Global Journal of Clinical Virology
Relevance of rapid, reliable and low-cost diagnostics in the current COVID-19 pandemic
S.Gayathri, S.P. Mounika, Kauser Banu, Bhawana Rai, Geethakumari, Soniya H and Bhairab Mondal*
Cite this as
Gayathri S, Mounika SP, Banu K, Rai B, Mondal B, et al. (2020) Relevance of rapid, reliable and low-cost diagnostics in the current COVID-19 pandemic. Glob J Clin Virol 5(1): 001-009. DOI: 10.17352/gjcv.000007COVID-19 virus has emerged as a global pandemic. This zoonotic virus has known to be originated in China, which within few weeks has spread across the world. The virus has infected millions of people and lakhs have died due to this virus. It has high transmissibility with a reproductive number (Ro) of 2.2. Many cases are reported asymptomatic, hence making the control of the infection a herculean task. Many countries have resorted to lockdowns to flatten the curve of infection, leading to shutting of many businesses and pushing the global economy into severe recession. India’s GDP is said to be in negative territory, as expected by Reserve Bank of India. The review summarizes about the global efforts that are been made to develop novel, rapid, reliable and affordable molecular diagnostic assays such as nucleotide and antigen-based detection assays and serological assays. Many of them are under developing stage and few have received an Emergency Use Authorisation (EUA) or Conformite Europeene (CE) certification for their application in different hospitals and clinical settings. For confirmatory diagnosis, such category of molecular diagnostics needs to replace the presently employed Real-Time PCR assays that are high in installation, testing cost and expertise intensive normally resulting in delay for obtaining results. These limitations do not permit an exhaustive testing needed under the current pandemic situation. The new category of rapid, reliable, low cost diagnostic once deployed would effectively help in arresting the community transmission of the disease to a larger extent, thereby easing of lockdown situations and contributes towards revival of economy.
Introduction
Coronaviruses are a family of RNA viruses originally identified in 1960s. The β-coronaviruses are known to cause respiratory diseases that can range from common cold to severe disease conditions like Severe Acute Respiratory Syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV) and are likely to be of zoonotic origin. The lethal virus was named “SARS-CoV-2” as it was found to be related to Severe Acute Respiratory Syndrome (SARS-CoV) [1].
The disease induced by the virus is known as ‘COVID-19’. The early cases of COVID-19 were identified in Wuhan City of the Hubei Province, China [2]. On March 11th, the Director of WHO declared COVID-19 as a “global pandemic” [3]. The virus has high transmissibility with an estimated reproductive number of 2.2, which connotes that an infected person can spread the virus to another 2.2 individuals within an incubation period of 5.8 days [4].
The disease has spread globally and has infected over 6.0 million people with around 3.63 lakh deaths till the end of May 2020. Many asymptomatic cases are reported which challenges the control of infection. This virus outbreak has challenged the economic, medical and public health infrastructure all over the world [5]. It has pushed the global economy into severe recession and several countries have resorted to lockdowns to flatten the curve of infection causing shutting of many businesses and disruption in public services [6]. The economic losses in the Asia ranges from $1.7 trillion to $2.5 trillion during containment scenario, accounting to 30 percent of overall decline in the global output [7]. In India, the steep drop-in service activity has accounted to 52 percent of its GDP [8]. The initial 21 days of lockdown costed nearly $4.5 billion every single day [9]. The outbreak has led to an increase in the demand for hospital beds and medical equipments, while the medical and other supporting staffs are themselves getting infected [10]. Vaccine developments are underway at an accelerated speed, yet it might take a year to come out with a good vaccination programme which makes diagnostics and therapeutics are the only option available for containment of the disease and bringing the economy back in track.
SARS-CoV-2 is large enveloped, positive-sense single-stranded virus ((+) ssRNA) that encode four essential proteins like Spike (S) glycoprotein, small Envelope (E) protein, Membrane (M) protein, Nucleocapsid (N) protein and several accessory proteins that interfere with the host innate immune response [11]. Based on these structural information, researchers across the globe are working round the clock to come up with diagnostic kits for the early detection of the infection and development of appropriate novel vaccine and therapeutics. The review discusses about the probable vaccine and therapeutic approaches in current situation and various diagnostic assays and kits based on PCR, Isothermal assays and various types of immunoassays that are commercially available worldwide.
Probable vaccine and therapeutic approaches for virus containment
World-wide efforts for developing novel therapeutics and vaccines is at war front. Vaccine development is a lengthy, complex and expensive process [12]. It involves enormous research, followed by trials, regulatory clearances and huge co-operation from both public and private sectors. The study of the virus is a very important component as the virus is prone to frequent mutations [13].
Numerous strategies are implemented for the development of coronavirus vaccines. Spike glycoprotein (S protein) is targeted as it plays a major role in inducing protective immunity during infection by eliciting neutralizing antibodies and T-cell response [14]. Recombinant protein containing receptor binding domain (RBD) and recombinant vectors encoding RBD can be used for the development of effective SARS-CoV-2 vaccines. Immunoinformatics methods are utilized for the identification of epitopes for the inclusion in SARS-CoV-2 vaccine [15].
Scientists at Vaccine Research Centre (VRC) of National Institute of Allergy and Infectious Disease (NIAID) are developing a vaccine expressing SARS-CoV-2 S protein in the mRNA vaccine platform technology. It is expected to undergo clinical testing in the coming months [16]. A Chinese vaccine company, Cansino Biologics Inc has claimed that their vaccine candidate was able to generate immune response against the virus. The early-stage trials are over and requires proof for its effectiveness which requires trials in thousand more people. Among the world’s fastest-moving experiments on COVID-19 vaccines, University of Oxford and AstraZeneca Plc are set to start the second phase or advanced human studies for its ChAdOx1 nCoV-19 vaccine. In India, ICMR and Bharat Biotech International Limited (BBIL) are jointly working towards the development of vaccines. They are working towards creating a killed virus vaccine which provides good immunogenicity. In Thailand, Thai mRNA vaccine is developed by National Vaccine Institute and Chaulalongkorn University’s vaccine research centre. The experiments showed promising results on mice and the research has moved towards testing on monkeys [17].
