Global Journal of Ecology
Eumelanic coloration and age interact to influence breath rate following a boldness test in urban pigeons
Sophie Dupont1, Emmanuelle Baudry2, Pauline Juette2 and Julien Gasparini1*
2Université Paris-Saclay, CNRS, AgroParisTech, Ecologie Systématique et Evolution, 91405, Orsay France
Cite this as
Dupont S, Baudry E, Juette P, Gasparini J (2020) Eumelanic coloration and age interact to influence breath rate following a boldness test in urban pigeons. Glob J Ecol 5(1): 115-119. DOI: 10.17352/gje.000029The rapid urbanization of the past decades has forced numerous species to adapt to their new environments over a very restricted time scale. Previous studies suggested that individuals living in urban areas have specific characteristics as compared to those living in rural areas. In feral pigeon populations (Columba livia), individuals living in cities are more melanic than those living in less urbanized areas. In this study, we tested whether the degree of eumelanin-based coloration in pigeons reflects certain adaptations to urban areas such as boldness and tolerance to stress. Hence, we examined the relationship between eumelanin-based coloration and three variables of boldness (arrival to a new food source, arrival latency, and flight distance) and tolerance to stress (breath rate). Our results show that the eumelanin-based coloration of individuals is not correlated with the boldness variables, but tends to be positively related to breath rate in adults. Therefore, eumelanin-based coloration could reflect the capacity of adult individuals to manage stress in an urban environment, but not the boldness. The higher frequency of eumelanic pigeons observed in cities might thus be due to urban selective forces that favor individuals with a higher response to stress than bolder individuals.
Introduction
Nowadays, urban areas constitute environments of particular interest in ecology and evolution. The rapid urbanization of the past decades as part of global change has forced numerous species to adapt to these new environments over a very restricted time scale. In the context of growing worldwide urbanization (increase of up to 1.2 million km2 between 2000 and 2030, [1], it becomes crucial to understand how natural species adapt to this new environment and, more generally, how this ecosystem functions.
While few studies focus on the adaptation of populations to the urban environment, they suggest that individuals living in urban areas have specific characteristics as compared to those living in rural areas [2]. The combination of particular traits would enable individuals to successfully exploit urban environments. This urbanization syndrome or synurbization [3] may include high reproduction rates, higher levels of aggressiveness and boldness, attenuated physiological responses to stressors, and increased tameness toward humans [5]. Moreover, the degree of melanin-based coloration has been frequently reported to covary with life-history, morphological, physiological, and behavioral traits, as well as with the sign and magnitude of these covariations fluctuating in time and space [6]. These results suggest that the degree of melanin-based coloration might reflect adaptations to different environmental conditions [7]. A recent review highlighted potential candidate genes pleiotropically affecting both the degree of individual melanization and other phenotypic traits, including aggressiveness, tolerance to stress, energy homeostasis, and anti-inflammatory immune reactions [8], suggesting that melanin-based coloration could be associated with several aspects of the urbanization syndrome. Accordingly, a recent study in urban pigeons (Columba livia) showed that the degree of eumelanin-based coloration was positively correlated with the urbanization rate [9]. If this hypothesis is true, we should observe a correlation between eumelanin-based coloration and certain aspects of the urbanization syndrome.
In this study, using the feral pigeon (Columba livia), we tested this prediction by investigating the association between the eumelanin-based coloration of pigeons and two potential aspects of the urbanization syndrome: boldness – the tendency of individuals to take risks, particularly in novel contexts – and tolerance to stress. Urban pigeons display alternative melanin-based coloration, which varies in terms of both melanin types and pigmentation patterns [10]: red coloration is due to pheomelanic pigments and black coloration to eumelanic pigments. These two types of melanic pigments can vary in intensity in feathers, ranging from a total absence of pigmentation (white coloration) to intermediate and even dark melanin coloration. In Paris (France), a preliminary observational study based on photos (n = 3041) revealed that pheomelanic morphs are rare (7 %) as compared to eumelanic morphs (93 %) (Lisa Jacquin and Julien Gasparini, personal observation). The main color polymorphism concerns the quantitative variation of eumelanin present in feathers [9]. A cross-fostering experiment also showed a strong heritability (0.88), suggesting a strong genetic control of this phenotype [11].
