Global Journal of Cancer Therapy
The effects of thymoquinone and cytozine arabinoside on apoptosis and cell proliferation in acute myeloide leukemia
Aslı Altun1, Nurten Kara1, Şengül Tural1*, Alişan Yıldıran2 and Leman Tomak3
2Child Health and Diseases, Allergic Immunology Faculty of Medicine, Ondokuz Mayıs University, Turkey
3Department of Biostatistics, Faculty of Medicine, Samsun, Ondokuz Mayıs University, Turkey
Cite this as
Altun A, Kara N, Tural S, Yıldıran A, Tomak L (2022) The effects of thymoquinone and cytozine arabinoside on apoptosis and cell proliferation in acute myeloide leukemia. Glob J Cancer Ther 8(1): 040-045. DOI: 10.17352/2581-5407.000047Copyright License
© 2022 Altun A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Purpose: The aim of this study was to investigate the effects of a chemotherapeutic agent Cytosine Arabinoside (Ara-C) and a natural anticancer agent of Thymoquinone (TQ) on apoptosis and cell proliferation of AML cell lines (Kasumi-6) both alone and in combined form.
Material and method: Kasumi-6 AML cells were treated with three different doses of Ara-C (0.1, 0.5 and 1 µmol) and TQ (25, 50 and 100 µM) for 48 and 72 hours incubations. After Annexin V and Propidium Iodide (PI) staining, apoptosis, viability, and cell proliferation were evaluated for each group in flow cytometry.
Results: As a result, AML cell lines showed a statistically significant difference in a single treatment of the active substances. Their combined treatment showed an increase in apoptosis and a decrease in viability in both groups at 48 and 72 hours incubation times (p < 0.001). In each group, it was observed that apoptosis was increased and viability was decreased and consequently cell proliferation was suppressed.
Conclusion: Ara-C was used for the first time in this study with TQ in AML. It was determined that the combined use of TQ and Ara-C did not have a synergistic effect on apoptosis.
Introduction
AML is a group of malignant diseases that occur with the uncontrolled and clonal proliferation of cells with increased proliferation rate and decreased apoptosis compared to normal cells and it occurs when these rapidly proliferating cells invade the bone marrow [1,2].
The main consideration for the treatment of AML is an effective combination of cytosine arabinoside (Ara-C) and chemotherapy. It’s known that the aim of chemotherapy is to restore the production of normal blood cells and to achieve complete remission in the patient. However, chemotherapy causes the death of normal healthy cells, and therefore, AML patients experience side effects such as nausea, weakness, and a high risk of infection [3]. The standard treatment is highly toxic and the development of resistance to the chemotherapeutic drugs used hinders the treatment. Therefore, the development of new therapeutic agents and protocols is needed to improve outcomes in AML patients. Ara-C used in AML is an effective apoptotic drug. Thymoquinone (TQ) is an extract of black cumin, a natural product with anti-carcinogenic effects. Its seeds can be used as a spice and in the treatment of many diseases in traditional medicine. It is reported that it can be used in alternative medicine together with chemotherapy in cancer patients [3,4]. TQ is a herbal anti-carcinogen with no side effects and has an important effect on cancer cells in terms of showing cytotoxic effects against tumor cells [5]. It has been reported to inhibit TNF-induced NF-kappa-B activation in human chronic myeloid leukemia cells through inhibition of TQ phosphorylation and nuclear translocation [6].
Our aim is to examine the effects of TQ and Ara-C on apoptosis and cell proliferation when administered alone or combined in AML cell lines (Kasumi-6).
Material and methods
AML cell line and reagents
Kasumi-6 (ATCC No: CRL-2775) myeloblast cells were used for AML-M2 cell culture. Ara-C and TQ 99% (Nigella sativa) were used as chemotherapeutic and apoptotic agents. Three different concentrations of Ara-C (0.1, 0.5 and 1 µmol) and TQ (25, 50 and 100 µM each) were applied to the cells taken into the original medium for 48 and 72 hours. After Annexin V and PI staining, apoptosis, viability, and cell proliferation were evaluated for each group in flow cytometry.
