Global Journal of Ecology
The ecological impact on the distribution of Pangolins in Deng-Deng National Park, Eastern Region, Cameroon
Melle Ekane Maurice*, Esong lionel Ebong, Nkwatoh Athanasius Fuashi, Ichu Ichu Godwill and Amos Fang Zeh
Department of Environmental Science, University of Buea, P.O. Box 63, Cameroon
Conservation Action Research Network (CARN) for the Congo Basin, Central African Region, Cameroon
Cite this as
Maurice ME, Ebong El, Fuashi NA, Godwill II, Zeh AF (2019) The ecological impact on the distribution of Pangolins in Deng-Deng National Park, Eastern Region, Cameroon. Glob J Ecol 4(1): 008-014. DOI: 10.17352/gje.000009One of the major impediments to studying wild pangolins has been the difficulty in locating them. In many areas where biodiversity surveys have been conducted, no pangolins were recorded, despite extensive nocturnal searches. The main objective of this survey was to assess the role of ecological factors on the distribution of pangolins in deng-deng national park. The research data collection method used for this study constituted the laying of fifteen 2-kilometre transects in the study area. The collection of relevant data such as pangolin feeding-material signs, burrows, trails, foot-prints together with ecological parameters such as vegetation type, weather conditions, landscape and forest canopy type. The survey revealed that weather conditions and Pangolin-sign encounter rate were significant, χ2 = 6.125df=9 P<0.05. Moreover, a significant association was found between canopy-types and the animal species encounter rate, χ2 = 27.006 df=8 P<0.05. There was also a significant link between the age of pangolin-sign and the forest-type, χ2 = 29.995 df=30 P<0.05. The recent pangolin-sign recorded the highest encounter rate frequency 42.67%, while the fresh-signs encounter rate recorded 34.67% in the survey. The gentle and steep slopes both recorded an animal occurrence frequency of 41.3%, as compared to the very steep slope landscape 17.3%. This study has revealed that the pangolin species in deng-deng national park have an ecological distribution influence. Hence, the protection of these pangolin species would be much enhanced by the proper conservation and national park management strategies put in place by the Cameroon government.
Introduction
African pangolins inhabit tropical and subtropical forests, dry woodlands, and open savannah regions in tropical and inter-tropical regions of the continent [1]. Although they can occur on livestock farms if afforded protection from human persecution and have been found on palm oil and rubber plantations, they avoid areas of extensive agriculture and human settlements [2,3]. Another study by Kingdon et al. [4], indicated that most reviewed literature shows that there is some degree of uncertainty on the major and most favorable ecosystem preferred by pangolins. This is a hindrance to the conservation process, and as such there is need for studies in order to unravel the mystery behind pangolin ecology. In Africa, pangolins have a wide distribution, covering an array of natural and man-made habitats including tropical rain forests, subtropical thorn forests, deciduous forests, open scrub-lands, grasslands, cultivated lands and human settlements [5]. According to Yang et al. African pangolins equally inhabits different types of tropical forests including wet evergreen forests, moist forests, dry deciduous, thorn and scrub forests, and grasslands up to mid-elevations [5].
In a comparative study of four habitat types; secondary natural forest, pine-dominated forest, rubber plantations and tea-dominated home gardens, associated with a tropical lowland wet evergreen rainforest in southwest Sri Lanka, Pabasara et al. and Perera et al. [6,7], observed an evidence of the presence of pangolins in pine-dominated forest. It should be noted that the main factors determining the presence of pangolins is the availability of prey and water (depending on the species) [3]. The two arboreal species, Manis tetradactyla and Manis. tricuspis are found in both primary and secondary tropical rainforest and require the availability of large trees for shelter. Manis temminckii is the only African species that lives in arid regions, primarily woodland savannah with moderate to dense scrub [8], although both species of African ground pangolin (Manis Temminckii and Manis tricuspis) are absent from desert and semi-desert habitat [9]. Pangolins are unlikely to be detected through monitoring approaches that are effective for other mammals, and are often not well-covered in general wildlife surveys because of their different ecologies [10,11]. Pangolins are widely distributed in primary and secondary tropical forests, limestone forests, bamboo forests, grasslands and agricultural fields (Gurung, 1996; Suwal, 2011) which digs its own burrows, or enlarges passages made by termites.
