Global Journal of Ecology
Impact of spatial patterns on arthropod assemblages following natural dune stabilization under extreme arid conditions
Ittai Renan1,2, Amnon Freidberg3, Elli Groner4 and Pua Bar Kutiel1*
2Hamaarag - Israel National Ecosystem Assessment Program, and The Entomology Lab for Applied Ecology, The Steinhardt Museum of Natural History, Tel Aviv University, Israel
3School of Zoology, The George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel
4Dead Sea and Arava Science Center, Mitzpe Ramon, 8060000, Israel
Cite this as
Renan I, Freidberg A, Groner E, Bar Kutiel P (2020) Impact of spatial patterns on arthropod assemblages following natural dune stabilization under extreme arid conditions. Glob J Ecol 5(1): 079-087. DOI: 10.17352/gje.000024Background: The cessation of anthropogenic activities in mobile sand dune ecosystems under xeric arid conditions has resulted in the gradual stabilization of dunes over the course of five decades.
Our objective was to analyze the spatial patterns of arthropod assemblages along a gradient of different stabilization levels, which represents the different stages of dune stabilization - from the shifting crest of the dune to the stabilized crusted interdune.
The study was carried out at the sand dunes of the northwestern Negev in Israel. Data was collected using dry pitfall traps over two consecutive years during the spring along northern windward aspects. Four dunes were chosen, characterized by three significant landscape units: shifting crest, semi-stabilized slope and stabilized interdune.
Results: We identified three significant assemblages of arthropods along the gradient. The shifting dune crests are populated by psammophilic species found almost exclusively in sandy habitats in Egypt and the western Negev in Israel. The crusted, stabilized inter-dunes are populated mainly by loess-dwelling species, which are common in most of the Negev loess plains and have a wide distribution range, and the semi-stabilized slopes host species of both extreme landscape units but is distinguished by four species that show significant affinity to it.
Conclusions: Our results demonstrate functional arthropod heterogeneity and emphasize the risk of regional species homogenization. Heterogeneity is a key property in maintaining sand dune biodiversity. Homogenization, as a result of sand stabilization, may lead to loss of psammophilic species.
Introduction
Scientific background
Habitat loss and habitat destruction are considered to be the main factors in reducing biodiversity worldwide [1,2]. Most studies on this issue focus on the direct and indirect impacts of human activity [3-5]. However, in some cases, lack of human activity is responsible for habitat loss. The cessation of traditional activities, such as grazing, hunting, clearing and burning of forests, which have been carried out on dunes for thousands of years, may lead to a dramatic change in the landscape and the loss of local plant and animal communities [6,7].
The sand dunes in Israel are an example of a habitat that has been devastated during recent decades due to three main processes: urban development [8], agricultural development, and sand stabilization due to human inactivity [9-12]. The stabilization process ensued from the cessation of traditional activities, such as grazing and cutting, which had been practiced along the Israeli Mediterranean coastal plain and the northern Negev for thousands of years. These types of activities were suspended during the last several decades due to local policies [13-15].
Studies conducted along the Israeli coastal dunes (400-450 mm mean annual rainfall) have shown that the stabilization process, caused by an increase in shrub coverage, has led to a significant decrease in fauna and flora that are characteristic to mobile and semi-stabilized dunes [9,12,16].
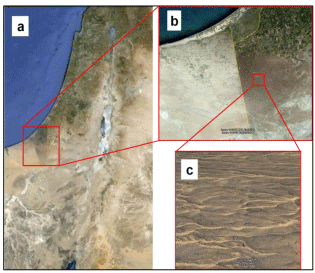
Dune stabilization has also been observed in the northwestern Negev. Sand was deposited throughout the region during a windy era between 23,000 to 12,000 years ago. Later on, a decrease in wind velocity stopped the shifting of sand and allowed stabilization processes to commence [17-19]. In arid regions, biological crusts, formed by cyanobacteria that glue sand and loess grains together, also stabilize dunes [20]. During the Byzantine era, active sand dunes covered the northwestern Negev, home to large populations of shepherds. The grazing and trampling herds damaged the crust layer, and the dunes became active for an additional 2,000 years. A time-line analysis reveals that in 1948 the sand dunes on both sides of the Israel-Egypt border were almost the same: mobile dunes with low, sparse vegetation cover. Since the establishment of the State of Israel in 1948 until 1982, the vegetation coverage on the Israeli side oscillated due to invasion of Bedouin livestock from the Egyptian side of the border. In 1982 the border, became impassable for livestock for the first time due to the installation of fences. This fact and the subsequent shepherd exclusion policy in Israel caused re-stabilization of most of the northwestern sandy Negev [19,21]. The vegetation on the Israeli side increased gradually, while decreasing on the Egyptian side [18]. In the absence of human activity and the prevalence of weak winds, which characterize this area, the dunes started gradually to stabilize. This process of stabilization was followed by extensive development of biological crusts in the interdunes due to accumulation of the relatively high content of fine-grained particles in the top soil, as well as the high vegetation cover (perennial herbaceous and shrub plants) along the slopes. Well-developed ripple marks on the dune crests are clearly indicative that these parts are very active and unstable under present-day conditions [22]. At present, on the Israeli side of the border, more than 70% of the former sandy region is stabilized and only 7% is bare sand. However, the dunes on the Egyptian side are still active due to continuous interference by Bedouin-owned herds (Figure 1a,1b) [17].
