Global Journal of Fertility and Research
Sperm immobilization factor of Candida albicans: A proposed mechanism of infertility in female mice
Sareeka Kumari, Aditi Chauhan, Deepali Thaper and Vijay Prabha*
Cite this as
Kumari S, Chauhan A, Thaper D, Prabha V (2019) Sperm immobilization factor of Candida albicans: A proposed mechanism of infertility in female mice. Glob J Fertil Res 5(1): 001-006. DOI: 10.17352/gjfr.000014Candida species are the most frequently isolated fungi, responsible for blood stream and urinary tract infections. Various studies have shown Candida albicans to be associated with impediment of sperm parameters. Therefore to determine the underlying mechanism, the standard strain of Candida albicans (MTCC 1637) was employed and it was found to cause complete immobilization of mouse spermatozoa in vitro. Further, sperm immobilization activity was shown by only cell-free supernatant, indicating that the sperm immobilization factor might be released extracellularly, as no activity was observed with the washed cells. The sperm immobilization factor (SIF) from supernatant was precipitated out with ammonium sulphate at the saturation of 60% to 80% and was purified by gel permeation chromatography followed by ion exchange chromatography. The molecular weight of SIF was found to be ~29 kDa. Further, SIF at a concentration of 50µg was capable of causing 100% immobilization of mouse spermatozoa within 30min of incubation at 370C, whereas at 100µg concentration resulted in complete loss of viability. Scanning electron microscopy showed profound morphological alterations with decapitation in mouse spermatozoa after the treatment with SIF. Also, total loss of Mg2+ ATPase activity of mouse spermatozoa was observed at a concentration of 75 and 100µg/ml. Further, in vivo study revealed that the intravaginal application of SIF (10µg) before mating completely avert conception in female mice as no pregnancy related changes were observed in comparison to female mice receiving PBS.
Introduction
Candida albicans, a dimorphic fungus, is usually encountered as a normal inhabitant of human mucosal surfaces [1]. Apart from being residing as a harmless commensal as a normal microbiota, it also has the notoriety of being implicated in superficial (oral and vaginal) and systemic candidiasis, giving rise to severe morbidity in millions of individuals worldwide. This transformation from normal harmless commensal to dangerous pathogen is due to slight alteration in the physiological state of the host (long term antibiotic treatment and compromised local immune/barrier defence) [2]. C. albicans possesses various virulence attributes that contribute to general survival, fitness and persistence within the host cell as well as specific factors associated with adhesion, invasion, cell damage and induction/evasion of host response (Calderone and Fonzi) [3]. Adhesion is one of the essential step for colonization and establishment of Candida infections in the host cell and it is accomplished by various adhesins [4]. Following this invasion fungal hydrolases drive the active penetration of fungus into the host cells [5]. Another important feature of C. albicans is its ability to form biofilm and phenotypic switching, one of the important virulence determinants which play an important role in its pathogenesis [6]. Along with the infection C. albicans has also been associated with sperm impairing property [7-9]. The sperm impairment is not only due to the culture, but the soluble factors of fungal metabolism (SFFM) as well as its quorum sensing molecule, farnesol which directly affect human sperm parameters [9]. In an earlier work done in our laboratory, infertility as a consequence of intravaginal inoculation with sperm impairing S. aureus, E. coli, S. marcescens and their corresponding factors has been observed [10-12]. Apart from bacterial strains, the fungus strain, C. albicans also resulted in loss of fertility in female mice. Except infertility, no other clinical manifestation could be seen apparently or histologically. Therefore, the mechanism for infertility was conjectured to be the sperm immobilization property of C. albicans [12]. Hence, the present study was intended with an aim to isolate and purify the sperm immobilization factor from C. albicans and to evaluate its effect on fertility outcome of female BALB/c mice.
Materials and methods
Microorganism
The standard strain of Candida albicans (MTCC 1637) used in the present study was already available in the laboratory.