Therapeutic drugs like remdesivir and lopinavir or ritonavir are taken in combination with interferon-β and convalescent plasma. Many anti-CoV agents like cobicistat, remdesivir, darunavir, oseltamivir, ritonavir, lopinavir and ASC09F (HIV protease inhibitor) are under phase III trial for COVID-19 disease [18]. Repurposed drugs are the potential therapeutic option for COVID-19 disease management. Lopinavir/ritonavir and interferon-1β possess in vivo anti-MERS-CoV activity. The in vivo study conducted in non-human primate model showed better outcomes [19].
Recently, the anti-viral efficiency of remdesivir and chloroquine were found to be highly effective in controlling COVID-19 in vivo [20].
Current nucleotide-based diagnostics for SARS-CoV-2
With fast advancement of medical diagnosis detection of viruses on bases of nucleic acid has become a rapid and reliable technology. PCR (Polymerase Chain Reaction) is a laboratory technique that is used for the amplification of segment of the interest DNA. Over the years, different types of PCRs are developed for varied applications. Real-Time PCR (RT-PCR) or Quantitative PCR (Q-PCR) is now considered the gold standard for the diagnosis of viral respiratory disease [21]. Globally this technique is currently in use for the detection of SARS-CoV-2. It is a simple and specific quantitative assay. Fluorescent dye or fluorescently labelled DNA probe or a quencher molecule is used to monitor the amplification of the DNA [22]. One-step procedure is the chosen approach for the detection of SARS-CoV-2. It is rapid, requires less bench time and reduces the chances of pipetting errors and cross-contamination. The only constrain of this technique is the risk of many false-positive and false-negative results [23]. However, the sensitivity and specificity of this technique is not hundred percent reliable.
The decrease in assay performance can be attributed towards the generation of mutated strains due to rapid evolution and genetic diversity, causing mismatches with the probe [24,25. The overall procedure of RT-PCR requires highly skilled personnel, high installation cost and a long time to deliver the test results.
Several types of RT-PCR kits are being developed that promises to overcome the inherent limitations. Globally diversified healthcare company, Abbott has developed a fully automated amplification and detection system for nucleic acids using 5´- nuclease technology [26]. Abbott’s Real-Time SARS-CoV-2 assay is engineered for dual targeting of RdRp and N-genes [27]. CE certified “ViroQ SRS-CoV-2” kit developed by BAG diagnostics from Germany, is designed for the detection of E gene and RdRp in the suspected samples. The RNA is converted to cDNA in one-step PCR procedure and then amplified according to the Real-Time PCR protocol. The test results are delivered in 90 minutes [28]. DiaSorin Molecular from Cypress, South California has developed “SimplexaTM COVID-19 Direct assay system” which is a Real-Time PCR system that enables the direct amplification of the virus from nasal or nasopharyngeal swabs. The assay targets the ORF1ab region and S gene. The time taken is little more than one hour. This test is authorized by FDA (Food and Drug Administration) under EUA (Emergency Use Authorization) [29]. “Lyra SARS-CoV-2 assay” is another assay developed in U.S.A. which is a Real-Time RT-PCR for the qualitative detection of nucleic acid from SARS-CoV-2 in nasal, nasopharyngeal or oropharyngeal swab specimens from the suspected patients. The identification of the virus is through using target specific primers and fluorescent-labelled probe (FAM - Fluorescein amidites) that hybridizes to the conserved region of the non-structural polyprotein (pp1ab) of the SARS-CoV-2 virus [30].
Other potential nucleic acid diagnostic assays
Other PCR based approaches like DNAzyme, and Multiplex PCRs are established to have sensitivities similar to Real-Time PCR but with added advantages of achieving low cost of testing, and can be used in hospitals, clinical labs and at point of entries. Multiplex PCR is used for the amplification of multiple targets in a single PCR experiment. Multiple primers are added in the reaction mixture. The technique is used to screen viral pathogens in many clinical samples. It has been proven to be a powerful and cost-effective tool in different epidemiological studies [31]. Many investigators across the globe are working on multiplex PCR for SARS-CoV-2 detection. GSD NovaPrime ® SARS-CoV-2 is a Real-Time multiplex PCR developed by Eurofins Technologies for direct qualitative detection of SARS-CoV-2 pathogens [32]. The Pixel by LabCrop COVID-19 utilizes multiplex PCR using two primers and probe sets to detect two regions of N gene and one primer and probe set for detection of human RNase P (RP) [33].
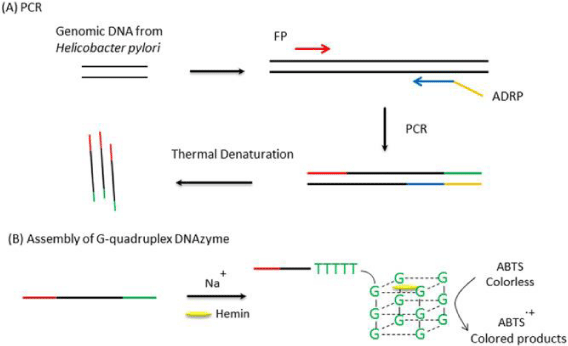
DNAzymes is yet another low-cost, reliable and rapid diagnostic assay. DNAzyme is a nucleic acid G-quadruplex structure that binds hemin to yield a complex that mimics peroxidase activities. The DNAzyme binds to its specific substrate and catalyses the reaction thereby resulting in the formation of coloured product which can be detected through colorimeter. This DNAzyme phenomenon has been exploited to detect SARS-CoV-2 in suspected patient samples. The RNA from the virus is extracted and subjected to PCR. DNAzyme synthesis in PCR is achieved by introducing uniquely designed primers. A study done by Wu and co-workers reported that they utilized DNAzyme to target 5`- untranslated region (UTR) of SARS genome to suppress SARS-CoV replication. The results have shown efficient cleavage of the substrate in vivo and its subsequent inhibition of the expression in mammalian cells [34] Figure 1.