The Morphology and behavior of pangolins is another interesting aspect of pangolin characteristics. Pangolins have an elongated tapering body covered with large overlapping scales. Scales are absent on ventral side of the body, head, inner surfaces of limbs and foot pads [12]. These moveable scales are shed periodically. The number and the pattern of scales may show intraspecific variations [12,13]. According to published descriptions, the number of rows of body scales can vary from 11 to 18 [13]. The color of scales varies from shades of brown to white, and often depends on the color of the soil associated with the habitat of the animal. The terminal scale on the ventral side of the prehensile tail of African pangolin is a distinct feature to differentiate them from other pangolin species [12]. Unlike most mammals, hair is virtually absent on dorsal surface of pangolins except some thick, short hair present between scales. However, thin, long, light colored hairs are present on the bare parts underneath (Kotagama and Goonatilake, 2013).
African pangolins are largely fossorial, but they are swift climbers as well. While walking on the ground, the tail and trunk are kept parallel to the ground with the back slightly arched. The hind legs are used to stand upright and search or sniff the surrounding air to detect prey [14]. African pangolin’s forelegs are specifically adapted for burrowing and digging. The three central claws are long and slightly curved. When climbing, forelimbs are used to tightly grip the tree, while the hind limbs are used to push the body upwards, and the tail helps to balance when arboreal [15]. The African pangolin can quickly roll itself into a compact ball in self-defense, exposing only its scales to a predator, and hissing loudly to scare off the predator. It can secrete a fluid with an irritating odor from their anal glands when disturbed or distressed by predators or humans [16].
In terms of pangolin burrowing characteristics, a recent study by Mahmood et al. [17] suggests that the African pangolin digs two types of burrows; living burrows and feeding burrows. Parameters such as burrow depth and diameter as well as the presence of remains of prey items and presence of fecal matter are considered as important signs in distinguishing the two types of pangolin barrows [18]. During the day time, pangolins sleep curled inside a “living burrow”, which may have several outlets sealed with loose earth [15]. Burrows are usually made under large rock boulders or sometimes in tree bases. The depth of the burrow varies depending on the soil type; 1.5 to 2m in rocky soils, and up to 5m or more in loose soil [19]. Pangolin burrows (diggings excavated deep enough for a pangolin to sleep in) were described as unmistakable for those of other species and as having a uniquely round entrance [20]. Talking about the diurnal or nocturnal character of pangolins, without precedent as white-bellied pangolin (Phataginus tricuspis), black-bellied pangolin (Phataginus tetradactyla), and Temminck’s ground pangolin (Smutsia gigantean) are known to be active in the day [21]. The current understanding is that giant ground pangolins are solitary [4]. For pangolins to thrive there should be the natural abundance of termites and ants in a forest that will serve as the pangolin primary feeding source. However, no unique environmental factor has been documented for favoring pangolin growth.
They appear to be highly selective of the ants and/or termites they prey upon and show seasonality in their diet selection, both are factors which may reduce trophic competition [3]. Foraging specificity is a contributing factor for the poor success in keeping pangolins in captivity. Larger species such as the giant ground pangolin, also consume other arthropods in low quantity [3]. Pangolins have highly developed olfactory organs and likely rely on sense of smell to forage. Pangolins will open termite and ant mounds using their front claws, although insect colonies are not destroyed and recover easily after pangolin raids [3]. An investigation conducted using fecal analysis of Indian pangolins in four districts of Potohar Plateau, Pakistan by Irshad et al. [18], found that ants (Camponotus confusion, and Camponotus compresses) are the major prey of Indian pangolin in the studied habitat while termites (Odonto termisobesus), bugs, wood fibers and grasses constituted other major components of the diet. Though considered as myrmecophagous, Indian pangolins may opportunistically feed on beetles, cockroaches, maggots and larvae of insects as revealed by gut content analysis studies [12,17] and according to local knowledge [6,22]. Though considered as myrmecophagous, pangolins may opportunistically feed on beetles, cockroaches, magotts and larvae of insects as revealed by gut content analysis studies [17] and according to local knowledge [6,22]. The eggs of the ants are more preferred by the Indian pangolins over ants, and they prefer feeding on prey found by burrowing rather than the prey species found on the soil or rock surfaces [12].