Perry [16] found that plant assemblages on these dunes vary among dunes at different states of stabilization. The effect of biogenic crusts on vascular plant establishment, coverage and composition on sandy soils is under strong debate [23-27]. Little is known about the impact of the landscape’s substrate character modification, i.e., the shift to stabilized dunes, on faunal assemblages [28,29].
Soil type is considered to be the main environmental variable affecting the distribution of terrestrial beetles and other arthropods [30-34], however, studies that have examined this factor assessed large scales of adjacent regions. Columbus [34], who studied the sand dunes of the northwestern Negev, found that the effects of perennial vegetation cover and dune topography determine beetle composition and community structure. In this study, we sampled arthropod assemblages along windward slopes at three landscape units, which differ in their vegetation cover and topography location.
Since we collected data for only 2 years, diversity loss with time due to dune stabilization cannot be shown. The process of dune stabilization has been taking place for decades. Data on arthropod diversity does not exist for this length of time and we therefore performed space-for-time substitution [35] by comparing the assemblage’s diversity of arthropods in various landscape units, which differ in their stabilization levels. Space-for-Time substitution can be applied when the studied ecological process (species turnover in our case) is compared to changes in the environment (dune stabilization) [36]. Active sand dune crests represent the most active landscape units and are characterized by mobile sand, low plant cover, and no biological crust. Interdune landscape units represent the stabilized area.
Heterogeneity is apparent in different degrees of sand stabilization, geomorphological characteristics, topography, plant cover and other characteristics of the landscape unit, but these are all considered “measured heterogeneity” . The fauna of these units may be differential or indifferent to these differences. If the abundance, distribution and community structure of fauna show heterogeneity (beta diversity), it is considered “functional heterogeneity” [37].
Study objective
Our objective was to analyze the spatial patterns of arthropod assemblages along a gradient of different stabilization levels, stretching from the dune’s shifting crest to the stabilized crusted interdune in order to establish how stabilization changes biodiversity. These landscapes represent different levels in the process of dune stabilization. The purpose of our study was to demonstrate the danger of the stabilization process on diversity loss.
Materials and methods
Study area
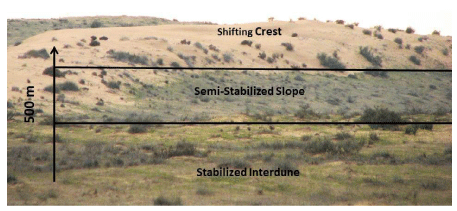
The study was conducted in the Haluza linear sand dunes, which are in the central part of the northwestern sandy Negev desert, covering 1,300 square kilometers (Figure 1). These dunes are considered to be the northeastern extent of the large sand field of northern Sinai. The climate is classified as arid to hyper-arid. The mean yearly rainfall in the study area is about 90 mm [38]. The research area is characterized by vegetated stabilized linear dunes, whereas some of the dunes maintain a shifting crest (Figure 1). The dunes are separated by wide, extremely crusted, stabilized interdunes. Most of the vegetation of the region is Saharo-Arabian [16,39,40]. We divided the area into three landscape units according to their stabilization status: shifting sand dune crests, semi-stabilized slopes, and stabilized interdunes (Figure 2).
The vegetation cover on the crest is 6-9% and is predominantly perennial species (Artemisia monosperma, Heliotropium digynum, Echinops polyceras and Stipagrositis scoparia); the vegetation cover on the dune’s semi-stabilized slopes is between 8-12% and is composed of the dominant perennial species (Noaea mucronata, Licium shawii and Retama raetam), randomly distributed biogenic crusts and annual species during the rainy winters; biogenic crusts (mainly cyanobacteria) cover 75% of the interdune areas. The only woody perennial species is Anabasis articulate.
