Global Journal of Cancer Therapy
Surgery, Chemotherapy and Radiotherapy May Promote Cancer Growth Speeds and Shorten Patient Lives
Jianqing Wu1* and Ping Zha2
2Independent Researcher, USA
Cite this as
Kazoba F, John J (2021) Surgery, Chemotherapy and Radiotherapy May Promote Cancer Growth Speeds and Shorten Patient Lives. Glob J Cancer Ther 7(1): 046-049. DOI: 10.17352/2581-5407.000043Copyright License
© 2022 Kazoba F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Medicine fails to find predictable cures for cancer in more than a century, and we explored the feasibility of controlling cancer growth speed by using lifestyle factors. After conducting an extensive literature review, we conducted simulations for cancer growth courses to see the feasibility of controlling cancer growth speeds.
We found that (1) medical treatments are often accompanied by three to four lethal factors: treatment side-effects, emotional distress, and chronic stress, reduced exercises and physical inactivity, and excessive nutrition in some cases; (2) clinical trial exaggerates treatments short-term benefits and underestimates the slow-delivering adverse side effects as a result of statistical averaging, interfering effects of personal lifestyle factors and insufficient follow-up times; (3) the benefits of medical treatments are limited by chain comparisons, where surgery may work as a negative standard relative to the best alternatives for resolving cancer; (4) the strategy of destroying the tumor or killing all cancer cells is unworkable; (5) medical treatments can turn natural cancer growth curve into approximately doubly exponential curve; (6) multiple-factor non-medical measures are potentially much more powerful than medical treatments in controlling cancer growth and metastasis speeds; and (7) cancer early diagnosis and over treatments are unwise strategies in light of discoveries. Based on huge increases in cancer growth rate constants, substantial loss of vital organ functional capacity, and severe systemic aging-like cellular damages, we concluded that medical treatments may promote cancer growth and metastasis speeds and shorten patient lives in most situations, and the claimed benefits are caused by triple biases of clinical trials. By using the same method to explore how several lifestyle factors affect cancer growth rates, we concluded that the better strategy for ending the global cancer epidemic in the future is changing caner treatment strategy from killing cancer cells to slowing down cancer growth rates by using various lifestyle factors in combination. This study in part explains why cancer can self-resolve.
Introduction
President Nixon declared a war on cancer in 1971 with his signing of The National Cancer Act. Half a century later, no cure has been found. We have heard time and again about “ground-breaking cancer research.” One thing that has never changed is the approach used in cancer research and the cancer treatment model. A recent meta-review shows that the complete response rates for the remission of later-stage cancer are around 7.4% [1]. The complete response does not preclude cancer from returning, implying the actual performance could be worse. Chemotherapy has severe drug side effects and causes cancer relapses at much faster speeds. A systematic review of thyroid cancer treatment performance found that the response rate was 22.1% to 27.1%, with complete response rates being 2.5% to 3.4% [2]. A retrospective cohort study conducted a systematic evaluation of cancer approvals by the European Medicines Agency in 2009-13 and found that most drugs entered the market without evidence of benefits on survival or quality of life [3]. At a minimum of 3.3 years after market entry, there was still no conclusive evidence that these drugs either extended or improved life for most cancer patients. This is similar to another finding: “The overall contribution of curative and adjuvant cytotoxic chemotherapy to 5-year survival in adults was estimated to be 2.3% in Australia and 2.1% in the USA [4].
Cancer researchers started seeking other kinds of drugs and target drugs around 1980. Beta-blockers, originally antihypertensive drugs, was thought to block cancer growth, as a new alternative for cancer adjuvant chemotherapy. Half or more of people who start taking a beta-blocker for controlling high blood pressure stop within a year [5], presumably due to well-known side effects such as fatigue and shortness of breath. The latest meta-study of 36 studies involving 319,006 cancer patients shows that beta-blockers have nearly no benefits on cancer outcomes [6]. Another meta-review similarly found dubious or marginal benefits and small negative impacts, depending on cancer types [7]. A meta-review on the effects of angiogenesis blockade for the treatment of gastric cancer shows mixed benefits [8]. Small benefits are found for only certain types of cancers and certain types of patients.