Materials and Methods
Description of the study area
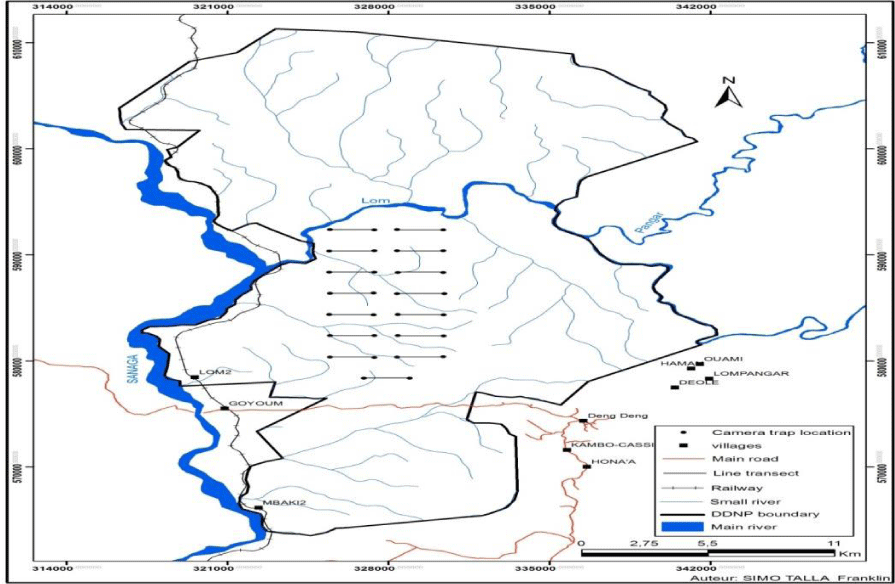
The Deng-Deng National Park is found in the East Region of Cameroon and covers a surface area of approximately 68,264 hectare. It is located between 5° 21’ 2.8” N to Longitude 13° 26’ 30.4” E [23]. It has mean monthly daily temperatures of about 34oc during the dry season. During the wet season, temperature falls to a daily mean of about 22oC, with the peak of rainfall recorded at about 2500mm in August [23]. The park has savanna ecosystem and forest vegetation found at the northern and southern portions of the park. The national park equally has diverse species of wildlife population, such as gorillas, chimpanzees, monkeys, antelopes, golden cats, porcupines, squirrels, red river hogs, duikers, and several others. This has made the park an attractive zone for research and exploration [23] (Figure 1).
Establishment of transects
In the study area 2-kilometre transects were laid using the GPS, 100m tape, and a compass. And a total number of fifteen 2-kilometre transects were laid in the study area. With the help of the GPS, the transect end point could be located from our position. This point was trailed using the compass by following the orientation of the angle given by the GPS. And where necessary, a cutter was used to trim impeding portion of the vegetation along transects to ensure easy access and identification of pangolin signs. The total area covered during the different phases of this study was approximately 1/6 of the entire park. In other words, the area covered was approximately 16.67% (1/6 x 100).
Collection of pangolin signs
Along transects identification of potential pangolin signs were recorded with the help of the hunters. The main signs recorded were the feeding materials, the burrows, and the claw marks on the trees. Since there was no evidence assuring that these signs were really those of pangolins, serious attention was needed in sign consideration, hence, only the feeding material signs located on dead woods that seemed to be widely used by pangolins and tails were considered. For each sign encountered, we noted its GPS point, the distance traveled, the perpendicular distance to the transect, its age and the species. We also characterized the environment of each sign by noting: the forest-type, the undergrowth vegetation, the canopy-cover, the undergrowth visibility, and the landscape slope. All burrows encountered along transects were characterized by the measurement of the following parameters: diameter, length, width, depth and orientation. These were located on the ground, and at the tree trunks. Analysis of the information gave potential information on the size and possible age of pangolins signs. Also, the random sampling of termite mount areas was considered to be important in the study because pangolins are ant-feeders, especially the termites.