Sampling methods
A block design of four dunes was chosen; each dune contains each of the landscape units (shifting crest, semi-stabilized slope and stabilized interdune). Arthropods were sampled using pitfall traps during the spring. Traps were laid out in the four dunes for 24 hours every two weeks for two years during the spring (March and April); in total 16 days per year were observed (Table 1). The traps, empty plastic cups with 11 cm diameter openings, were placed at a depth of 13 cm. Their opening was level with the soil surface. We placed the traps far from the edges of the adjacent landscape units (reducing the “edge effect”). In each landscape unit, we placed 20 traps: 10 traps under bushes and 10 traps in the inter-shrub area. The traps were placed at least five meters from each other, to prevent “competition” between neighboring traps on the same specimens [41]. Trapped beetles and ants specimens were identified to the species level; other arthropods were identified to the morphospecies level. Specimens identified in the field were released in situ.
Data analysis
In order to determine the level of similarity between the different landscape units, the species composition and the specimens’ numbers were compared to all other sampling landscape units by an ordination test. By DCA (Detrended Correspondence Analysis) ordination, the level of similarity between the sampled landscape units (three landscape units at each of the four dunes) was determined by comparing the species composition and individual abundances of arthropods in them (Figure 3). Graphs were processed using Principal Component Analysis (PCA). Statistical tests to examine differences between the landscape units were processed by Redundancy Analysis (RDA). The “environmental variables” were the landscape units (crest, slope, interdune). This test included only species of which at least ten specimens were trapped. Each sampling year was analyzed separately due to the differences in the species composition and individual abundance between the two years.
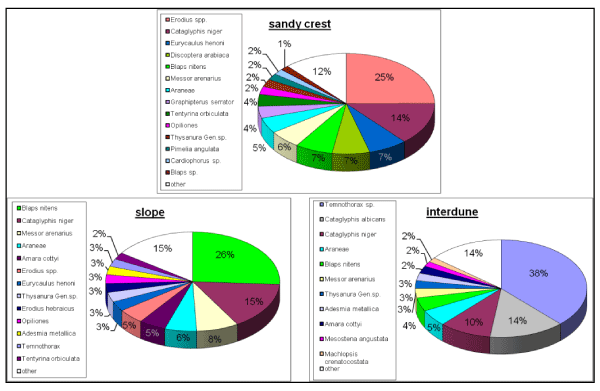
In order to identify the affinity of a species to one of the landscape units, we examined its abundance in each unit as a percentage of the species total abundance (Figure 4). Only species for which more than 50 individuals were caught in both seasons combined were examined.
Examination of statistical significance at the affinity analysis was performed with the block-design one-way ANOVA method using STATISTICA 7 software; the significance level for a two-sided test is 0.05.
In order to statistically examine the extent of affinity of a species to different landscape units, the degree of statistical significance was examined for the presence of a species in different units, according to the individuals caught. We examined all of the 47 species that were represented by over 10 individuals (taxons higher than genus were not examined).
Results
General results
We caught 10,590 individuals belonging to 122 species and 17 orders. The most commonly encountered order, Coleoptera (beetles), was represented by 5,613 individuals (53%), belonging to 72 species (59%) of 14 families. The most represented family, by abundance and species number, was the Tenebrionidae (darkling beetles), which was represented by 36 species and constituted 81% of all beetle individuals. Other common orders were the Hymenoptera, mainly Formicoidea (ants), and Thysanura (silverfish and alike).
Abundance was highest at the semi-stabilized slopes, followed by the shifting crest, and lowest on the interdune units (Table 2). Abundance was significantly different between the landscape units (one-way Anova: F(2, 21)=4.4409, p=.0.025), with the semi-stabilized slope having significantly higher specimen numbers than the interdune units (Table 2). When analyzing the two sampling years separately, the difference is still significant in 2008 (F(2,6)=8.2 p=0.019) but not in 2009 (F(2,6)=4.54 p=0.063), although the trend of the shifting crest and the semi-stabilized slope was the same as in the previous year.
The numbers of species found in the landscape units were similar (Table 2). Of the interdune’s total arthropod abundance, ants comprised 64%, of which 50% were small ants, mainly Temnothorax sp. and Cataglyphis albicans. Small ants were completely absent or very rare in the other two sampled landscape units (Figure 4 and Table 2). Relatively low proportions of Tenebrionid individuals were found in the interdune unit (12%) compared to the semi-stabilized slope (40%) and the shifting crest (48%).