Another meta-review also found that such drugs do not extend overall survival for biliary tract cancer [9]. The use of target therapy with radiotherapy compared to standard therapy increased the chance of severe adverse events while yielding comparable survival in glioblastoma multiforme patients [10]. The addition of targeted drugs to chemotherapy (TEM + RAD) did not improve the overall survival of patients with glioblastoma multiforme; however, it had some effect of stopping cancer progression for patients treated by cilengitide and the rate of adverse effects was higher in the experimental group than in the placebo group [11].
The general picture is that a vast number of patients do not fully respond to cancer drugs; none of the chemotherapy, adjuvant drugs, and target drugs can predictably cure cancer; and no drugs can reduce the risk of cancer returns. The situation of cancer pandemic is stated in one review article: “The claimed ‘targeted’ therapies that may or may not extend remission of cancer for a few months should not be accepted any longer as ‘cure’ by oncologists, scientist or patients….” and designer drugs cost between $100,000–$1000,000 [12]. Numerous surveys show that few doctors would consider using radiotherapy on themselves because it can cause new cancer, and that 75% of doctors would not consider using chemotherapy on themselves (N1, Sup.). Those little benefits are under challenge here.
Methods
The author assumes that «killing cancer cells» is a wrong strategy, and tried to evaluate treatment effects by using cancer growth rate constant -- daily cell net gain from the balance between cell dividing rate and cell death rate. We assume that cancer cannot be cured by killing all cancer cells, but is presumably cured by decreasing cancer cell daily net gain to negative. Therefore, we wanted to develop a methodology for comparing medical treatments with non-medical measures.
We systematically evaluated the performance of medical treatments from many angles such as treatment history, cancer theories, treatment performance data, medical models, recent performance studies, and meta-reviews. We will evaluate treatment benefits by focusing on how they affect cancer growth rates.
To analyze the adverse impacts of medical treatments, we extracted several important factors such as systemic inflammation, tissue loss, cell damage, chronic stress, physical inactivity, exercises, exessive nutrition, etc. from each of the medical treatments. From cancer research literature, we extracted data that show how each of such factors affects cancer growth rates. We then analyzed how medical treatments affect those factors singularly or in combination and how those factors may collectively affect cancer growth rate.
To determine how lifestyle factors affect cancer growth rates, we reviewed the findings from a large number of studies and estimated the effects of non-kinetic data on cancer growth rates. The non-kinetic data we used include incidence data, hazard ratios, survival times, etc. We assume safely that all non-kinetic data reflect cancer’s inherent development rate and thus can be used to estimate cancer growth rate. In estimating the impacts of cancer growth rates, we will show why clinical trial outcomes are biased and how to estimate their true effects.
Results
A. Medical treatments were guided by obsolete cancer theories and were never compared with non-medical measures
One flaw in medical treatment development is revealed in Figure 1. The figure shows most medical treatments are developed before all key influences factors were understood. Figure one shows the times for various cancer theories (from pre-1800 to 2020), the start times for increasing uses of surgeries (1846), the start time for using radiotherapy (about 1900) and the start time for using chemotherapy (1946), the start time of discovering cancer cause-related factors and influencing factors (mainly after 1980), and the start time for discovering exercises effects (mostly after 2000). This table shows that chemotherapy and the use of surgeries to remove cancer have been accepted as cancer treatment standards long before all key influence factors were known. The key influence factors, which include risk factors, causal factors, and influencing factors, fall within six large classes: the side-effects of medical treatments, emotional distress and chronic stress, exercises, and inactivity, diet and nutrition, cancer-fighting natural compounds, and other lifestyle factors. Those factors are shown in the top box in Figure 1.
The cancer theory history reflects how cancer treatments were developed. It was once believed that cancer is caused by a milk clot in a mammary duct, acidic lymph fluid, cancer poison, hormone, chronic irritation, infections, tobacco snuff, etc. [13]. Some theories include humoral theory (Hippocrates’s belief), lymph theory (Stahl and Hoffman), blastema theory (Johannes Muller, 1838), chronic irritation theory (Virchow), trauma theory (widely accepted belief from the late 1800s until the 1920s), infectious disease theory (Zacutus Lusitani, 1575-1642, and Nicholas Tulp, 1593-1674) [14]. All old cancer theories are clearly wrong or inaccurate for most types of cancer but are presumed to have influenced the developments of cancer treatments.