We used a boldness test in captivity to quantify the behavioral variables of pigeons (i.e., arrival to a new food source, latency of this arrival, and flight distance) as well as a physiological variable related to stress tolerance (i.e., breath rate) and then correlated these variables with eumelanin-based coloration, the main color polymorphism in pigeons.
Material and methods
In June 2015, we captured 39 feral pigeons (25 adults and 14 juveniles, sex undetermined) in four different locations in Paris (France): Gare Montparnasse (14th district), Eglise Saint-Sulpice (7th district), Notre-Dame de Paris (1st district), and Place Bernard Halper (5th district). The pigeons were then kept in outdoor aviaries (dimensions 310 x 200 x 240 cm) with bamboo perches, a shelter, and a bath at the CEREEP field station (Centre de recherche en Ecologie Expérimentale et Prédictive – Ecotron Ile-de-France, UMS 3194, Saint Pierre lès Nemours, France). They were hosted in similar conditions for 10 days before the boldness test. During this acclimation period, pigeons were fed ad libitum with a mix of maize, wheat, and peas, as well as mineral supplements and water. Upon their arrival at the aviaries, the birds were weighed and their coloration score determined (percentage of black coloration on wings) following the method described in Jacquin, et al. [12]. This score is a good approximation of the eumelanin content of feathers [13].
The subsequent testing period lasted 8 days: 3 days of environmental acclimation and 5 days of testing. During this period, all individuals were housed separately in a new aviary and fed with a restricted diet. Individuals were given a daily diet of 10 grams of a wheat and corn mix (delivered every morning). Because of the restricted caloric intake of this diet and the stress associated with captivity, individuals’ weight was regularly monitored. Individuals whose weight decreased too much were excluded from the experiment and re-fed ad libitum. During the 5 days of testing, birds were submitted to two boldness tests followed by two breath rate measurements, the first in the morning and the second in the afternoon (78 trials for 39 individuals). For the boldness tests, two protagonists were considered, namely a feeder who was known to the pigeons and an unknown observer. First, the feeder placed some wheat and corn mix on the floor of the aviary. The latency (in seconds) between the setting down of the food and the beginning of its consumption was measured using a chronometer. The trial was stopped if the animal did not eat the food after a time lapse of 20 minutes. When the individual started to eat, the observer, initially located 10 meters away from the aviary, walked in straight line toward it. As soon as the pigeon moved away from the seeds, the observer stopped walking. The distance between the observer and the food source was called the flight distance. After measuring the flight distance, each individual was captured to evaluate its stress level based on the breath rate [14]. The time taken to capture the bird (latency of capture) was noted because of its possible effect on this measurement. The number of respiratory movements in 1 minute was determined by placing the pigeon on its back in the hands of the observer. In summary, the 78 trials allowed us to obtain 4 variables of interest for 39 individuals: the arrival (or not) of the birds at the food site, the latency of this arrival, the flight distance, and the breath rate (a proxy of stress during this test).
We performed four mixed models to investigate whether the coloration and age (adults/juveniles) of birds and their interaction impacted the four variables of interest. All statistical analyses were performed using SAS (version 9.4). First, the arrival was coded as a binary variable (0: the bird did not come to eat the food; 1: the bird came to eat food). We used a mixed model with binomial distribution (logit link function) with the arrival as the dependent variable and the age (juveniles vs. adults), the period of the day where the trial has been done (morning vs. afternoon) and eumelanic coloration (percentage of black coloration on wings) in addition to their interaction as the explanatory variables. We included the location of capture and the individual nested within the location as random factors to take pseudoreplication into account. Then, similar mixed models were run on the logarithm of the arrival latency, flight distance, and breath rate with normal distributions. We log-transformed the arrival latency to better satisfy the assumptions of normality. For the breath rate model, we also included the latency of capture as a covariate. Non-significant interactions were removed step-by-step to obtain the final model.