Experimental animals
Sexually mature, 4 to 5week old female (22±2g) and 5 to 6week old male (25±2g) BALB/c mice were used in the present study. The mice were initialized under standard laboratory conditions in propylene cages bedded with clean rice husk at 20 to 25ºC, in well aerated Central Animal House, Sector-14, Panjab University, Chandigarh. All the animals were given standard pellet diet (M/s Ashirwad Industries Pvt. Ltd.) and water ad libitum. Animals were habituated in the new housing and experimental conditions for at least one week. All the experimental protocols were approved by the institutional Animal Ethics Committee of the Panjab university, Chandigarh vide number IAEC 52, 2017. The experiments were performed in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Extraction and purification of sperm immobilization factor (SIF)
The standard strain of C. albicans was grown in Sabouraud dextrose broth for 72h under shaking conditions (150rpm) at 28°C. The culture was centrifuged at 10,000rpm for 20min at 4°C and the supernatant was collected and subjected to ammonium sulphate precipitation to get a final saturation of 20, 40, 60, 80 and 100%. The precipitates were collected by centrifugation at 10,000rpm for 20min at 4°C. The pelleted protein was resolubilized in minimum amount of PBS (50mM, pH-7.2) and dialyzed against PBS at 4°C. The resolubilized and dialyzed protein was then concentrated against Polyethylene glycol at 4°C and checked for sperm immobilization activity.
Molecular sieving
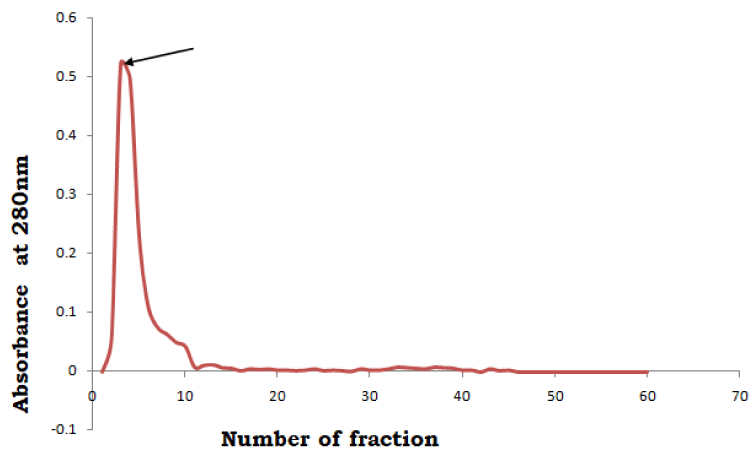
For purification of SIF, the precipitated (60% to 80%), dialyzed and concentrated protein was loaded on to the Sephadex G-200 column (2cm x 31cm) and the elution was carried out with PBS. The fractions of 3ml each were collected and absorbance was taken at 280nm. The fractions (10 to 12) showing immobilization of spermatozoa were pooled and concentrated against Polyethylene glycol at 4°C.
Diethylaminoethyl (DEAE) cellulose column chromatography
The pooled and concentrated fractions obtained by gel permeation chromatography (G-200) were applied directly onto the DEAE cellulose column. Before final elution, 40ml of elution buffer was allowed to run from the column. Final elution was carried out with PBS (50mM, pH-7.2) containing 0.05, 0.1, 0.2, 0.4 and 0.6M NaCl. Fractions of 4ml each were collected and absorbance was taken at 280nm. The fractions showing immobilization of spermatozoa were pooled and concentrated against Polyethylene glycol at 4°C.
Determination of molecular weight of SIF
The molecular weight of SIF was estimated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using standard molecular weight markers. A 7% gel was prepared and after the completion of run, the gel was silver stained and molecular weight was estimated.
Impact of SIF on various mouse sperm parameters: in vitro studies
Extraction of mouse spermatozoa: Male mice were sacrificed by the method of cervical dislocation. To collect spermatozoa the abdominal cavity was cut opened and both the vas deferens were gently pulled out and teased with the help of scalpel in pre warmed Phosphate buffer saline (50mM PBS, pH-7.2). The final count was adjusted to 40x106 spermatozoa ml-1 for further use in the experiments.
Sperm motility: The sperm motility was determined by the method given by Emmens [13].
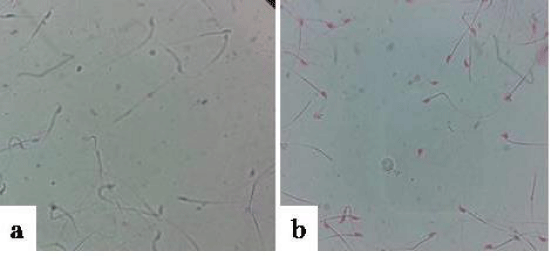
Sperm viability: For determining the sperm viability an equal volume of SIF and sperm suspension were mixed and incubated at 37°C and at different time intervals, the mixture was gently mixed with 0.5% Eosin Y and observed under a bright field microscope at 400X magnification.