Isothermal assays
A huge scope is available for developing and employing isothermal PCR based molecular assays for further easing out the cost of testing and requirement of highly skilled personnel.
There are several types of isothermal nucleic acid amplification methods such as Loop-Mediated Isothermal Amplification (LAMP), Recombinase Polymerase Amplification (RPA), Nucleic Acid Sequence-Based Amplification (NASBA), Rolling Circle Amplification (RCA), Polymerase Spiral Reaction (PSR), Transcription Mediated Amplification(TMA) and Helicase-Dependent Amplification (HAD) [35,36].
Abbott has developed the “ID NOW COVID-19” test system, which is a lightweight and portable, molecular point-of-care test system. It works on the principle of isothermal amplification of nucleic acid. The test can be accomplished within 5 minutes. The template is created to amplify a unique region of the RdRp segment. Fluorescently labelled molecules are then used to identify each of the amplified RNA targets. The test has received Emergency Use Authorisation (EUA) in the US [37].
LAMP assay is a widely attempted isothermal assay that has been standardized for the detection of large number of microorganisms specifically for disease diagnostics. The assays conventionally employ four set of primers to recognise eight distinct regions of target. Strand displacement is carried out by using Bst polymerase enzyme at standardized temperature that ranges between 60-65°C for an amplification time of close to 1 hour. In order to detect RNA viruses, LAMP assay can be modified by incorporation of reverse transcription step. This technique has generated a great interest among investigators and is believed to have the potential to be used as point-of-care diagnostics with higher sensitivity and can be performed in a decentralized test facility [38]. It has been used for the detection of Avian influenza virus which is a zoonotic virus like SARS-CoV-2. Huang’s research group have found a nucleic acid visualization technique that combines with RT-LAMP technique and a vertical flow visualisation strip (RT-LAMP-VF) to detect N gene of MERS-CoV [39]. In India, Sree Chitra Tirunal Institute for Medical Sciences & Technology developed LAMP diagnostic test for detection of SARS-CoV-2 and the kit is commercially called as ‘Chitra Magna’[40] Figure 2.
Nucleic Acid Sequence-Based Amplification (NASBA) is a robust amplification technology developed for the detection of RNA viruses. The methodology utilizes three enzymes: reverse transcriptase, T7 RNA polymerase and RNase H. The final amplification product is a single-stranded RNA with a polarity opposite to that of the target. An isothermal testing methodology known as Mango NASBA (Nucleic Acid Sequence-Based Amplification) has been undergoing for coronavirus diagnosis. The technique was named Mango after the aptamer, because it is specific for the RNA sequence bind and the brightly coloured fluorescent dye become excited and glow. It allows the detection of RNA and can be studied under a microscope [41].
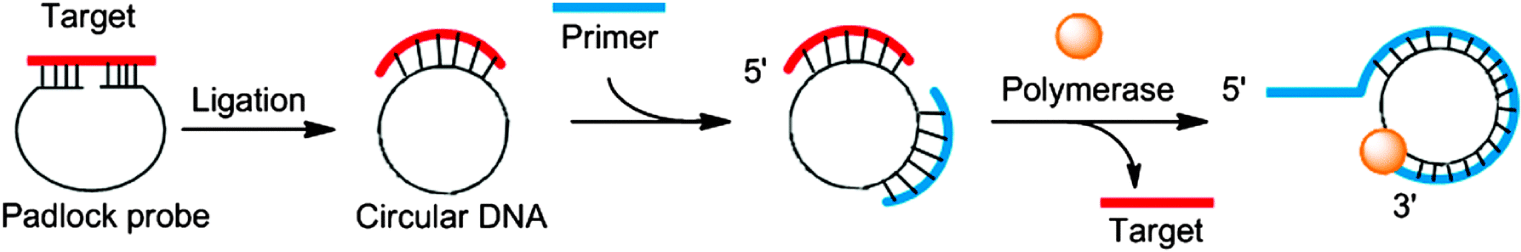
RCA is an isothermal enzymatic amplification assay, developed for sensitive diagnosis for a variety of targets including nucleic acids, DNA or RNA. A short RNA primer and RNA polymerase are employed to amplify circular RNA or DNA template to give rise to concatemer product containing tens to hundreds of tandem repeats that are complementary to the circular template. RCA is a unique procedure among isothermal reactions, requires a very fine approach for standardisation that needs to be further explored for large number of viral diseases. However, it has not been deployed for the detection of SARS-CoV-2 at this point [42].