Results
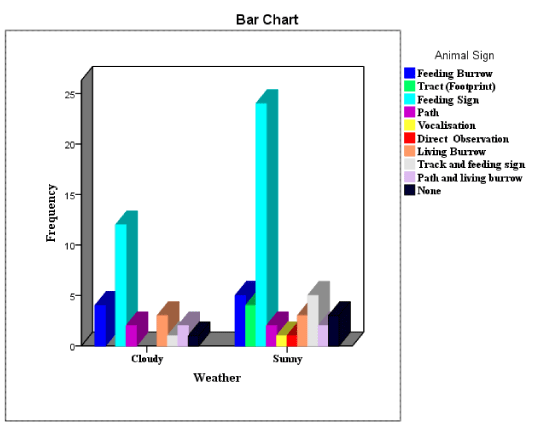
The results revealed that the weather conditions and pangolin sign-encounter rate were significant, χ2 = 6.125df=9 P<0.05 (Figure 2). Weather condition is an ecological parameter well known in determining wildlife behavior both socially and individually. Feeding and other animal social behaviors are known to increase in a warm bright weather temperature as compared to the wet cold weather condition. The Pangolin animals have shown a warm weather activity increase observed from their fresh-signs encounter rates during the study. The sunny weather condition facilitated the visibility of the signs and their proper identification 66.7%, as compared to the cloudy weather that impeded visibility of the signs 33.3% (Table 1).
The study revealed topography as another ecological determinant to the distribution of pangolins and other animal species population in the national park. The gentle and steep-slopes both recorded an animal occurrence frequency of 41.3%, as compared to the very steep-slope landscape 17.3%. Very steep-slope landscape have not been observed very often housing wildlife species except the birds and bats that can fly and perch on clips in the wild (Table 2).
The feeding sign of pangolins had the highest encounter rate frequency 48.0%, while vocalization had the least 1.3% (Table 3). The identification of frequent pangolin feeding signs than any other animal signs might be an evident that the population of this nocturnal rodent is in the increase in the national park. Night hunting by the local people in most part of Cameroon is with many challenges, needs a hunting lamp to enhance visibility, the hunters needs to be physically energetic and with a lot of experience. For this reasons most hunters avoid the night hunting, thus, animals like Pangolin rodents can only be killed by snare-traps. The nocturnal behavior of this animal species made possible for vocalization not to be commonly recorded by the diurnal data collection program of the research team, the reason why it was the least recorded.
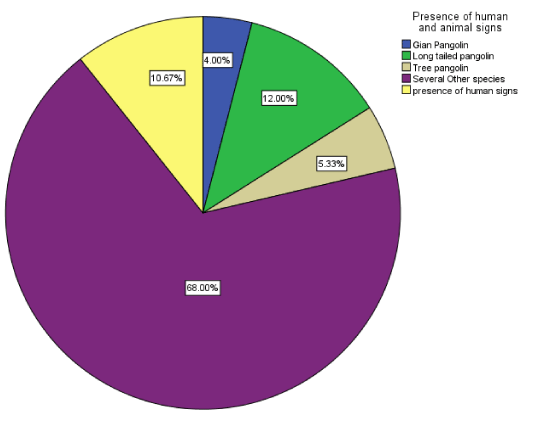
This survey recorded signs of three pangolin species endemic in the national park, white-bellied pangolin (Phataginus tricuspis)12.00%, black-bellied pangolin (Phataginus tetradactyla) 5.33%, and giant ground pangolin (Smutsia gigantea) 4.00% (Figure 3). From this observation the population of long-tailed pangolin is believed to be higher than the other two species. The presence of other wildlife had a frequency rate of 68.00%. Pangolin rodents are harmless and their territory is often shared with other wildlife species like duikers, antelopes, monkeys, and other species of rodents. Almost all the wildlife species are very territorial in the wild and hardly tolerate co-existence especially within the same species except for the purpose of procreation. Co-specific competition in wildlife is innate resulting to fight for feeding sites and mating partners.
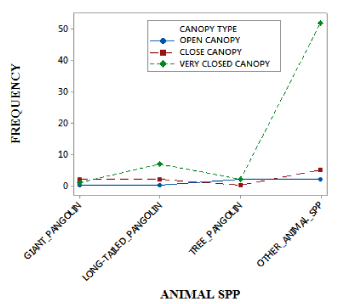
A significant association was found between canopy-types and animal species, χ2 = 27.006 df=8 P<0.05 (Figure 4). The rainforest of deng-deng national park is characterized with a dense continuous canopy architecture that shields the animal population from being spotted by distance predators. The dense forest canopy is a hide-out for birds, arboreal primates, the tree-climbing pangolins and also serves as a protection for forest moisture. The density of the canopy architecture in most areas in this national park determines the density of the understory. A dense rainforest canopy also provides a rich feeding ground to wildlife population, especially the arboreal species like rodents, primates, and birds. In addition, the close-canopy cover recorded the highest presence of animal population 52.0% (Table 4).