Assemblage compositions
The similarity levels of the assemblages’ compositions between the three landscape units, based on the number of individuals of any species in each sample landscape unit, were significantly different (Figure 3, Table 3).
Species affinity
Figure 5 and Table 4 show the various species and their affinities to the three landscape units. Most species are found in one of the colored triangles (yellow, green or brown) of Figure 4 indicating that species tend to belong mostly to one landscape unit with very few generalists. Species found on the slopes are found on the other two landscape units, while species associated with the other landscape units are more restricted to them. Table 4 lists the affinity of species to the landscape units. Examining species’ affinity to each landscape unit revealed that 53% of the dominant species showed either a significant preference for one landscape unit type or were significantly absent from one landscape unit type. For example: a total of 249 specimens of the ground beetle Discoptera arabica (Coleoptera: Carabidae) were caught, 240 of them (96%) on the shifting crest, 9 on the semi-stabilized slope and none on the interdune. A total of 72 specimens of the darkling beetle Pimelia angulata syriaca were caught, 71 (98%) on the shifting crests, one specimen on the semi-stabilized slopes and none on the interdune.
Discussion
Three different landscape units within the scale of a dune and its adjacent interdune were analyzed in order to characterize arthropod assemblages and their affinity to different units. The landscape units are distinctly different in terms of vegetation coverage, plant composition, and topography. The results indicate that although the number of species is almost the same in all three landscape units, the average abundance of arthropod species, and mainly their assemblage, differs among the landscape units. Similar results were obtained for the arthropod assemblages at the coastal sand dunes when comparing three dune types which differ in stabilization levels [12].
Affinity
When examining the affinity of the different arthropod species to the three landscape units, 85% of all species were found to “discriminate” between the landscape units and show preference of more than 50% to one of them. More than half of the species (that were represented by more than 10 caught individuals) showed a significant preference to one of the landscape units. It is important to emphasize the short distance between the three landscape units; the slope width is only 5-20 meters, and theoretically, most sampled species could cross this distance in a short time.
Three main traits distinguish between the three communities: the dominant species composition in each community; the number of individuals and species composition of the darkling beetles; the high proportion of ants caught in the interdune and the dominant ant species relative to the sandy crest and the slope landscape units (Figure 4). Characterization of the arthropod community’s landscape units, based on the dominant species that showed a statistically significant affinity to one of the landscape units, clearly demonstrates three distinctive communities.
Shifting-crest species
Unfortunately, lack of knowledge on the local arthropod fauna limited a wider biological and biogeographical description. However, the sandy crest’s dominant species are psammophilic species that tend to be restricted to sandy landscape units between the Sahara and the Arabian Peninsula deserts. For example, the fast running and digging diurnal ground beetle Graphipterus serrator is restricted to shifting sand habitats between the Nile delta and the Negev desert [42]; Discoptera arabica, a ground beetle which has adapted to dive and swim in the sand, is distributed throughout Egypt, Israel’s western Negev and Saudi Arabia [43]. The darkling beetle Pimelia angulata, which is adapted to locomotion and digging in the sand, is distributed throughout sandy habitats in Sudan, Egypt, Sinai, Jordan, and Israel [44,45].
The sandy desert habitat presents extreme environmental conditions, especially the aridity and the physical and mechanical properties of the sandy substrate [46]. Due to the grainy structure, the dune is dynamic, and wind over a certain velocity threshold causes grain movement. The grainy substrate also limits animal movement and burrowing. Quartz, which is the main component of sand, has a high solar radiation reflection and a high specific heat [47]. These conditions impact animals in various ways: instability of the substrate cover incumbers burrow construction and food collection; movement is challenging; and radiation and temperatures are high during the day. However, there are some advantages for animals who have adapted to the sandy environment. The structure of the coarse grains is highly porous, hence, the sand is thermally isolated and water permeable. These attributes create a habitable, subsurface micro-environment that provides protection from predators and the harsh climate [48,49]. The extreme environmental conditions of sandy habitats lead to extreme morphological and behavioral adaptations of sand-dwelling arthropods [48,50-52]. These specific adaptations cause these psammophilic arthropods to almost exclusively prefer sandy habitats, as has been shown in various studies [30,32,52,53,54,55].