RPA is relatively a new technique for developing isothermal assays and holds a better promise compared to other isothermal based assays due to its rapidity and specificity. It is a field deployable assay with minimal sample requirement, low operation temperature ranging from 25-42°C and is user friendly [43]. The assay is carried out by employing recombinase enzyme which forms complexes with the oligonucleotide primers and pair of primers with their homologous sequence present within DNA. The displaced template DNA is stabilised by single stranded binding protein and helps in formation of D-Loop. Amplification would be initiated by primers, only if the target sequences are present in the template.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) characterizes a family of nucleic acid sequence found in prokaryotic organisms. These recognized sequences are cut by a set of bacterial enzymes known as CRISPR-associated enzymes, represented as Cas9, Cas12 and Cas13. Sherlock Biosciences company has developed SHERLOCK method that uses Cas13 that cleaves the fluorescent RNA reporter when activated by target sequence [44]. Another assay developed by Mammoth Biosciences “DETECTR” uses Cas12 to specifically detect E and N genes of the RNA sequence. The target then undergoes isothermal amplification, resulting in visual readout with fluorophore. This technique does not require complex instrumentation and can be read using paper strip to detect SARS-CoV-2 without the loss of specificity or sensitivity. These tests have a great potential for point-of-care diagnosis and have the advantage of being low cost and less detection time of one hour [45].
Viral RNA extraction kit using magnetic beads
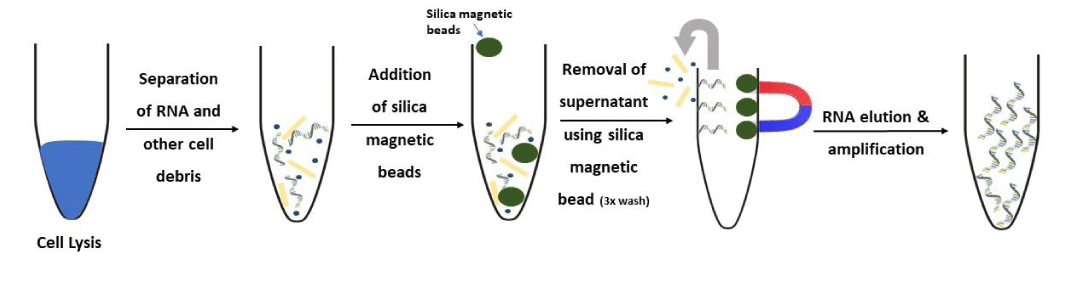
The first step of any detection test involves isolation of high quality and good concentration of RNA without degradation. This step is crucial as it is critical for the proper outcome of PCR or any another assays. Isolating viral genome using magnetic beads has risen a lot of interest among many researchers. The basic principle in viral isolation by magnetic beads involves addition of magnetic beads conjugated with specific binding agent, followed by magnetic separation of the bound particles from the mixture. This is a popular approach for extraction of nucleic acids due to high potential for automation. The extraction efficiency is much higher, and the time taken for the isolation is less. Simple procedure and avoidance of organic solvents makes it an attractive method for routine diagnostic purposes [46]. For the present COVID-19 crisis, Zymo research have come up with Quick-DNA/RNA Viral MagBead Kit for total RNA isolation for RT-PCR. The kit contains DNA/RNA Shield reagent that has been demonstrated to inactivate MERS-CoV, Parvovirus, HIV, HSV-1, HSV-2 and other pathogens [47] Figure 3.
Serological and immunological assays
Immunoassays for the recognition of antigens of microorganisms has emerged as an important tool for diagnosing and managing infectious diseases. Antigen detection is a useful diagnostic tool as it is rapid, simple one-step assay and requires less reaction time. These rapid diagnostic tools are convenient, relatively low-cost and does not require much laboratory equipment. They are generally produced at a lower cost than PCR and can potentially be scaled up to test millions of people [48].
Several Rapid Antigen Detection Tests (RADTs) are available commercially for the diagnosis of acute respiratory infection due to respiratory syncytical virus [49]. Serological tests are indirect way of diagnosing as it detects the antibody to SARS-CoV-2 in the body rather than detecting the presence of virus. The tests are based on the principle that the immunoglobulins produced by the immune system recognize, bind and neutralize foreign substance in the body [50].
These tests are aimed to distinguish patients with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection [51]. These are in high demand in order to quantify the number of COVID-19 cases. However, FDA has recommended that serological test should not be used as a sole basis for the diagnosis of SARS-CoV-2 because antibodies may not develop until two weeks of post symptom onset [52].
Although currently WHO does not recommend the use of antigen-detection for rapid diagnostic tests for patient care, research in their potential diagnostic efficiency is highly encouraged [53]. On 9th of May 2020, the U.S. Food and Drug Administration (FDA) issued the first Emergency Use Authorization (EUA) for SARS-CoV-2 antigen test. The test is swift in detecting the fragments of proteins found on or within the virus by testing the nasal swabs. The test is authorized for their use in point-of-care testing facilities and in high and moderate complex laboratories certified by Clinical Laboratory Improvement Amendments (CLIA) [54]. The antigen detection tests could be specific for virus, but not as sensitive as PCR tests. South Korean company, SD Biosensor have launched a kit called Standard Q COVID-19 Ag detection kit. The kit is highly specific and can detect 99.3 percent and 100 percent true negatives. Its ability to detect true positives ranges from 50.6 percent and 84 percent which a depended on the viral load in the patient [55].
Several immunoassays have been developed to enable the detection of IgM or IgG antibodies specific for viral antigens that includes S1, S2 subunits and receptor binding domain (RBD) of spike glycoprotein and nucleocapsid protein. The major categories of serological test available are Laboratory-based immunoassays like ELISA, CLIA, Rapid Serologic Test, and Virus Neutralization Test.
Enzyme-Linked Immunosorbent Assay (ELISA), is a sensitive and point-of-care diagnostic method capable of multiple sample analysis. It is a microwell, plate-based assay technique. In case of direct ELISA, the plate wells are coated with viral protein. If the antibody to the antigen is present in the sample, it will bind to the form a complex. This complex can then be detected using additional tracer antibody to produce a colorimetric or fluorescent-based readout [24]. “Platelia SARS-CoV-2 Total Ab” is a one-step antigen capture ELISA (Enzyme-linked Immunosorbent Assay) for qualitative detection of IgM/ IgA/ IgG in human serum or plasma [56]. The antibody test by Abbott detects the IgG antibody to SARS-CoV-2. The test is used on Abbott’s i1000SR and i20000SR laboratory instruments, which can run up to 100-200 tests per hour [57].