There was a significant link between the age of pangolin signs and the forest-type, χ2 = 29.995 df=30 P<0.05, (Figure 5). Interestingly, the forest-types were found to determine the presence and duration of the pangolin sign location. The frequency of recent pangolin sign-encounter rate recorded 42.67%, while old signs had 20.00%. (Figure 5). However, the pangolin population estimate could not be done by the sign-encounter rate frequency. In addition, the study showed a strong association between the forest-type and the pangolin sign-age, χ2 = 29.995 df=30 P<0.05, (Figure 5). The tropical rainforest in Cameroon is endemic to the pangolins, unfortunately, its population status is not known and the exploitation of the animals for local consumption and commercial scale-trade by foreign markets makes the population of this animal species to be threatened. Mixed and closed forest was characterized by very fresh-signs while secondary and open forest characterized by old signs. The recent-pangolin signs recorded the highest encounter frequency 42.67%, while the fresh-signs recorded 34.67% in the survey (Figure 6). The duration of pangolin signs as observed in the field during the data collection process could also explain to an extent the proximity of pangolin presence and its population in the location of the sign.
Discussion
All pangolin species have been subject to exploitation historically, which continues today, but little is known about their natural history, status or conservation needs [24]. The African pangolin (Manis pentadactyla) is an insectivorous scaly bodied small mammal with a wide historical distribution across eastern, southern and southeast Asia, but populations across its range are now severely threatened by hunting and illegal wildlife trade [25,26]. Although none of the previous surveys reviewed in this study specifically searched for pangolins within a given area, the low numbers of confirmed records from these surveys indicate that pangolins are largely missed by conventional biodiversity monitoring and require a specific detection and census methodology. The scarcity of detailed and accurate data (like altitude, and habitat type) recorded with pangolin is also a key problem. As expected, this study revealed the pangolin presence across different forest canopies showing there is a link between vegetation type and the intensity of pangolin presence. Habitat features such as tree species composition, vegetation cover and geological features (such as presence of rock boulders, water sources, and soil characteristics) are important parameters worth considering in habitat characterization [6,27]. Findings conducted by Kaspal, recorded thirty five species of vegetation and the distribution of burrows in different plant species does not differ. This study revealed the presence of pangolins in different forest types. According to Chakkaravarthy [5], Indian pangolin inhabits different types of tropical forests including wet evergreen forests, moist forests, dry deciduous, thorn and scrub forests, and grasslands up to mid-elevations. This ecological characteristic has been different from the observations of this study as pangolins and other animal species were identified across different forest habitats in deng-deng national park (DDNP).
In terms of forest landscape the encounter rate of the smaller pangolins (white-bellied and black-bellied) have been greater in DDNP as a result of the moist terrain with open understory often encountered in the park where even the slightest signs could be visibly noticed. The forest visibility has equally proven to be a vital ecological parameter in determining pangolin status [6]. In assessing pangolin status and population, estimation of the age of diggings and burrows could enable a surrogate measure of pangolin density to be developed, although occupant identity would need to be confirmed. Based on the images obtained using cameras and the signs of pangolins encountered at different ages, it is right to conclude in the study that pangolin population is dwindling. The true measure of population size would require knowledge of the rate of new burrow digging and also territory size, but comparative studies need only record number of active burrows per unit area in different habitats or sites. Similar approaches, based on relative burrow density, have been used to compare porcupine populations [28]. For pangolins, such efforts would also be best invested during the rainy season, when these species may be more active and/or signs of their activity more easily visible.
Even though pangolins are used for several purposes, all the purposes for which they are used are however not important in ensuring its regeneration and sustainability. Pangolins are important and valuable resources and conservationists need to engage with commitments in its adequate monitoring and conservation. For the sake of conservation and reviving the endangered species, the use of pangolins for the purpose of its delicacy and raw material it provides is not seen important, but rather its significance in ecological function and human health. Traditional medicine has been described by the World Health Organization (WHO) as the surest means to achieve total health coverage for the population. However, the fact that pangolins are now critically endangered and are the brink of extinction has led to a complete ban in trade of pangolins. Efforts are now being put to revive the species and it calls for a collective action of all stake holders [29]. Furthermore, the burrowing activity of pangolins creates a breeding habitat and shelter for many other animals [30]. Pangolins also excavate deep burrows for sleeping and nesting. Burrowing animals are sometimes referred to as “ecosystem engineers” as their burrows may be used by other animal species. Pangolin claws are used to pierce boils or skin abscess, and ointments made of pangolin scales to heal wounds and inflammations [31]. In Nepal, some communities consider pangolin flesh as a remedy for asthma and rheumatic fever, while oil extracted from pangolin scales is used to treat bone and muscle disorders [13]. Pangolin scales are also used by tribal ethno-medical practitioners in treating infertility in women [32,33].