Semi-stabilized and stabilized-dune-dwelling species
The semi-stabilized slope is the most productive landscape [39], but because of its location between the two extreme landscapes, its intermediate sand and crust characteristics and its narrow width of 5-20 meters, its holds only two species with significant affinity: the darkling beetles Arthrodeis rotundatus and Erodius hebraicus, and Blaps nitens with a strong trend affinity (p = 0.008). Like the sandy crest, the stabilized interdune holds seven species that show significant affinity. The distribution of these species is typically wider and more diverse than the sandy crest species. For example, the distribution of the darkling beetle Zophosis punctata is from northwestern Africa to west and east Europe and west Asia and it is common throughout Israel [45]. The ant Cataglyphis albicans is distributed from north and east Africa to western Iran [10], and in the central and western Negev on loess soils (Ionescu-Hirsch, personal communication).
The small ants Temnothorax sp. and Cataglyphis albicans are almost completely restricted to the crusted interdune and are completely absent from the crest. Small ant species do not dwell on the sandy crest because digging nests in coarse-grained sand is difficult for ants with small mandible openings. Most of the ants’ nests in the interdune are located under big shrubs, where fine-grain loess is common (pers. obs.). Some species, such as the darkling beetle Machlopsis crenatocostata and the ant Cataglyphis savigni, showed no affinity to any of the landscape units (Figure 5). In order to understand the community structure of the three landscape units, we looked for Israeli biogeographic patterns of the dominant species that showed a statistically significant affinity to one of the landscape units (Table 4). We found that the dominant species that inhabit the stabilized and semi-stabilized landscape, slope and interdune, are common in the loess regions of the adjacent central Negev [32,56-58].
Stabilization
Our results demonstrate that the measured heterogeneity, which represents the structure of landscape units in the western Negev sand dune area, has a functional impact on the local fauna. Indeed, the functional heterogeneity of arthropods showed different assemblages in each of the landscape units. While there were a few generalist species (4 out of 25) that were indifferent to the measured heterogeneity, most species showed affiliation to one of the landscape units. Hence, as stabilization of the sands continues, community structure will shift from the sandy assemblage to the stabilized assemblage. High spatial heterogeneity creates high β-diversity, which is the variation in composition among different local assemblages [12,59]. Conversely, homogenization of habitats will decrease β-diversity, resulting in a loss of γ-diversity in northwest sand dunes of the Negev. The dune stabilization will also cause a loss of the psammophilic species associated with shifting sand dunes.
Conclusions
Oral evidence, photographs and the contemporary shifting sand dunes on the Egyptian side of the border indicate that shifting sands dominated the northwestern Negev prior to 1982. We conclude that for the past 2,000 years, as long as the northwestern Negev was used by shepherds for grazing, the region was characterized by shifting dunes and was inhabited by psammophilic arthropods. Since 1982, as a result of grazing exclusion policies on the Israeli side of the border, biogenic crusts have expanded and sand dunes have been experiencing ongoing stabilization. The crusted soils provide a different habitat and psammophilic species were pushed to the remnants of shifting sand on the higher dune crests. Generalist arthropods invaded from the adjacent loess plains of central Negev into the expanded landscape. We found that the cessation of traditional land-use led to a decline of unique psammophilic species.
Removal of vegetation, as part of active management practices, which aimed to maintain the spatial heterogeneity patterns and conservation of psammophilic species were conducted in this region as well in Nizzanim coastal sand dune in Israel. The temporal dynamic of the arthropod responses observed showed that slight changes at the assemblage compositions had leveled-off at a new, slight different state [34,60-62].
We wish to thank all the people and institutions that helped us in this work: The Israel Nature and Parks Authority (Aviv Ohayon, Eran Hyams), those who helped with beetle identifications, The Steinhardt Museum of Natural History, Tel Aviv University (Vladimir Chikatunov, Leonid Leib Ariel Friedman, Oz Rittner), with ant identifications (Armin Ionescu-Hirsch), and to Thorsten Assmann, and Martin Lillig. Our field volunteers (Adi Weiss, Tal Saidman, Rotem Fux, Michal and Dror Peleg,) and Sharon Renan. We wish to thank the Ministry of Science and Technology for their support and Michelle Finzi for the scientific editing.