Chemiluminescent Immunoassay (CLIA) is a type of immunoassay technique like ELISA where the label is a luminescent molecule. The luminophore markers used are acridinium and ruthenium esters. The enzymatic markers used are horseradish peroxide (HRP) with luminol or its derivatives, alkaline phosphate with adamantyl 1,2-dioxetane aryl phosphate (AMPPD) [58]. A novel chemiluminescent immunoassay (CLIA), “MAGLUMITM 2000 Plus” was made by New Industries Biomedical Engineering Co., Ltd, China. It uses magnetic microbeads and ABEI (Amino-Butyl-Ethyl-Isoluminol) labels which improves the stability and sensitivity of the reagents. The expected output is up to 180 tests/ hour [59]. Cai and co-workers have developed a magnetic chemiluminescence enzyme immunoassay using synthetic peptide antigens from orf1ab, spike and nucleocapsid proteins. The peptides were labelled with biotin and was bound to streptavidin-coated magnetic beads [60].
Rapid Serologic Test, also known as Rapid Diagnostic Test or Lateral flow assay (LFA) is typically a small, portable, point-of-care, qualitative chromatographic assay. The results can be obtained within 10-30 mins. The operation principle is same as ELISA, the assay is a paper-based platform for the detection of analytes in complex mixture. The test strip is composed of sample pad, conjugate release pad, membrane with immobilized antibodies and absorbent pad, which are fixed on inert backing material. The sample deposited on the sample pad moves to conjugate where the conjugated antibody binds to the target analyte and migrates to test line where immobilized antibodies react with analyte bound to conjugated antibody [61]. Many Lateral Flow IgG/IgM kits are manufactured, to a name a few, Cellex qSARS-CoV-2 IgG/IgM Cassette Rapid Test kit by Cellex Inc (United States Of America), OnSite COVID-19 IgG/IgM Rapid Test by CTK Biotech Inc (USA), GenBody COVID-19 IgM/IgG by GenBody Inc (Korea) and SARS-CoV-2 Antibody Test (Lateral Flow Method) by Guangzhou Wondfo Biotech Co Ltd (China) [62].
Virus Neutralization Test, also known as plaque reduction neutralization test (PRNT) is used to measure the titer of neutralizing antibody against a virus. The test usually takes 3-5 days to complete and includes three main elements namely, cultured cells, viable virus and patient’s specimen to determine if the patient has antibodies that can neutralize or prevent the virus from infecting the cells in the cell culture [63]. Ethos laboratories in partnership with GenScript, USA are first in the world to offer a quantitative surrogate viral neutralization test to quantify the neutralizing capacity of antibodies against SARS-CoV-2. The test is marketed as Tru-Immune [64].
Immunofiltration assay is one of the serological assay that involves flow of fluid containing the analyte through a porous membrane and into an absorbent pad. Over the past few years, this assay have been designed with various modifications for detection of antibodies, serum proteins, antigens from human samples. This assay was first utilized during Ebola Haemorrhagic fever outbreak in Central Africa targeting monoclonal antibodies to the EBOV matrix protein (VP40), which previously had been found to work in a conventional enzyme-linked immunosorbent assay [65]. Immunofiltration combined with magnetic detection has various advantages such as quantification and sensitive detection. This assay is found to have a sensitivity similar to that of the widely used antigen-detection ELISA and relatively less sensitive than RT-PCR assay. Turklab, a Turkish firm based in Aegean province of Izmir has developed a test kit relying on immunofiltration assay which will yield results within five minutes [66].
Flow Cytometry (FCM) is a technique used to detect and measure physical and chemical characteristics of cells or particles. The basic principle involves that the cell components are fluorescently labelled and then excited by the laser to emit light at varying wavelengths. Flow virometry is a recent and exciting development of flow cytometry. Flow virometry has now been used to characterize an expanding array of additional viruses, including lambda phage, herpes simplex virus 1 (HSV-1), Mouse Hepatitis Virus (MHV), human immunodeficiency virus (HIV), Nipah virus, Junin virus, vaccinia virus, dengue virus, Human Cytomegalovirus (HCMV), and giant viruses [67]. The fluorescence signals of the small genome-sized RNA viruses (7.4-14.5 kb) were found at the limit of detection. The main disadvantage of flow virometry is the inability to distinguish between infectious and non-infectious particles [68].
The antibody tests do not have much value for disease diagnostics but do have merits in retrospectively confirming infection, for epidemiological studies to asses community spread and to evaluate herd immunity.
Indian efforts for development of diagnostics
India has been greatly dependent on other countries for diagnostics kits. Many imported kits are faulty and are sent back as they reported inaccurate results in many places [69].
Many clinical laboratories are using Real Time-PCR version developed by IIT Delhi and Mylab. The use of indigenous kits has lowered the cost of RT PCR testing [70]. With growing number of cases, the labs are staying selective in picking up cases of only severe category of disease. This trend is still been followed in highly developed countries of Europe and America despite possessing huge resources. RT-PCR systems are limiting the accessibility to other categories of infected individuals that are under asymptomatic or exhibiting mild symptoms. As individuals stay infectious, they would keep contributing towards the transmission of infection in the community. Owing to this inherent limitation of presently available RT PCR testing methods, control of disease is technically difficult to achieve despite large scale lockdowns [71]. Lockdowns are simply assisting in relatively slow spread of the infection, rather than an effective containment of the disease with these available testing and isolation protocols [72].