Most influential cancer theories include Somatic Mutation Theory (SMT) [15], somatic evolution theory [16] and revolutionary cancer theory [17]. None of the modern cancer theories can explain all causal factors, risk factors, and influencing factors. The SMT theory cannot explain the most striking fact that most mutations take place at birth and new mutations are added at a similar pace each year, cancer incidences strike mainly people above 60. It does not explain the roles of emotion, personal lifestyles, and personal habits.
In the last half a century, cancer research slowly discovered that cancer is accompanied by changes in a large number of biochemical and cellular processes. Some of such changes are well reflected in The Hallmarks of Cancer by Hanahan & Weinberg [18]. Cancer is considered to be also caused by the mismatch between modern lifestyles and what human genes were adapted to [19]. Inferring from known causal factors, risk factors, and influencing factors, cancer is a result of changed biochemical and cellular processes associated with misfitted lifestyles. Changed biochemical and cellular process patterns further imply that cancer cannot be cured by cutting off the detected tumor or killing all cancer cells. Thus, surgery, chemotherapy, and radiotherapy developed by relying on old and obsolete cancer theories are deemed to fail in most cancer. This is probably why the current treatments could not reliably cure cancer.
The unsettled performance of cancer surgery can be explained by examining its development history. The “benefits” of surgery for “curative” treatment of breast cancer were “recognized” by the Greek physician Galen of Pergamum (130–200 A.D.) and Scottish surgeon John Hunter (1728-1793). A century later, matured anesthesia art (e.g. diethyl ether in 1846) promoted its use. It later became standard treatment. This standard gained wide acceptance long before any remotely right cancer theory had been developed. Its use in treating rectal cancer was prompted by anesthesiological techniques. In 1908, William Ernest Miles introduced the basis of modern rectal cancer surgery with improved surgical options [20]. Thus, the rationale of using surgery is based on an unproved or most probably wrong notion that a tumor can be cut off and killed. It is like an attempt to change biochemical and cellular processes by cutting reactant media. An obvious reason for its continued use is that surgery can reduce the cancer burden and patients can survive for several months to several years. This perceived benefit would have been obvious in ancient times when cancer patients were not enabled to fight cancer using a large number of influence factors. All that cancer patients could do were taking more rest, eat better, and do less, all of which would accelerate cancer growth.
Chemotherapy started gaining acceptance around 1946 when Gustaf Lindskog’s study on non–Hodgkin’s lymphoma was published. It had been heavily influenced by old cancer theories on infection. The “chemotherapy” was a term used for treating infectious diseases in the early 1900s. Penicillin was initially thought to have anti-tumor properties. Actinomycin D, an antibiotic, was considered to have significant anti-tumor properties and enjoyed considerable use in pediatric tumors in the 1950s and 1960s. Medicine has slowly developed clinical trials as a standard for evaluating the effectiveness of drugs [21,22]. A key requirement for clinical trials is that human subjects are randomly assigned to a control group or a treatment group. However, a large number of factors relating to lifestyles cannot be controlled. When clinical trials are used as the standard, medicine essentially excludes as cure anything that cannot be controlled and anything that requires patients’ active involvement. What is excluded include mind regulation (changing emotional state, reducing stress, avoiding fears, changing faith, being happy, etc.), changing lifestyles, getting rid of bad habits, using special diets, doing exercise, raising body temperature, altering body mechanical properties, etc. Moreover, the use of placebos in cancer trials is not feasible because cancer can cause deaths, and treatments for the control are thus selected by using best-available-therapy [23]. The generally accepted strategy is to kill all cancer cells. Since it is assumed that all non-medical measures such as emotion, diet, exercises, etc cannot kill cancer cells, they cannot be used as treatments in clinical trials.
Accepting clinical trials and the best-available-therapy as controls essentially narrows cancer treatment options to only things that can be swallowed without distinctive tastes and anything that does not grab the attention of the human subjects. By using the narrow comparison options, the best treatment candidates are naturally synthetic drugs, radiation, and things that can be wrapped in small sizes for easy administration.