AML cell line and viability assay
AML-M2 cells were thawed in a 37 °C water bath. After dissolving, it was taken into a 15 ml falcon tube, and 1 ml of RPMI 1640 medium containing bovine serum (FBS) and antibiotics were added. It was centrifuged twice at 1200 rpm for 10 minutes. The supernatant was discarded and 1 milliliter (ml) of the antibiotic-free medium was added to the obtained pellet. Ten microliters (µl) of this suspension was taken and pipetted onto a thoma slide. Viability was determined by adding trypan blue at the same rate. Live and dead cell ratios were measured in the automated cell counter (Bio-Rad). As a result of the measurement, 22% vitality was detected. The number of viable cells of the ready-made AML cell line was measured as 1.02 × 106 cells/ml.
Cell culture
Due to the low number of cells, multiple passages were applied. AML cells were seeded into flasks in 4 ml RPMI 1640 medium and cultured. AML cells were checked under the microscope and their cell densities were examined. All flasks were collected in a single 50 ml falcon tube and washed in accordance with the previously applied wash procedure. Thirty-six ml of medium was added to the cells remaining at the bottom of the tube. Ten µl was taken from it and the measurement was made by an automated cell counter device for the determination of viability. As a result of standard cell culture, a total of 7 × 106 cells/ml was obtained.
The cells suspended in a nutrient medium were seeded into 12-well cell culture dishes at 2 × 105 cells per milliliter and left in the incubator for 24 hours without adding any substance. TQ (25, 50, 100 µM each) and Ara-C (0.1, 0.5, 1 µmol each) were added to all samples at the determined rates at the end of the 24th hour and DMSO was added to the control groups at the same rates. It was incubated again and at the end of the 48th hour, the cultures were taken into 5 ml falcon tubes and prepared for staining for apoptosis and viability determination by flow cytometric analysis. The same procedures were repeated for the 72-hour groups at the end of the 72nd hour.
Apoptosis measurement by flow cytometry
Cells are washed 2 times with cold PBS. It is suspended in 1 × Binding Buffer. 100 µl of the obtained suspension is transferred to culture tubes. 5 µl Annexin V and 5 µl PI pipetted and vortexed. And following incubated for 15 minutes in the room. Following 400 µl of 1 × Binding Buffer was added. It was read within 1 hour. With this staining technique, four different phenotypes are determined; viable cells (stained with annexin-V and PI), early apoptotic (stained only with annexin-V), late apoptotic (stained with annexin-V and PI) and dead/damaged cells (stained with PI only).
Statistical analysis
MINITAB Statistical Software 15.0 Two proportion test was applied. p < 0.05 was accepted as statistically significant.
Results
Cell culture and viability
The number of viable cells of the ready cell line was measured as 1.02 × 106 cells/ml. A total of 7 × 106 cells/ml were obtained as a result of the normal cell culture performed to multiply the cells before the anti-carcinogen application.
Flow cytometry results
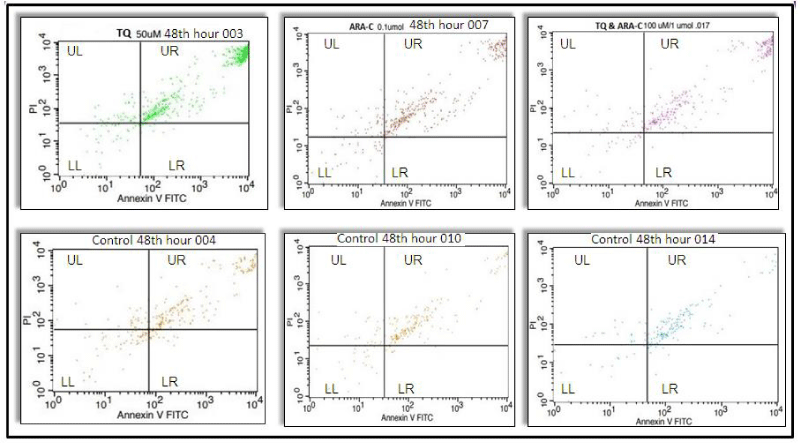
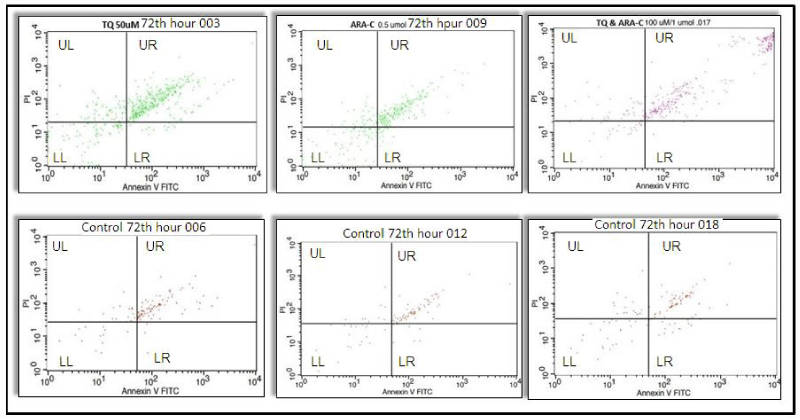
It was observed that the number of apoptotic cells at three different TQ concentrations (25, 50 and 100 µM each) increased significantly (p < 0.001) at the 48th hour, when the cells in the TQ-applied experimental group underwent the first mitosis (p < 0.001), and the viability decreased in the experimental groups compared to the controls (p < 0.001), (Figure 1, Table 1). In the 72nd hour incubation of this group, it was observed that the number of apoptosis increased (p < 0.001) and viability decreased (p < 0.001) when the experimental groups were compared with the controls (Figure 2, Table 1).