- Fahrig L (1997) Relative effects of habitat loss and fragmentation on population extinction. Journal of Wildlife Management 61: 603. Link: https://bit.ly/34L1Gos
- Wilson EO (1992) The Diversity of Life. Harvard University press. Link: https://bit.ly/2GNP1cu
- Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biological Conservation 128, 231-240. Link: https://bit.ly/2SOaqEU
- Tilman D, May RM, Lehman C, Nowak M (1994) Habitat destruction and the extinction debt. Nature 371: 65-66. Link: https://go.nature.com/3nFR2rQ
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth's ecosystems. Science 277: 494-499. Link: https://bit.ly/3nD6Tr4
- Naveh Z, Kutiel P (1990) Changes in vegetation of the Mediterranean Basin in response to human habitation. In: The Earth in Transition. Patterns and Processes of Biotic Impoverishment. Ed. G. Woodwell. Cambridge Press 259-300.
- Naveh Z (1998) From biodiversity to ecodiversity – holistic conservation of biological and cultural diversity of Mediterranean landscapes. In: Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosystems. Ed. P.W. Rundel, G. Montenegro and F.M. Jaksic. Ecological Studies (ECOLSTUD, Vol. 136), Shpringer 23-53. Link: https://bit.ly/31dRDHN
- Achiron-Frumkin T, Frumkin R, Rudich R, Maloul A, Levin N, et al. (2003) Conservation of the sands of the coastal plain Ministry of the Environment. SPNI, NRPA, JNF, HS, JIIS.
- Kutiel P, Peled Y, Geffen E (2000) The effect of removing shrub cover on annual plants and small mammals in a coastal sand dune ecosystem. Biological Conservation 94: 235-242. Link: https://bit.ly/33N3k9Y
- Kutiel P (2001) Conservation and management of the Mediterranean coastal sand dunes in Israel. Journal of Coastal Conservation 7: 183-192. Link: https://bit.ly/33RQszs
- Tsoar H, Blumberg DG (2002) Formation of parabolic dunes from barchan and transverse dunes along Israel's Mediterranean coast. Earth Surface Processes and Landforms 27: 1147-1161. Link: https://bit.ly/3lu6RQu
- Bird LFT, Dorman M, Ramot A, Bouskila A, Bar (Kutiel) P, et al. (2017) Shrub encroachment effects on habitat heterogeneity and beetle diversity in a Mediterranean coastal dune system. Land Degradation & Development 28: 2553–2562. Link: https://bit.ly/3lD3Op7
- Levin N (2006) The Palestine Exploration Fund Map (18711877) of the Holy Land as a Tool for Analysing Landscape Changes: the Coastal Dunes of Israel as a Case Study. The Cartographic Journal 43: 45-67. Link: https://bit.ly/34LCo9H
- Rosen S (2006) Desertification and pastoralism: a historical review of pastoral nomadism in the Negev region. In Land Use and Land Cover, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK. Link: https://bit.ly/3lu72v8
- Levin N, Ben-Dor E (2004) Monitoring sand dune stabilization along the coastal dunes of Ashdod-Nizanim, Israel, 1945-1999. Journal of Arid Environments 58: 335-355. Link: https://bit.ly/3dle4zC
- Perry M (2008) Studying perennial plant impact on annual diversity in sand dunes in different spatial scales. M.A. Thesis. Ben Gurion University of the Negev. Beer-Sheba, Israel (English).
- Qin Z, Li W, Mao K, Karnieli A, Berliner P (2003) Quantitative estimation of main land cover patterns in arid environmental ecosystem across Israel-Egypt border using remote sensing data. IGARSS 2003 International Geosciences and Remote Sensing Symposium, III: 1879 – 1881, July 21-25, 2003, Toulouse, France. Link: https://bit.ly/2FlyYBT
- Tsoar H (2008) Land use and its effect on the mobilization and stabilization of the northern-western sand dunes. In: Arid Dune Ecosystems – The Nizzana Sands in the Negev Desert, W. Breckle, S., A., Yair, M., Veste, Eds,, Springer-Verlag Berlin Heidelberg, 79-92. Link: https://bit.ly/3k2YMSg
- Roskin J, Porat N, Tsoar H, Blumberg DG, Zander AM (2011) Age, origin and climatic controls on vegetated linear dunes in the northwestern Negev Desert (Israel). Quaternary Science Reviews 30: 1649-1674. Link: https://bit.ly/2SKU1B9
- Verrecchia K, Yair A, Kidrom GJ, Verrecchia K (1995) Physical-properties of the psammophile cryptogamic crust and their consequences to the water regime of sandy soils, north-western Negev desert, Israel. Journal of Arid Environments 29: 427-437. Link: https://bit.ly/2SLyVT5
- Meir A, Tsoar H (1996) International borders and range ecology: The case of Bedouin transborder grazing. Human Ecology 24: 39-64. Link: https://bit.ly/3diJoyM
- Yair A, Veste MW, Breckle S (2008) Geo-ecology of the north-western Negev sand field. In: Arid Dune Ecosystems – The Nizzana Sands in the Negev Desert. S.-W., Breckle, A., Yair, M., Veste, Eds., Springer-Verlag Berlin Heidelberg 17-24. Link:
- Danin A, Bar-Or Y, Inka Y, Israely T (1990) Integrate of Cyanobacteria and vascular plants in sand stabilization in the Negev. Horizons in Geography 31: 169-178 (Hebrow).