The unwarranted trust in clinical trials naturally leads to this current drug-evaluating practice: the benefits of each drug are determined by comparing the drug with surgery or a previously approved drug, and the newest drug is compared with a previously approved drug. This practice can be seen in any clinical trial [24-27]. Most design of clinical trials can be found in the online database www.clinictrial.gov. The treatments in both control and treatment arms include surgery and one or more drugs. A common randomized clinical trial is to compare a new drug with the old drug on patients who have some type of cancer. The FDA approved panitumumab for extending mean time to disease progression or death by 36 days over the best available drugs (fluoropyrimidine, oxaliplatin and irinotecan). To save resources, the control arm can be shared among different clinical trials [28].
All cancer treatment studies focus on the treatment’s ability to remove or kill cancer cells. They reflect an unspoken presumption that all risk factors and influence factors can cause cancer or affect cancer growth speeds, but cannot be used to cure cancer. Even after tens of thousands of studies have been published to show the effects of a large number of lifestyle and environmental factors on cancer, they are recognized as measures for preventing cancer but not curing cancer. This presumption has frozen medical researchers’ mindset to selecting options from a small number of choices.
Surgery has escaped from being validated in the entire medical history. Since surgery has been used as the standard treatment, the true benefits of surgeries are unknown. Before 1980, most cancer-causing factors, risk factors, and influencing factors were largely unknown. The roles of lifestyles, life habits, hundreds of factors such as omega 3, vitamins, antioxidants, free radicals, apoptosis-inducing compounds, exercise, emotional distress, and chronic disease, etc. were unknown. Thus, the true benefits of surgeries could not be assessed against all non-medical measures that affect cancer growth. From 1980 to the present, more knowledge of cancer has been found, but medical researchers could not overcome the presumption that treatment must have sufficient power to destroy the tumor. With that presumption, ethical consideration further prevents anyone from using any unapproved non-medical measures as cancer treatments. Thus, potentially tens of thousands of non-medical measures are automatically precluded as potential cures. Medicine has not evaluated surgeries’ absolute performance against everything under the Sun.
Surgeries are often used as standard treatments [29] unless they would pose imminent risks in some patients. When surgeries were used as controls for chemotherapy and radiotherapy, the determined performance of chemotherapy and radiotherapy is relative to that of surgeries. All drugs and other treatments are evaluated by comparing them against surgeries directly or indirectly. If surgeries have large negative benefits over best references, the “determined” performance of drugs or other treatments can be still on the negative side. If surgeries do shorten lives dramatically, the drugs or other treatments could have similar effects. Surgeries may set up upper limits on the patient life. Whatever benefits of chemotherapy and radiotherapy exhibit in clinical trials may be only some improvements over life-shortening surgeries.
B. Four big lethal factors associated with medical treatments
Cancer treatments are often associated with four lethal factors: their side effects, emotional distress and chronic stress, lack of exercise and physical inactivity, and excessive nutrition which is often seen in cancer care. Radical or invasive medical treatments exert adverse effects by causing systemic damages and tissue loss and raising systemic inflammation. Emotional factors exert their adverse impacts by shocks, emotional distress, chronic stress, anger, etc. Long-term physical inactivity exerts its adverse impacts by speeding up aging-like health deterioration. Excessive nutrition improves the nutritional supply to cancer cells.
1. Kinetic methods for characterizing cancer growth rates: The purpose of this study is to establish a method for evaluating every potentially relevant factor on cancer growth rates. It is necessary to use a kinetic method to characterize cancer growth. Tumors often exhibit Gompertzian growth, but their growth rates depend on cell numbers. Thus, the first order law must be the main characteristic of kinetics [30-32]. Cell divisions among all cells are initially synchronized, once the clock control is off, their division timings will become out of phase after several division cycles, and the fractions in each phase of the cell cycle reach a steady state. After that, cells divide asynchronously with a different number of cells dividing at different times.
For convenience, daily gain or loss of cancer cells can be evaluated by cell cycles (or every 24 hours). The exponential growth curve of solid tumors will level off due to biological resource limits. The fraction of cancer cells that are dividing varies from day to day. The net growth rate constant (1/day) is equivalent to a fraction of cancer cells in the tumor that completes cell division each day and will be referred to as an apparent rate constant. In fighting cancer, what is important is the daily cell changes (the differential equation). The integrated equation can be used to only show the overall cancer cell number changing pattern.
2. Surgery raises cancer growth rates: Until Halsted (1908), the consensus was clearly that, unless forced by the circumstances, surgical resection should be avoided for disease much more advanced than very early-stage tumors (the cacoethes of Celsus) [33].