Sperm morphology: The effect of SIF on morphology of mouse spermatozoa was evaluated by using scanning electron microscopy (SEM). Equal volume of purified SIF and sperm suspension was incubated at 37ºC for 1h. 1.5% buffered gluteraldehyde was added gently to the mixture and incubated at 37ºC for 30min. The suspension was then washed twice in PBS (50mM, pH-7.2) by centrifugation for 5min at 550rpm. One drop of fixed and washed spermatozoa was placed on silver-painted adhesive tape mounted on brass stubs and air-dried and One hundred angstrom gold coating was done on a Jeol fine-coat ion sputter and the specimens were observed.
Sperm Mg2+ ATPase activity: Mg2+ ATPase activity of spermatozoa was studied according to protocol of [14] and Chappell with slight modifications. Sperm suspension containing 1×108/ml spermatozoa was washed in Tris-HCl (0.2M, pH-7.6) and was sonicated at 50Hz for 10min (10 cycles of 30sec with 1min interval) at 4ºC. To 0.2ml of sonicated sperm suspension, 0.2ml of Tris-HCl buffer (0.2M, pH-7.6), 0.2ml of MgCl2 (5mM) and 0.2ml of ATP (6mg/ml) and 0.2 ml of SIF (25, 50, 75 and 100µg) were added and the mixture was incubated at 37ºC for 1h. After incubation, 1ml of cold TCA (Trichloroacetic acid, 10%) was added to the reaction mixture in order to stop the reaction. This mixture was then incubated at 4ºC overnight for protein precipitation. The control tubes contained all the components of the reaction mixture but TCA was added in the beginning to stop the ATPase activity. Inorganic phosphorus (Pi) released was determined according to the method of Boyce et al., [15]. One unit of ATPase is expressed as µg of the Pi released after 1h of incubation.
Contraceptive efficacy of SIF on fertility outcome in female BALB/c mice: in vivo study
For evaluation of contraceptive efficacy of SIF, female mice synchronised in their oestrous cycles by Whitten effect were divided into three groups (Group I, II and III). The group I mice was administered intravaginally with single dose of 20µl PBS and served as control. Group II and III were intravaginally inoculated with SIF 10µg and 50µg, respectively. All the animals were mated overnight immediately after administration of SIF with proven breeder male mice (two female mice and one male mice in each cage). On next morning, mating was confirmed by presence of vaginal plug and mated animals were separated and kept under observation until the delivery of pups.
Results
The standard strain of C. albicans used in present study was capable of immobilizing the spermatozoa in vitro and this property of immobilization was found to be associated with cell free supernatant only.
Extraction and purification of sperm immobilization factor (SIF) from C. albicans
When the cell free supernatant of C. albicans was subjected to ammonium sulphate precipitation, the results showed that sperm immobilization factor could be precipitated out with ammonium sulphate at 60-80% saturation. The ammonium sulphate precipitated protein re-dissolved and dialyzed against PBS (50mM, pH-7.2), was subjected to purification by filtration through Sephadex G-200 column. The column chromatographic pattern showed that the immobilization activity was present in the fractions 10-12 with a peak value in fraction 11, where each fraction was of 3ml quantity (Figure 1). The fractions that showed immobilizing activity were pooled and concentrated using polyethylene glycol (PEG 6000). After gel filtration chromatography, the concentrated fractions so obtained were applied to DEAE-cellulose column. The results revealed that the bioactive component could be eluted with PBS (50mM, pH-7.2) in the fractions 3-5 with a peak value in fraction 3, where each fraction was of 4ml (Figure 2). These bioactive fractions were again pooled and concentrated by PEG-6000.
Purification status and molecular weight estimation of SIF using SDS PAGE
The purification status and molecular weight of SIF obtained after ion-exchange chromatography was estimated by SDS-PAGE. After denaturation, 20µl of SIF was loaded on the gel and electrophoresis was done using 7% separating gel. The silver stained gel showed a single band indicating the purified SIF with a molecular weight of approximately 29kDa (Figure 3).
Impact of SIF on various mouse sperm parameters: in vitro Studies
Motility: When the effect of different concentrations of SIF (10, 25 and 50µg) was checked on mouse spermatozoa, it was observed that SIF at a concentration of 50µg showed complete immobilization of spermatozoa within 30min of incubation.