Results
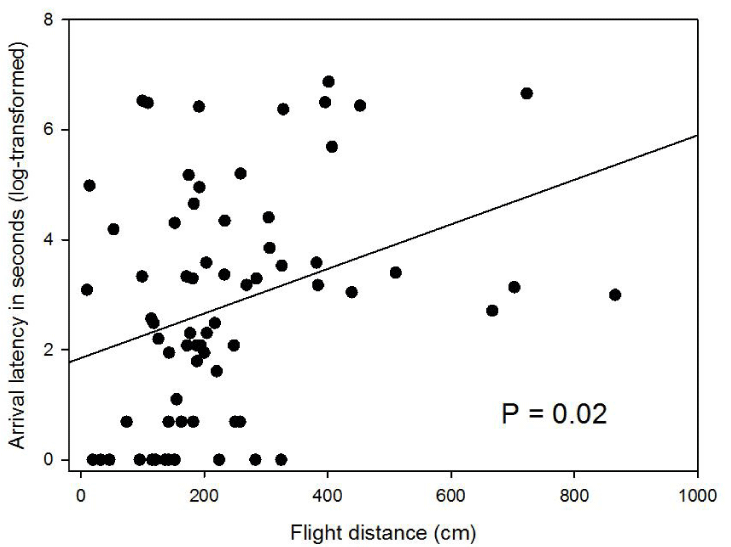
Among the 78 trials (n = 39), the birds decided to go to the food on 68 occasions. This decision was not affected by the age or coloration of the pigeons (Table 1), and only one individual never went to food. However, pigeons decided to go to the food more often in the afternoon (38 times over 39) as compared to the morning (30 over 39; Table 1). We then investigated the arrival latency, flight distance, and breath rate for these 68 trials involving 38 birds. We first found a positive relationship between arrival latency and flight distance (F1,28 = 6.05, P = 0.02, Figure 1). However, these two variables were not correlated with the breath rate (mixed models; arrival latency: F1,28 = 1.33, P = 0.26; flight distance: F1,29 = 0.12, P = 0.73). The arrival latency was not affected by coloration and age of the birds whether individually or in interaction (Table 1) but tend to be longer during trials done in the morning (365 seconds ± 83) than in the afternoon (134 seconds ± 42; non-significant trend, Table 1). The flight distance was longer for adults (206 cm ± 21, n = 24) than for juveniles (149 cm ± 25, n = 14; Table 1) and was longer during trials done in the morning (303 cm ± 39) than during trials done in the afternoon (185 cm ± 16; Table 1). The breath rate was significantly affected by the arrival latency and by the interaction between age and coloration (Table 1). The longer the time necessary to catch birds, the higher their breath rate was. In adults, darker pigeons tended to have higher breath rates than paler ones (F1,20 = 3.66, P = 0.07; Figure 2a). This trend was not observed in juveniles (F1,8 = 2.02, P = 0.19; Figure 2b).
Discussion
In the present study, we wanted to test whether a darker eumelanin-based coloration in pigeons reflects their better adaptation to urbanization, namely in terms of greater boldness and tolerance to stress. Our experiment provided contrasting results. When considering boldness variables and contrary to previous studies in other species [15], the results did not support our hypothesis as eumelanin-based coloration was not related to arrival, arrival latency, or flight distance (Table 1). In contrast, the breath rate, as a proxy of tolerance to stress, tended to be higher in darker adults, a trend that we did not observe for juveniles. This suggests that eumelanic adult pigeons tend to have a higher response to stress than paler ones immediately after capture and therefore a lower capacity to cope with stress. In contrast with our results, breath rate was found to be negatively correlated with the width of eumelanic black tail band in kestrels (Falco tinnunculus) [16] and with the eumelanic black spots numbers in barn owls (Tyto alba) [17,18]. Overall, our results are rather contrasted with previous studies [8,19] but it is possible that the direction of the relationship between response to stress and melanin-based coloration might depend on species as observed for others physiological component [8].