- Kutiel P (1998) Possible role of biogenic crusts in plant succession on the Sharon sand dunes, Israel. Israel Journal of Plant Sciences 46: 279-286. Link: https://bit.ly/34N9h5R
- Li XR, Jia XH, Long LQ, Zerbe S (2005) Effects of biological soil crusts on seed bank, germination and establishment of two annual plant species in the Tengger Desert (N China). Plant and Soil 277: 375-385. Link: https://bit.ly/33MchAi
- Prasse R, Bornkamm R (2000) Effect of microbiotic soil surface crusts on emergence of vascular plants. Plant Ecology 150: 65-75. Link: https://bit.ly/36Xa5Ik
- Ram A, Aaron Y (2007) Negative and positive effects of topsoil biological crusts on water availability along a rainfall gradient in a sandy and area. Catena 70: 437-442. Link: https://bit.ly/3jQnPbb
- Li XR, Chen YW, Su YG, Tan HJ (2006) Effects of biological soil crust on desert insect diversity: Evidence from the Tengger Desert of northern China. Arid Land Research and Management 20: 263-280. Link: https://bit.ly/34GG5xi
- Zaady E, Bouskila A (2002) Lizard burrows association with successional stages of biological soil crusts in an arid sandy region. Journal of Arid Environments 50: 235-246. Link: https://bit.ly/33RggLK
- Irmler U (2003) The spatial and temporal pattern of carabid beetles on arable fields in northern Germany (Schleswig-Holstein) and their value as ecological indicators. Agriculture Ecosystems & Environment 98: 141-151. Link: https://bit.ly/3lzfp8E
- Aloia A, Colombini I, Fallaci M, Chelazzi L (1999) Behavioural adaptations to zonal maintenance of five species of tenebrionids living along a Tyrrhenian sandy shore. Marine Biology 133: 473-487. Link: https://bit.ly/34QRpXZ
- Ayal Y, Merkl O (1994) Spatial and temporal distribution of tenebrionid species (Coleoptera) in the Negev Highlands, Israel. Journal of Arid Environments 27: 347-361. Link: https://bit.ly/3dj8cqe
- Thiele HU, Wieser J (1977) Carabid beetles in their environments: a study on habitat selection by adaptations in physiology and behaviour. Springer-Verlag Berlin. Link: https://bit.ly/36UJUlm
- Columbus U (2013) Ecological restoration of sand dunes at nort-western Negev: restoring aeolian activity and faunal response. P.Hd. Thesis, Ben Gurion University of the Negev, Beer Sheva, Israel (English).
- Pickett STA (1989) Space-for-Time Substitution as an Alternative to Long-Term Studies.,In: Long-Term Studies in Ecology – Approaches and Alternatives, Ed. G. E. Likens . Springer-Verlag Incl. New York 110-135. Link: https://bit.ly/3dj8m0O
- Damgaard C (2019) A critique of the Space-for-Time Substitution Practice in Community Ecology. Trends in Ecology and Evolution 34: 416-421. Link: https://bit.ly/34MQYhp
- Kolasa J, Rollo CD (1991) The heterogeneity of heterogeneity: a glossary. in Kolasa J and Picket STA Ecological Heterogeneity Springer-Verlag, New York 320.
- Littmann T, Berkowicz S (2008) The regional climatic setting. Pages 49-63 in S. W. Breckle, A. Yair, and M. Vences, editors. Arid Dune Ecosystems The Nizzana Sands in the Negev Desert. Springer, New York.
- Sigal Z (2009) Human influence, droughts, and multi-year climate fluctuations in Agur sands Reserve. Ben Gurion University of the Negev, Be'er-Sheva.