Viability: In order to assess the impact of SIF on sperm viability, dye-exclusion method using Eosin Y was performed. The results showed that SIF at concentration of 100µg could lead to complete sperm death as compared to control within 30min of incubation (Figure 4).
Morphology: The effect of SIF on morphology of mouse spermatozoa was evaluated by using scanning electron microscopy (SEM). The results revealed that SIF at 50µg concentration could induce profound morphological alterations in mouse spermatozoa followed by decapitation (Figure 5).
Mg2+ ATPase activity: The effect of SIF on Mg2+ ATPase activity of mouse spermatozoa was also examined. It was observed that SIF could significantly inhibit Mg2+ ATPase activity of mouse spermatozoa. The activity was reduced from 1320units (control) to 800, 200, 0, 0 units in the presence of SIF at a concentration of 25, 50, 75 and 100µg, respectively (Table1).
Effect of SIF on fertility outcome in female BALB/c mice: in vivo study
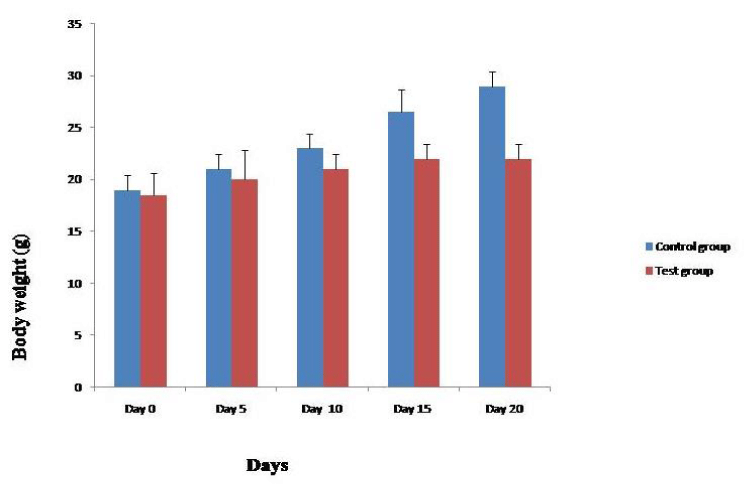
SIF was evaluated for its impact on fertility outcome of female mice. It was observed that Group I mice receiving 20µl of PBS remained fertile as evident by consistent weight gain and delivery of pups at the end of gestation period (Figures 6,7) and in case of mice treated with 10μg and 50μg of SIF, the results showed that SIF at concentration of 10μg could efficiently block conception in female mice as mice did not show any pregnancy related changes and failed to deliver pups at the end of gestation period.
Discussion
Fungal pathogens are the opportunistic commensal fungi that commonly colonize the mucosal surface of healthy host. The fungal pathogens cause minor infections in immunocompromised individuals, such as thrush in babies and vaginal infections in women. This infection turns to be fatal in immunocompromised individuals when it becomes systemic. Candida species now ranks as the fourth most common cause of noscomial blood stream infections worldwide with 50% attributable mortality rate [16]. About 70% of women experienced vaginal infections caused by Candida species and 20% of them also suffer from recurrence [17]. For decades, C. albicans has been associated with various superficial and systemic infections. C. albicans infects female genital tract where it damages the accessory organs of the female reproductive system with enormous detrimental effect on the process of fertilization and later it affects pregnancy and ultimately these consequences lead to infertility [9]. However, the role of C. albicans in relation to infertility is still being debated. There are reports regarding its in vitro role in impediment of sperm parameters, however, in vivo role is yet to be demonstrated [7,8]. In this regard, in an earlier work done in our laboratory, infertility was observed in female mice as a result of intravaginal administration of sperm immobilizing fungal uropathogen, C. albicans [12]. Hence, interest was generated to isolate and purify its corresponding sperm immobilizing factor and evaluate its role in fertility outcome. The cell free supernatant of C. albicans was capable of causing sperm immobilization, whereas the washed cells failed to do so. Hence, cell free supernatant was used to isolate the sperm immobilization factor (SIF) from C. albicans. SIF was extracted from culture supernatant upon precipitation with ammonium sulphate and further purification was carried out by subjecting to sequential chromatographic procedures using Sephadex G-200 and DEAE cellulose. The bioactive molecule was purified with a molecular weight of approximately 29 kDa. Sperm motility and viability are used as indicators of fertilizing potential of spermatozoa. So, if these parameters of spermatozoa are impaired than it would not be able to interact with oocyte and hence it accounts for infertility [18]. In this context, effect of SIF on sperm parameters was investigated in vitro. The results showed that SIF at