The obvious evidence against the use of surgery is that cancer is not a single tumor. Cancer may often come with different tumors of different sizes, with different detection times. After a primary tumor is removed or destroyed, the body does not stop any cancer cells or even normal cells from growing into new tumors. Cancer is not just one tumor in one site but may be followed by various tumors in pipe line. Surgeries with or without drugs and radiotherapy cannot stop micro- tumors in the pipeline.
Surgery often removes tumors with large tissue margins or removes a significant part of an organ. It reduces organ functional capacity. Organ reserve correlates with the outcomes of surgical treatments or chemotherapy as implied by a treatment-accelerated aging process [34]. It can safely be assumed that death occurs when a vital organ’s functional capacity is reduced to below a threshold of death. When cancer burden progressively reduces a vital organ’s functional capacity, further reduction of the organ functional capacity by removing margin tissues naturally invites an earlier death. Surgeries also exert adverse impacts by creating emotional distress. Patients may be disabled physically, lose dignity, and suffer emotional pain from abandoning their life plans and hopes.
Physical and emotional impacts in various degrees have not been used to appraise surgeries’ performance relative to best references performance.
Since patients do not immediately die, it creates an impression that a radical operation can extend life. No attempt has been made to understand how surgery affects patients’ lives over best reference lifespans that patients would live if their cancers naturally resolve OR are held in check by using non- medical measures. This is a question that cannot be answered by experiment.
We show that the notion that “tumor must be cut off with all cancer cells killed” is like an attempt to halt somatic cell revolution and the human aging process. To a reasonable person, killing all cancer cells is impossible. This incorrect presumption forces medical professionals to accept only invasive and harmful treatment methods and reject everything else. No valid evidence exists to show that approved medical treatments must be better than tens of thousands of other non-medical measures. No existing evidence can show that non-medical measures cannot safely slow down cancer growth rates. There is no basis to support a finding that medical treatments are best, can extend lives, or improve life quality.
Recent studies started to cast serious doubt on surgeries. One adverse effect of surgery is that it raises cancer growth rates of return cancer. Although some cancers recur many years after tumor surgical removal, a substantial fraction of patients develop overt metastases relatively soon after the removal of their primary tumors [35-37]. A prior surgery dramatically alters the body’s ability to resist future cancer [35,36,38,39]. Surgically operated patients experience a sharp rise in the risk of distant recurrence that begins 6 months after surgery and peaks between 12 and 18 months.
A recent study by Krall, et al. [38] provides conclusive evidence that surgical tumor removal triggers the outgrowth of otherwise-dormant metastases, leading to the synchronous pattern of relapse. The tumor incidence rate and tumor size are related to the severity of the wound. The study further found that the systemic wound-healing response triggers tumor outgrowth at distance sites. The study pinpoints the wound of surgery as at least one cause of faster cancer returns and cancer metastasis. This is consistent with the finding that inflammation promotes invasion and metastasis [40]. This finding also supports the fact that surgery can paradoxically augment the development of metastases [41].
Cancer growth time from tumor initiation to the time that a tumor is or could be detected is an important factor to be considered. For many types of cancers, cancer growth rates start picking up at about 50 to 55. The incidence rate of cancer at age is proportional to probabilities of occurrence of each mutation per unit time and the sixth power of the age [42]. Most patients are diagnosed at ages after menopause [27,29] while dormant cancer was frequently found from 80 to 85. The total growth times for most types of cancer is about 5 to 25 years while some types of cancer could take 50 to 70 years to reach a detectable size. A median growth time is about 15 years though it entirely depends on personal lifestyle. One surgical operation will shorten the next tumor’s growth time to one and half a year. This implies that surgery raises cancer’s apparent growth rate constant by as much as ten folds.
For a tumor of an initial size to reach a detectable size, the product of the rate constant k and time t is fixed. When k is raised by 10 times, the growth time for achieving the same final tumor size will be reduced to one-tenth. The rise in the growth rate constant by one order of magnitude is a game-ending adverse effect for cancer patients.