- Tielborger K (1997) The vegetation of linear desert dunes in the north-western Negev, Israel. Flora 192: 261-278. Link: https://bit.ly/2SKUPWH
- Ward D, New T, Yen A (2001) Effects of pitfall trap spacing on the abundance, richness and composition of invertebrate catches. Journal of Insect Conservation 5: 47-53. Link: https://bit.ly/3nOsXiL
- Renan I, Assmann T, Freidberg A (2018) Taxonomic revision of the Graphipterus serrator (Forskål) group (Coleoptera, Carabidae): an increase from five to 15 valid species. ZooKeys 753: 23-82. Link: https://bit.ly/3iQXZCT
- Assmann T, Boutaud E, Buse J, Chikatunov V, Drees C, et al. (2015) The ground beetle tribe Cyclosomini sl in Israel. Spixiana 38: 49-69. Link: https://bit.ly/3jQ74gx
- Elshewy D, Salem M (2016) Elmetwally, n.e. Checklist of the family Tenebrionidae (Coleoptera) in Egypt. Egyptian Journal of Agricultural Research 94: 39-57. Link:
- Lillig M, Pavlicek T (2003) The darkling beetles of the Sinai Peninsula: Coleoptera: Tenebrionidae (excl. Lagriinae et Alleculinae). Kasparek Verlag. Link: https://bit.ly/3dlvg7L
- Seely MK (1991) Sand dune communities. In G. A. Polis, editor. Desert Ecology Series: The Ecology of Desetr Communities. University of Arizona press 348-382.
- Pye K, Tsoar H (2009) Aeolian sand and sand dunes. Springer Verlag. Link: https://bit.ly/2ImJSbN
- Cloudsley-Thompson J (2001) Thermal and water relations of desert beetles. Naturwissenschaften 88: 447-460. Link: https://bit.ly/2GNQn73
- Ward D, Seely MK (1996) Adaptation and constraint in the evolution of the physiology and behavior of the namib desert tenebrionid beetle genus Onymacris. Evolution 50: 1231-1240. Link: https://bit.ly/30VHcbz
- D'Haese C (2000) Is psammophily an evolutionary dead end? A phylogenetic test in the genus Willemia (Collembola: Hypogastruridae). Cladistics 16: 255-273. Link: https://bit.ly/30Wp3u1
- Fet V, Polis GA, Sissom WD (1998) Life in sandy deserts: the scorpion model. Journal of Arid Environments 39: 609-622. Link: https://bit.ly/3dhwocA
- Henschel JR (1998) Dune spiders of the Negev Desert with notes on Cerbalus psammodes (heteropodidae). Israel Journal of Zoology 44: 243-251. Link: https://bit.ly/3536wOf
- Bonte D, Baert L, Maelfait JP (2002) Spider assemblage structure and stability in a heterogeneous coastal dune system (Belgium). Journal of Arachnology 30: 331-343. Link: https://bit.ly/36UEffd
- Maes D, Ghesquiere A, Logie M, Bonte D (2006) Habitat use and mobility of two threatened coastal dune insects: implications for conservation. Journal of Insect Conservation 10: 105-115. Link: https://bit.ly/36TUbOD
- Mattoni R, Longcore T, Novotny V (2000) Arthropod monitoring for fine-scale habitat analysis: A case study of the El Segundo sand dunes. Environmental Management 25: 445-452. Link: https://bit.ly/2GPeRN5
- Chikatunov VI (2007) Coleoptera of central Negev Catalog. Israel Natural History Collections, Unpublished, Tel-Aviv. Link:
- Krasnov B, Ayal Y (1995) Seasonal changes in darkling beetle communities (Coleoptera: Tenebrionidae) in the Ramon erosion cirque, Negev Highlands, Israel. Journal of Arid Environments 31: 335-347. Link: https://bit.ly/3disznD
- Krasnov B, Shenbrot G (1998) Structure of communities of ground‐dwelling animals at the junction of two phytogeographic zones. Journal of Biogeography 25: 1115-1131. Link: https://bit.ly/3nFxp35
- Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, et al. (2011) Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecology Letters 14: 19–28. Link: https://bit.ly/36VZv4g
- Fairfax Bird TL, Bouskila A, Groner E, Bar (Kutiel) P (2020) Can vegetation removal successfully restore coastal dune biodiversity?. Applied Sciences 10: 2310. Link: https://bit.ly/2SJVzuT
- Ramot A (2007) Effect of plant cover on arthropod community in Nizanim coastal dunes. M.A. Thesis. Ben Gurion University of the Negev, Beer-Sheba, Israel (Hebrow).
- Collingwood CA, Agosti D (1996) Formicidae (Insecta: Hymenoptera) of Saudi Arabia (Part 2). Fauna of Saudi Arabia 15: 300-385. Link: https://bit.ly/36W29af
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