concentration of 50µg leads to complete immobilization of mouse spermatozoa and at concentration of 100µg there is complete death of mouse spermatozoa. Recent observation by Barbonetti et al., [19], indicated release of an unidentified soluble factor by E. coli that inhibits mitochondrial membrane potential (ΔΨm), motility and vitality of spermatozoa. Burrello et al., [8], also reported the negative effects of experimentally induced C. albicans infection on motility. Rennemeier et al., [20], found a new aspect in the interaction of factors with male gamete where they demonstrated that quorum sensing molecules farnesol and 3-oxododecanoyl-L-homoserine lactone, released by C. albicans and the gram-negative bacterium P. aeruginosa, respectively, elicit multiple deteriorating consequences on mouse spermatozoa. Although visual analysis of spermatozoa by light microscopy allowed us to examine its motility and viability, however, it was found incapable of determining any morphological defects. Since scanning electron microscopy (SEM) offers advantage in the form of thorough insight into the structural and morphological features of spermatozoa, hence, SEM was carried out to examine the effect of purified SIF on mouse spermatozoa. The results showed that SIF at a concentration of 50µg could induce profound morphological alterations in sperm structure indicating that morphological defects might be responsible for the immobilization of spermatozoa. These results are in agreement with those of earlier studies by Prabha et al., [21], who revealed multiple and profound alterations in the superficial structure of spermatozoal head. The motility of spermatozoa requires energy in the form of ATP. Then possess cation dependant (Na+, K+, Mg++) enzyme dynein-ATPase, an intracellular motor for sperm motility, located on the axoneme of spermatozoa that account for the breakdown of ATP to release energy for flagellar contractile processes [22]. The importance of these dyenin arms in motility has clearly been established in several experiments showing a direct correlation between the quantity of dyenin arms present on the axoneme and the sliding velocity [23]. To address the possibility of involvement of cation dependant ATPases in SIF induced sperm impairment, the effect of SIF on Mg2+ dependent ATPase was studied; results showed the negative effect of SIF on Mg2+ dependent ATPase activity. Similar results have been reported by Arias et al., [24], who showed inhibition of the dynein-ATPase activity leading to sperm immobilization. As SIF exhibited noteworthy sperm immobilizing and spermicidal effect in vitro, hence an attempt was made to assess its impact on fertility outcome in female mice. When different concentrations of SIF (10μg and 50μg) were instilled in the vagina of female mice as a single dose before mating, it was observed that SIF at a concentration of 10μg/animal rendered female mice infertile. This result highlighted that SIF at the concentration of 10µg could induce infertility in female mice when applied intravaginally. These fertility studies suggest that presence of SIF in vagina may transform the female genital tract into a hostile milieu for spermatozoa and could play an important role in stimulation of infertility. These results are in concordance with earlier studies performed in our laboratory wherein sperm-impairing Staphylococcus aureus, E. coli and S. marcescens were shown to cause infertility without producing any adverse effects in female BALB/c mice [10-12]. From the above mentioned preliminary observations obtained in the present study, it can be concluded that SIF isolated and purified from a fungus i.e. C. albicans is capable of compromising various sperm parameters viz. motility, viability, morphology and Mg2+ dependent ATPase activity. Moreover, this factor also led to blockage of conception in female mice. Thus, this study revealed the enormous antifertility potential of SIF produced by C. albicans.