Apoptotic cell numbers increased significantly at three different Ara-C concentrations (0.1, 0.5, 1 µmol each) in the 48th hour, when the cells in the Ara-C applied experimental group underwent the first mitosis, compared to the controls (p < 0.001). It was observed that viability decreased in the experimental groups compared to the controls (p < 0.001), (Figure 1, Table 1). In the 72nd hour incubation of this group, it was observed that the number of apoptosis increased (p < 0.001) and viability decreased (p < 0.001) when the experimental groups were compared with the controls (Table 1).
It was observed that the number of apoptotic cells in the experimental group with three different concentrations, in which TQ and Ara-C were applied together, were significantly increased (p < 0.001) compared to the controls, at the 48th hour when they underwent the first mitosis, and the viability decreased in the study groups compared to the control groups (p < 0.001) (Table 1). It was observed that the number of apoptosis increased (p < 0.001) and the viability decreased when compared to the controls in the 72nd-hour incubation of this group (Table 1).
The increase in apoptosis and the decrease in viability, which we observed in all three groups, showed us that proliferation was suppressed. Apoptosis increased when the samples were compared with their control groups at 48 and 72-hour incubation periods when Ara-C (0.1, 0.5, 1 µmol) and TQ (25, 50, 100 µM each) were applied at three different concentrations. (p < 0.001) viability decreased (p < 0.001) (Figure 1). In addition, cell proliferation was suppressed. When the 48 and 72-hour incubation times and three different concentrations applied for the TQ, Ara-C, and TQ+Ara-C combined groups of AML cell lines were compared, it was observed that the number of apoptotic cells increased more and the viability decreased at the 48th hour compared to the 72nd hour (p < 0.001) (Table 1 and Figures 1,2).
Discussion
Different strategies are used in the treatment of AML. Apoptosis in cancer cells is carried out with the use of certain chemical drugs that are compulsorily administered [7]. Significant problems have been observed in patients during treatment with anti-carcinogenic chemical drugs. The anti-carcinogenic potential of natural products attracts the attention of clinicians and scientists [8]. The application of natural herbal products on humans is more advantageous than anti-carcinogenic drugs. It has been observed that phytochemicals used in cancer treatment today are a good alternative to synthetic drugs. In recent years, it has been found that TQ, an anti-carcinogenic and anti-mutagenic herbal product taken with diet, suppresses cell proliferation in studies [9]. The antitumor effect of TQ has been demonstrated in many studies covering lung [10] pancreatic [11] prostate [12] and colon [13] cancers. In our study, the effects of Ara-C and TQ, a natural herbal product, on apoptosis, viability, and cell proliferation in acute myeloid leukemia (Kasumi-6 cell line) cell lines were investigated. Uncontrolled growth and proliferation of cancer cells is an important feature in carcinogenesis and causes an increase in tumor size and problems in treatment. It has been shown that the expression and/or activity of regulators of cell cycle progression and cell proliferation can be inhibited by TQ [4-19].
