABS 2: 89-93. Link: https://bit.ly/2wQwF1V
- Šiljegovi J, Stojković D, Nikolić M, Glamočlija J, Soković M, et al. (2011) Antimicrobial activity of aqueous extract of Laetiporus sulphureus (Bull.: Fr.) Murill. Proc. Nat Sci 120: 297-303. Link: https://bit.ly/2MLVs1K
- Pârvu M, Andrei AŞ, Roşca-Casian O (2010) Antifungal activity of Laetiporus sulphureus mushroom extract. Contrib Bot 45: 65–70. Link: https://bit.ly/2IbOIGi
- Wang J, Sun W, Luo H, He H, Deng W, et al. (2015) Protective Effect of Eburicoic Acid of the Chicken of the Woods Mushroom, Laetiporus sulphureus (Higher Basidiomycetes), Against Gastric Ulcers in Mice. Int J Med Mushrooms 17: 619-626. Link: https://bit.ly/31sd0DH
- Lin CH, Kuo YH, Shih CC (2017) Eburicoic Acid, a Triterpenoid Compound from Antrodia camphorata, Displays Antidiabetic and Antihyperlipidemic Effects in Palmitate-Treated C2C12 Myotubes and in High-Fat Diet-Fed Mice. Int J Mol Sci 18: E2314. Link: https://bit.ly/2F4K6zW
- León F, Quintana J, Rivera A, Estévez F, Bermejo J (2008) Lanostanoid triterpenes from Laetiporus sulphureus and apoptosis induction on HL-60 human myeloid leukemia cells. J Nat Prod 67: 2008-2011. Link: https://bit.ly/2WBDv5K
- Yoshikawa K, Bando S, Arihara S, Matsumura E, Katayama S (2001) A benzofuran glycoside and an acetylenic acid from the fungus Laetiporus sulphureus var. miniatus. Chem Pharm Bull (Tokyo) 49: 327-329. Link: https://bit.ly/2wTmrh7

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