- Calderone RA, Clancy CJ (2011) Candida and Candidiasis. American Society for Microbiology Press 196-321. Link: https://tinyurl.com/t6vgxk8
- Naglik JR, Moyes DL, Wächtler B, Hube B (2011) Candida albicans interactions with epithelial cells and mucosal immunity. Microbes and Infection 13: 963-976. Link: https://tinyurl.com/v5vae4v
- Zhu W, Filler SG (2010) Interactions of Candida albicans with epithelial cells. Cell Microbiols 12: 273-282. Link: https://tinyurl.com/ruzqq96
- Naglik JR, Richardson JP, Moyes DL (2014) Candida albicans pathogenicity and epithelial immunity. PLoS Pathogens 10: 100-425. Link: https://tinyurl.com/vekpn2r
- Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, et al. (2016) Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532: 64-68. Link: https://tinyurl.com/vduq27y
- Kabir MA, Hussain MA, Ahmad Z (2012) Candida albicans: A model organism for studying fungal pathogens. ISRN Microbiology. Link: https://tinyurl.com/wmwogks
- Berktas M, Aydin S, Yilmaz Y, Cecen K, Bozkurt H (2008) Sperm motility changes after coincubation with various uropathogenic microorganisms: an in vitro experimental study. Int J Nephrol Urol 40: 383-389. Link: https://tinyurl.com/w7l8t3a
- Burrello N, Salmeri M, Perdichizzi A, Bellanca S, Pettinato G, et al. (2009) Candida albicans experimental infection: effects on human sperm motility, mitochondrial membrane potential and apoptosis. Reprod Biomed Online 18: 496-501. Link: https://tinyurl.com/sn3cpf4
- Reichman O, Sobel JD, Bentely G (2010) Chronic vulvar fissure a rare manifestation of mycosis fungoides. J Low Genit Tract Dis 14: 65-67. Link: https://tinyurl.com/qm3l79m
- Kaur K, Kaur S, Rishi P, Singh SK, Prabha V (2012) Evidence for the occurrence of receptor in sperm for spermagglutinating factor isolated from Escherichia coli. Gynaecol Endocrinol 34: 207-209.
- Kaur K, Prabha V (2014) Spermagglutinating Escherichia coli and its role in infertility: in vivo study. Microb Pathog 69: 33-38. Link: https://tinyurl.com/qo2s3m5
- Vander H, Prabha V (2015) Evaluation of fertility outcome as a consequence of intravaginal inoculation with sperm-impairing micro-organisms in a mouse model. J Med Microbiol 64: 344-347. Link: https://tinyurl.com/weu8up3
- Emmens CW (1947) The motility and viability of rabbit spermatozoa at different hydrogen ion concentration. J Physiol 106: 471-481. Link: https://tinyurl.com/yx3ntaft
- Kielley MW (1995) Mitochondrial ATPase In: Methods in Enzymology. Academic Press New York 593-595.
- Boyce A, Casey A, Walsh G (2004) A phytase enzyme based biochemistry practical particularly suited to students undertaking courses in biotechnology and environmental science. Biochem Mol Biol Educ 32: 336-340. Link: https://tinyurl.com/rz5yhnj
- Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, et al. (2014) Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PloS One 9: 101-510. Link: https://tinyurl.com/qw7szr4
- Fidel PL (2004) History and new insights into host defense against vaginal candidiasis. Trends Microbiol 12: 220-227. Link: https://tinyurl.com/tm8qbc2
- Cahill DJ, Wardle PG (2002) Management of infertility. BMJ: British Medical Journal 325: 28. Link: https://tinyurl.com/w6svlm2
- Barbonetti A, Vassallo MRC, Cinque B, Filipponi S, Mastromarino P, et al. (2013) Soluble products of Escherchia coli induce mitochondrial dysfunction-related sperm membrane lipid peroxidation which is prevented by Lactobacilli. PloS 8: e83136. Link: https://tinyurl.com/w4xtrb7
- Rennemeier C, Frambach T, Hennicke F, Dietl J, Staib P (2009) Microbial quorum-sensing molecules induce acrosome loss and cell death in human spermatozoaInfect Immun 77: 4990-4997. Link: https://tinyurl.com/ttw3nl5
- Prabha V, Sandhu R, Kaur S, Kaur K, Sarwal A, et al. (2010) Mechanism of sperm immobilization by Escherichia coli. Advances in Urology 2010. Link: https://tinyurl.com/r2goa4r
- Lishko PV, Miller MR, Mansell SA (2016) The Role of Sperm Ion Channels in Reproduction. In Ion Channels in Health and Disease 223-238. Link: https://tinyurl.com/tlfyn8d
- Mencarelli C, Lupetti P, Rosetto M, Mercati D, Heuser JE, et al. (2001) Molecular structure of dynein and motility of a giant sperm axoneme provided with only the outer dynein arm. Cell Motil Cytoskeleton 50: 129-146. Link: https://tinyurl.com/txyjbc6
- Arias RD, Vivenes CY, Camejo MI, Pinero S, Proverbio T, et al. (2015) ATPases, ion exchangers and human sperm motility. Reproduction 149: 475-484. Link: https://tinyurl.com/w6xbxef
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