Conclusion
The cultural belief system is most likely to be the driving force for the maintenance and continued use of pangolins in urban environments for medicinal purposes. Popular knowledge about the curative properties of pangolin body parts is an integral part of the local culture. This is to show that most cultures are inspired by the natural environment and demonstrates the necessity of carefully studying the use of pangolins for therapeutic practices to better understand the human cultural interaction. This has necessitated the undertaking of multidisciplinary studies to investigate the social, cultural, and economic aspects of pangolin use in traditional medicinal practices in Africa in order to develop sound conservation management strategies and action plans for these animal species, and maintaining the value of cultural attachments to pangolins. The importance and value of a particular resource is assessed based on its unique characteristic. The reviewed importance above is valued as it is for a sustainable exploitation. Reliable estimates of the status of an animal species are vital for its sustainable conservation management. Assessing the status of pangolins in deng-deng national park in particular and Cameroon in general remains a challenge because pangolins are difficult to study as they are nocturnal in nature. This study has shown that it is advisable to combine field and socio-economic techniques in efforts to assess pangolin poaching threats and status. This technique would greatly increase the chances of successfully obtaining pangolin data. Though the study was unable to estimate the actual number of pangolins in deng-deng protected area, field techniques have provided scientific evidence of the presence of pangolins, a baseline from which comparative studies could be carried out in future to monitor changes.
- Pangolín Special Group (2014) Scaling up Pangolin Conservation, Golbal Conservation Action Plan. Link: https://tinyurl.com/y2skkmkh
- Challender DWS, Waterman C, Baillie JEM (2014) Scaling up pangolin conservation. IUCN SSC pangolin specialist group conservation action plan. Zoological Society of London, UK. Link: https://tinyurl.com/y2skkmkh
- Gaubert P (2011) Family Manidae. In: Wilson DE, Mittermeier RA (Eds) Handbook of the Mammals of the World, Vol. 2: Hoofed Mammals. Lynx Edicions, Barcelona, Spain 82-103.
- Kingdon JS, Hoffmann M, Hoyt R (2013) Smutsia gigantean Giant Ground Pangolin. In: Kingdon JS, Hoffmann M (Eds) The mammals of Africa. Volume 5: Carnivores, pango-lins, equids, rhinoceroses 396-399.
- Chakkaravarthy QA (2012) Research and conservation needs of the indian pangolin (manis crassicaudata). Proceedings of Third Seminar on Small Mammals Conservation Issues 50-55. Link: https://tinyurl.com/yy7jz8by
- Pabasara M, Perera P, Dayawansa N (2015) A preliminary investigation of the habitat selection of indian pangolin (manis crassicaudata) in a tropical lowland forest in south-west sri lanka. Proceedings of 20th International Forestry and Environment Symposium, Sri Lanka. Link: https://tinyurl.com/yyoojot8
- Perera P, Wijesinghe S, Dayawansa N, Marasinghe S, Wickramarachchi C (2017) Response of tropical birds to habitat modifications in fragmented forest patches: A case from a tropical lowland rainforest in south-west Sri Lanka. Community Ecology 18: 175-183. Link: https://tinyurl.com/y2vzv3zq
- Heath ME, Coulson IM (1997) Preliminary studies on relocation of cape pangolins Manistemminckii. South African Journal of Wildlife Research-24-month delayed open access, 27: 51-56. Link: https://tinyurl.com/y4xczcg3
- Heath ME (1992) Manis temmincki. Mammalian Species 415: 1-5. Link: https://tinyurl.com/y4eblq8d
- Duckworth JW, Salter RE, Khounboline K (1999) Wildlife in Laos PDR: 1999 status report. IUCN, Vientiane. Link: https://tinyurl.com/y2nxnqda
- Shek CT, Chan CS, Wan YF (2007) Camera trap survey of hong kong terrestrial mammals in 2002-06. Biodiversity 15: 1-11. Link: https://tinyurl.com/y3pm66y2
- Heath ME (1995) Maniscrassicaudata. Mammalian Species Archive 513: 1-4. Link: https://tinyurl.com/yycxlv6q
- Kaspal P (2010) Saving the pangolins: Ethno zoology and pangolin conservation awareness in human dominated landscapes of nepal. Proceeding of the First One Day National Seminar on Small Mammals Issues 43-58.