Sree Chitra Tirunal Institute for Medical Sciences & Technology has developed a magnetic nanoparticle-based RNA extraction kit for PCR and LAMP diagnostic tests for SARS-CoV-2 called Chitra Magna. The test kit is being validated in larger test samples for ICMR approval [40]. They have also developed an innovative diagnostic test kit named, Chitra Gene LAMP-N for the diagnosis of SARS-CoV-2. The kit detects the N-gene of the virus using RT-LAMP (Reverse Transcription Loop-Mediated Isothermal Amplification) technique. It is highly specific that can detect two regions of the gene to ensure its effectiveness even if one region of the gene undergoes any mutation. The time taken from sample collection to the delivery of the result is less than two hours [73].
The National Institute of Virology, Pune has developed “COVID KAVACH ELISA” for antibody detection using isolated virus from positively- tested patients. The kit is approved by Indian Council of Medical Research (ICMR) and is manufactured by Zydus-Cadila, a leading global pharmaceutical company in India. The kit is reported to have a sensitivity of 98.7 percent and specificity of 100 percent but has no value for disease conformation. As per the ICMR officials, the kit will be used in the nationwide sero- surveillance to be conducted by the Health Ministry [74].
“Quanitplus COVID-19” is also an ICMR approved testing kit developed by Huwel Lifesciences from Hyderabad. The kit can deliver results within two hours. It is a single formulation which doesn’t require any addition of separate component while setting up the reactions [75-77].
SN Lifesciences, in collaboration with BBC (Bangalore Bioinnovation Centre) has developed five kits, namely SN isothermal, SN RPA isothermal, SN DNAzyme, SN RAMP, and SN Triplex PCR. These kits overcome the problem of the existing PCR assays by employing a novel RNA isolation method and provide a rapid molecular detection as alternative to existing molecular tests. SN RPA isothermal test and Isothermal test are rapid, reliable and sensitive. The time taken would be 90 mins from clinical sample processing to delivery of result. These are user-friendly and do not require any special expertise for its performance and are doable at any hospital or clinical setting, highly reproducible and comparable in terms of sensitivity similar to the presently used real-time PCR. They are highly affordable with a total cost of being less than Rs. 500 per sample testing compared to Rs. 4,500 for the real time PCR in use. These diagnostic systems can be installed at one-fifth the cost of installation of the existing real time PCR system.
SN DNAzyme uses primers that harbour antisense sequence of DNAzyme. DNAzyme is a single stranded nucleotide that mimics catalytic peroxidase enzyme that gives colour indication in the presence of virus in patient sample. The cost of testing is Rs. 500/- per test. The test would be do-able in any hospital or clinical laboratory with relatively far-less installation costs. Results would be available in 2-3 hours compared to days taken by the present strategy of using RT-PCRs. The above mentioned three kits are at validation stage.
SN RAMP assay is two step isothermal amplification process which uses primers that targets the open reading frame 1ab (ORF1ab) gene of the COVID-19 RNA virus. This is a colorimetric assay for rapid and reliable diagnosis of SARS-CoV-2 from any kind of clinical samples. The cost of testing is Rs. 650/- per test, and the test results would be available within 1-2 hours.
SN Triplex PCR assay uses the primers that particularly targets the highly specific regions of open reading frame 1ab (orf1ab) gene, spike glycoprotein (S gene) and membrane glycoprotein (M gene) of the COVID-19 RNA virus ensuring high specificity and high selectivity. The reliability of the triplex PCR test in additional revealed an internal amplification control (IAC) that has been incorporated in the test. The positive result is indicated by the presence of four amplicon products and negative result by one amplicon product that is of IAC. The cost of the test is Rs. 450/- per test. This test reliably diagnoses the patients within 3 hours from collection and processing of sample thereby initiating highly effective response mechanisms to check the spread of disease. This method is relatively more reliable than other PCR based assays, including real time PCRs which are presently in use.
With the spurt in cases happening each passing day and the requirements of the testing that is estimated to increase many folds, these test kits would be of enormous help not only in saving the government expenditure but also in arresting the spread of infection in more efficient ways. A single test alone doesn’t stand out to be 100 percent efficient, hence combination of tests like PCR and Serological test can be carried out to increase the diagnostic efficiency. Testing with low cost serological test available in a market can be used initial testing of patients showing any symptoms. Highly sensitive assays can then be used for confirmation of the results.