In our species, darker eumelanic individuals are more prolific in highly urbanized areas [9], suggesting that they are potentially better adapted to urban areas than paler ones. From this result, we hypothesized that one of this adaptation would be a better capacity to cope with stress for darker eumelanic pigeons and we predicted that darker eumelanic pigeons would have a better capacity to cope with stress as compared to paler ones. We tested this hypothesis in the present study, but our results found a relation opposite to our prediction. Indeed, in our study, paler pigeons tend to better tolerate stress following capture, at least in adults. Our results are nevertheless consistent with a recent study in pigeons showing that corticosterone response, as a physiological measure of stress, increased with the degree of eumelanin-based coloration in a rural population [20]. Previous studies suggest that urbanization selects animals that are more tolerant to stress [21]. Yet perhaps this speculation is incorrect, as urbanization would appear to select individuals that maintain a high level of stress response. Indeed, a higher stress response may have a positive impact on fitness in certain habitats such as those with a high predation rate [22]. Urban areas may also include several environmental stressors (anthropic stressors) that select individuals with a high stress response. In our pigeon population, such individuals seem to be eumelanic, which could explain their higher frequency in highly urbanized areas. This interpretation is based on our correlative result (Figure 2a). Future studies should thus experimentally validate this scenario and identify the environmental stressor in urban areas that favors individuals with a higher stress response. Furthermore, our correlative approach has been performed on pigeons alone in an aviary, which is not the same environment than those experimented by pigeons in cities (social interactions and/or other environmental urban factors). Therefore, our results could not be representative of the reality of what it happens in cities. Even if our approach enabled to standardize environmental factors, future studies involving approach in natura are required to confirm our results.
Even if the boldness measurements (arrival, arrival latency, and flight distance) were not correlated with eumelanin-based coloration in pigeons (our main hypothesis), we found an interesting positive relationship between arrival latency and flight distance (Figure 1). This result confirms that these two variables measured the same behavioral syndrome, notably boldness. Flight distance is a common method used to estimate boldness in birds and is applied in many studies [23,24]. By contrast, arrival latency is used less often when investigating boldness. Flight distance is a less invasive method, because its measurement does not depend on the restriction of food intake. As a result, future studies should favor this measurement when investigating boldness. However, arrival was not correlated to arrival latency or flight distance, and in our study, most pigeons decided to go to the food (68 out of 78 trials). Therefore, the low variation of this estimate did not enable us to distinguish between bold and less bold individuals. This low variation may be due to the starvation of birds during the experiment. Indeed, it has been suggested that hunger might increase risk-taking among individuals [25,26]. Hence, the food restriction of our study might explain why most of birds took the risk of going to the food. Accordingly, pigeons were more prone to go to the food in the afternoon than in the morning. Indeed, birds were daily fed in the early morning and therefore they were more starved in the afternoon than in the morning. Globally, we observed that this higher starvation increases the risk-taking: a higher frequency to go the food, a shorter latency to go the food and a shorter flying distance (Table 1). Future studies should therefore test the effect of food restriction on the different estimates of boldness to determine the most suitable, that is, the estimate for which food restriction does not alter the variation among individuals.
Conclusion
Our study shows that the eumelanin-based coloration of individuals could reflect the capacity of adults to manage stress in an urban environment, but not boldness. Therefore, the higher frequency of eumelanic pigeons observed in cities might be due to urban selective forces favoring individuals with a higher response to stress as opposed to bold individuals. Boldness is therefore not an aspect of the urbanization syndrome. By contrast, a higher response to stress could be part of the urbanization syndrome in pigeons.
Ethical note
This study was carried out in strict accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (revised Appendix A). All experiments and captures were approved by local authorities and the “Direction Départementale des Services Vétérinaires de Seine-et-Marne” (permit No. 77-05).
Data sharing and data accessibility
The data will be deposited in Dryad or other equivalent archives when accepted.
- Seto KC, Gueneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proceedings of the National Academy of Sciences of the United States of America 109: 16083-16088. Link: https://bit.ly/32FLUv3
- Evans J, Boudreau K, Hyman J (2010) Behavioural Syndromes in Urban and Rural Populations of Song Sparrows. Ethology 116: 588-595. Link: https://bit.ly/2GWmEsC
- Luniak M (2004) Synurbanization - adaptation of animal wildlife to urban development. Proceedings of the 4th International Urban Wildlife Symposium (ed by W.W. Shaw, L.K. Harris and L. Vandruff) 50-55. Link: https://bit.ly/3pm7yOu
- Gaston KJ (2010) Urban Ecology. Cambridge University Press.
- Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, et al. (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23: 960-969. Link: https://bit.ly/38F55ZK
- Roulin A (2004) The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol Rev Camb Philos Soc 79: 815-848. Link: https://bit.ly/3kvky0u
- Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecology Letters 7: 1225-1241. Link: https://bit.ly/32Dmqhy
- Ducrest AL, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol Evol 23: 502-510. Link: https://bit.ly/2ItFasO
- Jacquin L, Recapet C, Prevot-Julliard AC, Leboucher G, Lenouvel P, et al. (2013b) A potential role for parasites in the maintenance of color polymorphism in urban birds. Oecologia 173: 1089-1099. Link: https://bit.ly/3eT4Taj
- Johnston RF, Janiga M (1995) Feral Pigeons. Oxford University Press.
- Jacquin L, Haussy C, Bertin C, Laroucau K, Gasparini J (2013) Darker female pigeons transmit more specific antibodies to their eggs than do paler ones. Biological Journal of the Linnean Society 108: 647-657. Link: https://bit.ly/38R53hx
- Jacquin L, Lenouvel P, Haussy C, Ducatez S, Gasparini J (2011) Melanin-based coloration is related to parasite intensity and cellular immune response in an urban free living bird: the feral pigeon Columba livia. Journal of Avian Biology 42: 11-15. Link: https://bit.ly/3nkPDG9
- Gasparini J, Bize P, Piault R, Wakamatsu K, Blount JD, et al. (2009) Strength and cost of an induced immune response are associated with a heritable melanin-based colour trait in female tawny owls. J Anim Ecol 78: 608-616. Link: https://bit.ly/3eTieiK
- Torné-Noguera A, Pagani-Nunez E, Senar JC (2014) Great Tit (Parus major) breath rate in response to handling stress: urban and forest birds differ. Journal of Ornithology 155: 315-318. Link: https://bit.ly/3ltvvkv
- Mafli A, Wakamatsu K, Roulin A (2011) Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann’s tortoises. Animal Behaviour 81: 859-863. Link: https://bit.ly/2IvrjSW
- van den Brink V, Henry I, Wakamatsu K, Roulin A (2012) Melanin-Based Coloration in Juvenile Kestrels ( Falco tinnunculus) Covaries with Anti-Predatory Personality Traits. Ethology 118: 673-682. Link: https://bit.ly/2IwznmF
- van den Brink V, Dolivo V, Falourd X, Dreiss AN, Roulin A (2012) Melanic color-dependent antipredator behavior strategies in barn owl nestlings. Behavioral Ecology 23: 473-480. Link: https://bit.ly/3kt1r7s
- Peleg O, Charter M, Leshem Y, Izhaki I, Roulin A (2014) Conditional association between melanism and personality in Israeli Barn Owls. Bird Study 61: 572-577. Link: https://bit.ly/38ArsiV
- Roulin A, Ducrest AL (2011) Association between melanism, physiology and behaviour: A role for the melanocortin system. Eur J Pharmacol 660: 226-233. Link: https://bit.ly/3kpTWhC
- Corbel H, Legros A, Haussy C, Jacquin L, Gasparini J, et al. (2016) Stress response varies with plumage colour and local habitat in feral pigeons. Journal of Ornithology 157: 825-837. Link: https://bit.ly/2IzxK7e
- Partecke J, Schwabl I, Gwinner E (2006) Stress and the city: Urbanization and its effects on the stress physiology in European Blackbirds. Ecology 87: 1945-1952. Link: https://bit.ly/2InYd8o
- Bell AM, Henderson L, Huntingford FA (2010) Behavioral and respiratory responses to stressors in multiple populations of three-spined sticklebacks that differ in predation pressure. J Comp Physiol B 180: 211-220. Link: https://bit.ly/3pmPsff
- Blumstein DT (2006) Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Animal Behaviour 71: 389-399. Link: https://bit.ly/3ps76y7
- Carter AJ, Goldizen AW, Tromp SA (2010) Agamas exhibit behavioral syndromes: bolder males bask and feed more but may suffer higher predation. Behavioral Ecology 21: 655-661. Link: https://bit.ly/2IxLv6u
- Petersen A, Nielsen KT, Christensen CB, Toft S (2010) The advantage of starving: success in cannibalistic encounters among wolf spiders. Behavioral Ecology 21: 1112-1117. Link: https://bit.ly/2Iq9IMy
- Ariyomo TO, Watt PJ (2015) Effect of hunger level and time of day on boldness and aggression in the zebrafish Danio rerio. J Fish Bio 86: 1852-1859. Link: https://bit.ly/35tpwXA
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