In our study, three different doses of TQ (25, 50, 100 µM) and Ara-C (0.1, 0.5, 1 µmol each) were applied to AML (Kasumi-6) cell lines in 48 and 72 hours incubation periods. The effects of TQ and Ara-C on apoptosis and cell proliferation were investigated when used alone or together. When the three different concentrations of active ingredients were applied, apoptosis increased (p < 0.001), and viability decreased (p < 0.001) when the samples were compared with their control groups in 48 and 72-hour incubation periods (Table 1). In addition, cell proliferation was also suppressed. In some studies, different drug combinations were used together with TQ. The combination of TQ and doxorubicin has been shown to have a greater effect than the use of these agents individually [20]. A similar finding has been reported for combinations of TQ and 5-fluorouracil [21]. TQ and cisplatin [10]. In a study investigating the effect of TQ on prostate cancer cells, it was observed that TQ was effective on regulatory proteins that provide the transition from G1 to S phase and blocked cells that transitioned from G1 to S phase [12]. Similarly, the suppressive effect of TQ in the G1 phase of the cell cycle was observed in previous studies with cancer cell lines [22]. In studies with the anti-carcinogenic Ara-C used in the treatment of AML, it has been reported that Ara-C inhibits DNA polymerase and impairs DNA replication and repair, depending on the dose; in the samples, it is used. The incorporation of Ara-C into the DNA structure occurs only in the synthesis phase (S phase) of DNA synthesis [23-26]. In a study, TQ was investigated in Jurkat lymphoblastic cell line and showed that it has a synergistic effect in combination with DOX. This combination strategy can be an alternative way for more powerful anticancer effects [27]. Almajali, et al. examined the effects of TQ in HL60 leukemia cells and they reported that TQ significantly induced cycle arrest at G0-G1 phase [4]. Anti-apoptotic proteins (eg, Bcl-2 and survivin) have also been reported to regulate the release of cytochrome by mitochondria [28,29] also found that TQ had a significant apoptotic effect in the first 48 hours, unlike other incubation times, similar to our findings. TQ has been shown to be extremely unstable in aqueous solutions, with pronounced effects of both pH and light. Although we used 100% DMSO to dissolve TQ, it may not have shown its apoptotic effect for as much as 48 hours in the 72-hour incubation period due to the aqueous phase of the medium.
In this study, it was observed that apoptosis increased and viability decreased in AML cell lines induced by TQ and Ara-C. However, cell proliferation was decreased. The results of our study are consistent with other studies, for example, other studies such as human myeloblastic leukemia [14] human colon cancer [30] liver cancer [31] cell line. TQ has been shown to induce apoptosis in some cell lines. In our previous studies, we applied in vivo animal experimental study with antiepileptic drugs and we measured apoptosis and genotoxicity [32,33]. By novel studies and methodologies, the study results should be confirmed [34-36].
Conclusion
In conclusion, our findings showed that TQ can be used as an anti-carcinogenic phytochemical. However, it has been observed that it does not have an effective complementary effect when used with Ara-C. In further studies, observing the possible effects of anti-apoptotic and anti-carcinogenic treatment with TQ and Ara-C in AML cell lines in vitro in AML-produced experimental animals will provide the reliability and beneficial effect of such studies.
We thank OMU KITAM for cell counting support.
Funding
This research project was supported by Ondokuz Mayis University OMU BAP PYO.TIP.1904.15.001.
- Mezginejad F, Mohammadi MH, Khadem P, Farsani MA. Evaluation of LKB1 and Serine-Glycine Metabolism Pathway Genes (SHMT1 and GLDC) Expression in AML. Indian J Hematol Blood Transfus. 2021 Apr;37(2):249-255. doi: 10.1007/s12288-020-01329-1. Epub 2020 Aug 14. PMID: 33867731; PMCID: PMC8012466.
- Emamdoost F, Khanahmad H, Ganjalikhani-Hakemi M, Doosti A. The miR-125a-3p Inhibits TIM-3 Expression in AML Cell Line HL-60 In Vitro. Indian J Hematol Blood Transfus. 2017 Sep;33(3):342-347. doi: 10.1007/s12288-016-0733-4. Epub 2016 Oct 6. PMID: 28824235; PMCID: PMC5544632.
- Khader M, Eckl PM. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci. 2014 Dec;17(12):950-7. PMID: 25859298; PMCID: PMC4387230.