Conclusion
Research, technology development and deployment are currently the only defence to fight against SARS-CoV-2. Many vaccines are under development stages and few are under clinical trials. Even with the best of efforts from scientist across the world, the earliest genuine estimate of getting a vaccine still is 8-10 months away. Epidemiologists also are relying on to the fact that by the time vaccine appears, the disease would be under control owing to other critical factors. These include building of herd immunity, loss of virulence in strain due to successive passages of the strains in the susceptible population, environmental factors and likely appearance of therapeutic regimes. The easiest and more practical approach to contain the transmission of virus is still largely dependent on quick mass testing of suspected individuals and patients followed by isolations, quarantine or hospital treatments. Herein, a wide-range use of rapid, reliable, low-cost for installation and testing and easily deployable tests would play the critical role. As presented in this review, possibility of such relevant tests or kits entering the system is extremely high in the very near future. The government agencies throughout the world should encourage speeding up the processes of validations, reagent resourcing and deployment of such tests or kits at the maximum number of hospitals and clinical laboratory settings. This is bound to have a significant impact in arresting the transmission of the disease in the communities and when employed with effective isolation and quarantine procedures would ease out the lockdown situations thereby bringing the economy back on rails.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, et al. (2020) COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Human Vaccines and Immunotherapeutics 1-7. Link: https://bit.ly/3dzdfS5
- Wu D, Wu T, Liu Q, Yang Z (2020) The SARS-CoV-2 outbreak: what we know. Int J Infect Dis 94: 44-48. Link: https://bit.ly/3i1Jpcg
- Outside FOR, Us, THE and Only, C. (n.d.). LIAISON ® SARS-CoV-2 S1/S2 IgG. 2–5. Link: https://bit.ly/2Ns1gen
- Chen WH, Strych U, Hotez PJ, Bottazzi ME (2020) The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep 1-4. Link: https://bit.ly/31cnh9e
- Explained: How Covid-19 has affected the global economy. Link: https://bit.ly/3i0z5Bq
- COVID-19 Economic Impact Could Reach $8.8 Trillion Globally — New ADB Report. Link: https://bit.ly/3fVzG5p
- Indian economy after COVID-19: a positive outlook. Link: https://bit.ly/37ZNiKc
- Covid-19 has hurt India the most among the world’s top 10 economies. Link: https://bit.ly/37Y6Mit
- COVID-19 may double poverty in India. Link: https://bit.ly/2CztO3n
- COVID-19 in India: Poverty may be deadlier than the coronavirus. Link: https://bit.ly/3fZHCCX
- Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, et al. (2020) Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 14: 3822-3835. Link: https://bit.ly/2VbmlOD
- Lurie N, Saville M, Hatchett R, Halton J (2020) Developing Covid-19 vaccines at pandemic speed. New England Journal of Medicine 382: 1969-1973. Link: https://bit.ly/3dxNPUI
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, et al. (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281-292.e6. Link: https://bit.ly/2VejtRa
- Kim MH, Kim HJ, Chang J (2019) Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. PLoS One 14: e0220196. Link: https://bit.ly/2Z4t4Li
- Baruah V, Bose S (2020) Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol 92: 495-500. Link: https://bit.ly/2YtK4vn
- NIAID (2020) Developing therapeutics and vaccines for coronaviruses. Link: https://bit.ly/3eIWCEI
- Coronavirus (Covid-19) vaccine latest update: Cansino Biologics claims success in human trials; Indian vaccine unlikely soon. Link: https://bit.ly/3i43grh
- Li G, De Clercq E (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 19: 149-150. Link: https://bit.ly/2NuMzaG
- Chan JFW, Yao Y, Yeung ML, Deng W, Bao L, et al. (2015) Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 212: 1904-1913. Link: https://bit.ly/3hVXTut
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research 30: 269-271. Link: https://go.nature.com/2BD5ihz
- Mackay IM, Arden KE, Nitsche A (2002) Real-time PCR in virology. Nucleic acids research 30: 1292–1305. Link: https://bit.ly/3eviKTe
- Piiparinen H, Höckerstedt K, Grönhagen-Riska C, Lautenschlager I (2004) Comparison of two quantitative CMV PCR tests, Cobas Amplicor CMV Monitor and TaqMan assay, and pp65-antigenemia assay in the determination of viral loads from peripheral blood of organ transplant patients. J Clin Virol 30: 258-266. Link: https://bit.ly/3eviN1m
- Mo Y, Wan R, Zhang Q (2012) Application of reverse transcription-PCR and real-time PCR in nanotoxicity research. Methods in molecular biology (Clifton, N.J.) 926: 99–112. Link: https://bit.ly/37XTcf3
- Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, et al. (2020) Assay Techniques and Test Development for COVID-19 Diagnosis. ACS central science 6: 591–605. Link: https://bit.ly/3i23HTd
- Stellrecht KA (2018) The drift in molecular testing for influenza: mutations affecting assay performance. J Clin Microbiol 56: e01531-17. Link: https://bit.ly/2YvoeaK
- M2000 Realtime System. Link: https://abbo.tt/2NqTGR8
- ABBOTT REALTIME SARS-COV-2 ASSAY. Link: https://abbo.tt/3hVYts9
- ViroQ SARS-CoV-2 - the reliable rapid PCR test. Link: https://bit.ly/37XoySO
- Simplexa® COVID-19 Direct Kit. Link: https://bit.ly/37WM1Un
- Lyra SARS-CoV-2 assay. Link: https://bit.ly/3hZtM54
- Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE (2000) Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev 13: 559-570. Link: https://bit.ly/2B9aFoz
- Eurofins launches RT multiplex PCR test for COVID-19 detection. Link: https://bit.ly/37YpC96
- FDA OKs LabCorp's At-Home COVID-19 Test Kit. Link: https://bit.ly/3dtkA5L
- Wu S, Xu J, Liu J, Yan X, Zhu X, et al. (2007) An efficient RNA-cleaving DNA enzyme can specifically target the 5'-untranslated region of severe acute respiratory syndrome associated coronavirus (SARS-CoV). Journal of Gene Medicine 9: 1080-1086. Link: https://bit.ly/3dAIpse
- Gill P, Ghaemi A (2008) Nucleic acid isothermal amplification technologies—a review. Nucleosides, Nucleotides Nucleic Acids 27: 224-243. Link: https://bit.ly/2NpGrAt
- Zhao Y, Chen F, Li Q, Wang L, Fan C (2015) Isothermal amplification of nucleic acids. Chemical reviews 115: 12491-12545.