- Israel S, Grewal B, Hoefer H, Sinclair T (1987) Indian wildlife: Sri lanka, nepal, APA Publications, Prentice Hall.
- Prater S (1980) The book of indian animals bombay natural history society and oxford university press. Mumbai.
- Roberts T (1997) The mammals of pakistan (revised ed.) oxford university press. Karachi, Pakistan, 525.
- Mahmood T, Jabeen K, Hussain I, Kayani AR (2013) Plant species association, burrow characteristics and the diet of the indian pangolin, manis crassicaudata, in the potohar plateau, pakistan. Pakistan Journal of Zoology 45: 1533-1539. Link: https://tinyurl.com/yy9frg5o
- Irshad N, Mahmood T, Hussain R, Nadeem MS (2015) Distribution, abundance and diet of the nindian pangolin (manis crassicaudata). Animal Biology 65: 57-71. Link: https://tinyurl.com/y56a98n4
- Prater SH (1965) The book of indian animals, Bombay natural history society.
- Nowak RM (1999) Walker’s mammals of the world, Vol 2, 6th edn. John Hopkins University Press, Baltimore.
- Pietersen D, Waterman C, Hywood L, Rankin P, Soewu D (2014) Smutsia temminckii. Link: https://tinyurl.com/yyrl4r2f
- Karawita K, Perera P, Pabasara M (2016) Indian pangolin (manis crassicaudata) in yagirala forest reserve: Ethnozoology and implications for conservation. Proceedings of 21st International Forestry and Environment Symposium, Sri Lanka. Link: https://tinyurl.com/yxcu4cdq
- Maisels F, Ambahe R, Ambassa E, Fosso B, Pouomegne JB et al. (2010) Wildlife and human impact surveys of the Deng Deng National Park and UFA 10065, 2010. WCS Cameroon, Yaounde, Cameroon.
- Challender DWS (2011) Asian pangolins: Increasing affluence driving hunting pressure. Traffic Bulletin 23: 92-93. Link: https://tinyurl.com/yyzmkxhj
- Corlett RT (2007) The impact of hunting on the mammalian fauna of tropical Asian forests. Biotropica 39: 292-303. Link: https://tinyurl.com/y5z2765m
- Challender DWS, Baillie J, Ades G, Kaspal P, Chan B, et al. (2014) Manis pentadactyla. The IUCN Red List of Threatened Species, version Link: https://tinyurl.com/lx6be9
- Mahmood T, Irshad N, Hussain R (2014) Habitat preference and population estimates of indian pangolin (manis crassicaudata) in district chakwal of potohar plateau, pakistan. Russian journal of ecology 45: 70-75. Link: https://tinyurl.com/y4pk638v
- Sidique MM, Arshad M (2004) Relative density of porcupine (Hystrix indica) population in forest plantation by food sta- tion transect method. Pak J Biol Sci 7: 1745-1749. Link: https://tinyurl.com/y5adrl6f
- Soewu DA, Adekanola TA (2011) Traditional-medical knowledge and perception of pangolins (Manissp) among the Awori people, Southwestern Nigeria. J. Ethnobiol Ethnomed 7: 25. Link: https://tinyurl.com/y2oycdxd
- Hansell MH (1993) The ecological importance of animal nests and burrows. Functional Ecology 7: 5-12. Link: https://tinyurl.com/y5e9z24d
- Mohapatra RK, Panda S, Nair MV, Acharjyo LN, Challender DW (2015) A note on the illegal trade and use of pangolin body parts in india. Traffic Bulletin 27: 33. Link: https://tinyurl.com/y4zl2fra
- Katuwal HB, Neupane KR, Adhikari D, Thapa S (2013) Pangolins trade, ethnic importance and its conservation in eastern nepal. Small Mammals Conservation and Research Foundation and WWF-Nepal, Kathmandu, Nepal. Link: https://tinyurl.com/y6jl6q8b
- Mentor-Pop Report (2017) Pangolin Conservation in Central African Region.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