- Almajali B, Al-Jamal HAN, Wan Taib WR, Ismail I, Johan MF, Doolaanea AA, Ibrahim WN, Tajudin SA. Thymoquinone Suppresses Cell Proliferation and Enhances Apoptosis of HL60 Leukemia Cells through Re-Expression of JAK/STAT Negative Regulators. Asian Pac J Cancer Prev. 2021 Mar 1;22(3):879-885. doi: 10.31557/APJCP.2021.22.3.879. PMID: 33773553; PMCID: PMC8286695.
- Ivankovic S, Stojkovic R, Jukic M, Milos M, Milos M, Jurin M. The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp Oncol. 2006 Sep;28(3):220-4. PMID: 17080016.
- Sakalar C, Yuruk M, Kaya T, Aytekin M, Kuk S, Canatan H. Pronounced transcriptional regulation of apoptotic and TNF-NF-kappa-B signaling genes during the course of thymoquinone mediated apoptosis in HeLa cells. Mol Cell Biochem. 2013 Nov;383(1-2):243-51. doi: 10.1007/s11010-013-1772-x. Epub 2013 Aug 14. PMID: 23943306.
- Bauer J, Wekerle H, Lassmann H. Apoptosis in brain-specific autoimmune disease. Curr Opin Immunol. 1995 Dec;7(6):839-43. doi: 10.1016/0952-7915(95)80057-3. PMID: 8679129; PMCID: PMC7135830.
- Cakir Z, Saydam G, Sahin F, Baran Y. The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60: acute myeloid leukemia cells. J Cancer Res Clin Oncol. 2011 Feb;137(2):279-86. doi: 10.1007/s00432-010-0884-x. Epub 2010 Apr 18. PMID: 20401667.
- Rajput S, Kumar BN, Dey KK, Pal I, Parekh A, Mandal M. Molecular targeting of Akt by thymoquinone promotes G(1) arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013 Nov 13;93(21):783-90. doi: 10.1016/j.lfs.2013.09.009. Epub 2013 Sep 15. PMID: 24044882.
- Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010 Jul 1;29(1):87. doi: 10.1186/1756-9966-29-87. PMID: 20594324; PMCID: PMC2909169.
- Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009 Jul 1;69(13):5575-83. doi: 10.1158/0008-5472.CAN-08-4235. Epub 2009 Jun 23. Retraction in: Cancer Res. 2018 Sep 15;78(18):5468. PMID: 19549912.
- Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, Dou QP, Reddy GP. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007 Aug 15;67(16):7782-8. doi: 10.1158/0008-5472.CAN-07-1483. PMID: 17699783.
- Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B, Jurjus A, Roessner A, Schneider-Stock R. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008 Jan-Feb;12(1):330-42. doi: 10.1111/j.1582-4934.2007.00095.x. PMID: 18366456; PMCID: PMC3823493.
- El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005 Nov 10;117(3):409-17. doi: 10.1002/ijc.21205. PMID: 15906362.
- Richards LR, Jones P, Hughes J, Benghuzzi H, Tucci M. The physiological effect of conventional treatment with epigallocatechin-3-gallate, thymoquinone, and tannic acid on the LNCaP cell line. Biomed Sci Instrum. 2006;42:357-62. PMID: 16817634.
- Li QX, Yu DH, Liu G, Ke N, McKelvy J, Wong-Staal F. Selective anticancer strategies via intervention of the death pathways relevant to cell transformation. Cell Death Differ. 2008 Aug;15(8):1197-210. doi: 10.1038/cdd.2008.48. Epub 2008 Apr 25. PMID: 18437165.
- Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol. 2011 Apr;67(4):867-74. doi: 10.1007/s00280-010-1386-x. Epub 2010 Jun 26. PMID: 20582416.
- Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, Tan KH. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem Pharmacol. 2011 Sep 1;82(5):464-75. doi: 10.1016/j.bcp.2011.05.030. Epub 2011 Jun 14. PMID: 21679698.
- Baharetha HM, Nassar ZD, Aisha AF, Ahamed MB, Al-Suede FS, Abd Kadir MO, Ismail Z, Majid AM. Proapoptotic and antimetastatic properties of supercritical CO2 extract of Nigella sativa Linn. against breast cancer cells. J Med Food. 2013 Dec;16(12):1121-30. doi: 10.1089/jmf.2012.2624. PMID: 24328702; PMCID: PMC3868399.
- Woo CC, Hsu A, Kumar AP, Sethi G, Tan KH. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013 Oct 2;8(10):e75356. doi: 10.1371/journal.pone.0075356. PMID: 24098377; PMCID: PMC3788809.
- Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X, Dong W. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem Biophys Res Commun. 2012 Jan 13;417(2):864-8. doi: 10.1016/j.bbrc.2011.12.063. Epub 2011 Dec 20. PMID: 22206670.
- Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004 Apr;15(4):389-99. doi: 10.1097/00001813-200404000-00012. PMID: 15057144.
- Burke GA, Estlin EJ, Lowis SP. The role of pharmacokinetic and pharmacodynamic studies in the planning of protocols for the treatment of childhood cancer. Cancer Treat Rev. 1999 Feb;25(1):13-27. doi: 10.1053/CTRV.1998.0098. PMID: 10212587.
- Kufe D, Spriggs D, Egan EM, Munroe D. Relationships among Ara-CTP pools, formation of (Ara-C)DNA, and cytotoxicity of human leukemic cells. Blood. 1984 Jul;64(1):54-8. PMID: 6587917.
- Vaughan WP, Karp JE, Burke PJ. Two-cycle timed-sequential chemotherapy for adult acute nonlymphocytic leukemia. Blood. 1984 Nov;64(5):975-80. PMID: 6487807.
- Grant S. Ara-C: cellular and molecular pharmacology. Adv Cancer Res. 1998;72:197-233. doi: 10.1016/s0065-230x(08)60703-4. PMID: 9338077..
- Soltani A, Pourgheysari B, Shirzad H, Sourani Z. Antiproliferative and Apoptosis-Inducing Activities of Thymoquinone in Lymphoblastic Leukemia Cell Line. Indian J Hematol Blood Transfus. 2017 Dec;33(4):516-524. doi: 10.1007/s12288-016-0758-8. Epub 2016 Dec 8. PMID: 29075062; PMCID: PMC5640521.
- Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004 Mar 1;1644(2-3):229-49. doi: 10.1016/j.bbamcr.2003.08.009. PMID: 14996506.
- Yuan L, Wang J, Xiao H, Xiao C, Wang Y, Liu X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol. 2012 Nov 15;265(1):83-92. doi: 10.1016/j.taap.2012.09.022. Epub 2012 Sep 28. PMID: 23026832.
- El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010 Feb;15(2):183-95. doi: 10.1007/s10495-009-0421-z. PMID: 19882352.
- Rooney S, Ryan MF. Modes of action of alpha-hederin and thymoquinone, active constituents of Nigella sativa, against HEp-2 cancer cells. Anticancer Res. 2005 Nov-Dec;25(6B):4255-9. PMID: 16309225.
- Tural S, Tekcan A, Elbistan M, Karakuş N, Ozyurek H, Kara N. Genotoxic effects of prenatal exposure to levetiracetam during pregnancy on rat offsprings. In Vivo. 2015 Jan-Feb;29(1):77-81. PMID: 25600534.
- Tekcan A, Tural S, Elbistan M, Guvenc T, Ayas B, Kara N. Evaluation of apoptotic cell death on liver and kidney tissues following administration of levetiracetam during prenatal period. J Matern Fetal Neonatal Med. 2017 Feb;30(4):420-423. doi: 10.1080/14767058.2016.1174990. Epub 2016 Jun 3. PMID: 27255296.
- Niyaz L, Tural S, Eski Yucel O, Can E, Ariturk N, Celik ZB, Tekcan E, Kara N. Chromosomal microarray analysis of patients with Duane retraction syndrome. Int Ophthalmol. 2019 Sep;39(9):2057-2067. doi: 10.1007/s10792-018-1042-8. Epub 2018 Nov 26. PMID: 30478753.
- Michel M, Lucke-Wold N, Hosseini MR, Panther E, Reddy R, Lucke-Wold B. CNS Lymphoma: Clinical Pearls and Management Considerations. Biomed Res Clin Rev. 2022;7(2):121. Epub 2022 Jun 27. PMID: 35832688; PMCID: PMC9275513.
- Nwafor D, Radwan W, Lucke-Wold B, Underwood W, Gyure K, Marsh R. Follicular lymphoma presenting as scalp mass deformity: Case Report and Review of the literature. Biomed Res Clin Pract. 2018;3(1):10.15761/BRCP.1000155. doi: 10.15761/BRCP.1000155. Epub 2018 Feb 24. PMID: 30057944; PMCID: PMC6059655.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