- Detect Covid-19 In As Little As 5 Minutes. Link: https://abbo.tt/2Z2HnQy
- Thai HTC, Le MQ, Vuong CD, Parida M, Minekawa H, et al. (2004) Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J Clin Microbiol 42: 1956-1961. Link: https://bit.ly/2VgYUmU
- Huang P, Wang H, Cao Z, Jin H, Chi H, et al. (2018) A Rapid and Specific Assay for the Detection of MERS-CoV. Frontiers in microbiology 9: 1101. Link: https://bit.ly/2Nv55zr
- Sree Chitra Tirunal Institute develops magnetic nanoparticle-based RNA extraction kit for PCR and LAMP tests for COVID-19. Link: https://bit.ly/2Vgkb09
- Coronavirus testing kits using RNA imaging technology. Link: https://bit.ly/3dA9HyS
- Gu L, Yan W, Liu L, Wang S, Zhang X, et al. (2018) Research progress on rolling circle amplification (RCA)-based biomedical sensing. Pharmaceuticals 11: 35. Link: https://bit.ly/2Z4OQi9
- Daher RK, Stewart G, Boissinot M, Bergeron MG (2016) Recombinase polymerase amplification for diagnostic applications. Clinical chemistry 62: 947-958. Link: https://bit.ly/31eFh2B
- Zhang F, Abudayyeh OO, Gootenberg JS (2020) A protocol for detection of COVID-19 using CRISPR diagnostics. A protocol for detection of COVID-19 using CRISPR diagnostics 8. Link: https://bit.ly/2B0Viie
- Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, et al. (2020) CRISPR–Cas12-based detection of SARS-CoV-2. Nature Biotechnology 1-5. Link: https://go.nature.com/3fT5GY3
- Viral RNA isolation for COVID-19 research. Link: https://bit.ly/2A18BhY
- Quick-DNA/RNA Viral MagBead Kit. Link: https://bit.ly/2A2K1gP
- What is the role of rapid antigen detection in the workup of viral pneumonia? Link: https://wb.md/3eyfczw
- Chartrand C, Tremblay N, Renaud C, Papenburg J (2015) Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: Systematic review and meta-analysis. Journal of Clinical Microbiology 53: 3738–3749. Link: https://bit.ly/2ZlQoob
- Immunoassays for Diagnostics. Link: https://bit.ly/3eAFCki
- Vashist SK (2020) In vitro diagnostic assays for COVID-19: Recent Advances and Emerging Trends. Diagnostics 10: 202. Link: https://bit.ly/2YuDHYI
- FDA notes limitations of COVID-19 antibody tests; OKs first at-home diagnostic test. Link: https://bit.ly/2CC0B81
- Advice on the use of point-of-care immunodiagnostic tests for COVID-19. Link: https://bit.ly/2Zb8ysF
- Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients. Link: https://bit.ly/31bxfYt
- Explained : How rapid antigen test detects Covid-19, where it will be used. Link: https://bit.ly/2NoJ19Q
- SARS-CoV-2 Total Ab. BioRad. Link: https://bit.ly/2VxoXGT
- Abbott Launches Covid-19 Antibody Test. Link: https://abbo.tt/37ZPFg4
- Cinquanta L, Fontana DE, Bizzaro N (2017) Chemiluminescent immunoassay technology: what does it change in autoantibody detection?. Auto- immunity highlights 8: 9. Link: https://bit.ly/386NJmc
- Immunoassay C, System C (2000) MAGLUMI TM 2000 Plus Outstanding Technology Power of MAGLUMI TM.
- Cai X, Chen J, Hu J, Long Q, Deng H, et al. (2020) A Peptide-based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Corona Virus Disease 2019 (COVID-19). medRxiv. Link: https://bit.ly/2VgEmv3
- Koczula KM, Gallotta A (2016) Lateral flow assays. Essays in biochemistry 60: 111–120. Link: https://bit.ly/3i4b38O
- COVID-19 test kits included in the ARTG for legal supply in Australia. Link: https://bit.ly/2Vbqli7 (accessed on : 21.06.2020)
- Jin Y, Yang H, Ji W, Wu W, Chen S, et al. (2020) Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12: 372. Link: https://bit.ly/2BG2TlX
- World’s First Quantitative Surrogate Viral Neutralization Test Evaluates COVID-19 Protective Immunity in US. Link: https://bit.ly/3i0oHcP
- Lucht A, Formenty P, Feldmann H, Götz M, Leroy E, et al. (2007) Development of an immunofiltration-based antigen-detection assay for rapid diagnosis of Ebola virus infection. J Infect Dis 196: S184- S192. Link: https://bit.ly/3dwpleE
- Turkish firm develops 5-minute COVID-19 test. Link: https://bit.ly/2BD85Y5
- Lippé R (2018) Flow virometry: a powerful tool to functionally characterize viruses. Journal of virology 92. Link: https://bit.ly/31eHaMJ
- Zamora JL, Aguilar HC (2018) Flow virometry as a tool to study viruses. Methods 134: 87-97. Link: https://bit.ly/31enw3z
- Quick, Effective: Indigenous Covid-19 Test Kits at One-fourth Cost to Cut India’s Dependence on China. Link: https://bit.ly/3fUEsjz
- Here's how Mylab developed cost-effective and time-saving COVID-19 test kits for India. Link: https://bit.ly/2NoKhK6
- Bustin SA, Nolan T (2004) Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech 15: 155-156. Link: https://bit.ly/2ZawmNd .
- Why ‘flattening the curve’ may be the world’s best bet to slow the coronavirus. Link: https://bit.ly/3evmiow
- Indian scientists develop low cost diagnostic test kit for COVID-19. Link: https://bit.ly/37WtgAq
- Covid-19: ICMR clears first batch of key ELISA antibody testing kits made in India. Link: https://bit.ly/2NrDQWL
- Hyderabad firm's Covid-19 test kit gives result in 2 hours. Link: https://bit.ly/2Yx3uzF
- Liu Z, Yao C, Wang Y, Zheng W (2018) Visual diagnostic of Helicobacter pylori based on a cascade amplification of PCR and G-quadruplex DNAzyme as a color label. J Microbiol Methods 146: 46-50. Link: https://bit.ly/31hXzQC
- Yan L, Zhou J, Zheng Y, Gamson AS, Roembke BT, et al. (2014) Isothermal amplified detection of DNA and RNA. Molecular BioSystems 10: 970-1003. Link: https://rsc.li/3g0PXWZ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