3. Chemotherapy and radiotherapy promote cancer growth rates: Chemotherapy and radiotherapy have been known for raising cancer growth rates for decades. One well-known old puzzle is the rapid return of cancer after the administration of chemotherapy and radiotherapy. Rapid regrowth of cutaneous or pulmonary metastases has been observed [30,31] and in non-small cell lung cancer [43]. The change is characterized by a much shorter doubling time (“DT”) which is the time required to double cancer cells. In 31 human metastases in which it was measured, the value of this ratio ranged from 2.5 to 5. Since DT*K=Ln(2), the reductions of DT are equivalent to 2.5 to 5 times increase in the apparent rate constant. Similarly, untreated and unresponsive patients had a growth fraction of less than 4% for myeloma, but relapsing patients had growth fraction ranging from 14% to 83% [44]. Growth fraction is closely related to DT, it is estimated that the rate constant increases by about 3.5 to 20.75 times.
Observed cancer growth rate depends on existing cancer cell numbers. This is true even if a large number of other factors such as geometry, nutrition, daily food intake, daily physical activity, etc. affect cancer dividing rates. Assuming that a cancerous aggregate of 100 cells becomes a detectable tumor of 1 billion cells in 10 years, it would be equivalent to a daily net addition rate of 0.004416 (1/day). This is equivalent to a kinetic process were about 4.4 new cells per 1000 cells in the tumor. The times for 100 cells to reach 1 billion cells under various rate constants are shown in Table 1.
If the rate constant arises, the final cancer cell number from an initial number in a given time will be increased by a multiplier M. This multiplier M can be estimated by M=Exp((n-1)*kt), assuming that the tumor grows in the same pace (N2, Sup.). For example, by raising the rate constant by 2.5 times, a returned or a secondary tumor could generate 1413 times more final cancer cells within the same 3 years (N3, Sup.). This is why returned cancer is often terminal if no measures can stop cancer from growing. While cancer division rate can vary from day to day and true rate constants fluctuate from day to day, its daily values are critically important.
The final cancer cell numbers depend on cell dividing cycles and rate constants. An increased apparent rate constant or reduced doubling time can lead to much larger final cell numbers. The tumor will become much larger with each day passing. This problem should be viewed in light of another problem that multiple tumors may erupt in various organs or tissues at dramatically increased rates (even though they are not detected). Due to differences in tissue ecosystem, one year difference in detection time is natural. The adverse effects of increased rate constants lie in compounding effects. It is like multiple mortgage loans compounded at variable daily interest rates. A slight rise in the daily rates for a loan may bankrupt the debtor because the increased loan balance can affect each of thousands of subsequent compounding cycles.
Most treatment protocols of chemotherapy cannot kill all cancer cells by batch applications; the half-lives of a super majority of cancer drugs are short [N4, Sup.]. We estimate that they lose 90% concentrations in just 1 to 3 days. In each hiatus between two administrations, cancer cells could generate new cells even though the new cells cannot be accurately detected.
The scope of side effects of cancer drugs was underestimated. If the drug causes any symptoms in any part of the body, a proper presumption should be that the drug affects reach the body because the same drug is circulated in every part of the body. However, some parts of the body can tolerate the drug side effects better and thus need more time for damages to show up. If the drug is slowly diminishing an organ’s functional capacity, its side effects will not be felt until the person’s health has deteriorated to a point that the organ’s functional capacity is insufficient to support life. The scope of adverse effects is reflected in cancer survivors’ aging-like cellular damages and lost lifespans [34,45-47].
Our findings refute findings that chemotherapy and radiotherapy have a few percents contribution to the 5 years survivals. Cancer treatments were driven by the presumption of “killing cancer cells.” That strategy is obsolete. All prior studies are based on chain comparisons using surgery as a starting reference. If surgery shortens patient lives by various big margins, a few percents improvements over such a bad control as determined by 5 years survival rate cannot turn their net effects to the positive sides. Clinical trials are unfit for studying slow-delivering side effects, and statistical analysis of clinical data is meaningless when controls are improper. After those flaws are corrected, we predict that the true effects of chemotherapy and radiotherapy are negative relative to the best references.
4. Adverse emotional factors promote cancer growth rates: Emotional distress, chronic stress, and other emotional factors speed up cancer initiation, growth, and spread [48-52]. The evidence, taken as a whole, is conclusive. Adverse emotional factors also dramatically speed up cancer metastasis.
The study of Sloan, et al. Sheds light on the magnitude of effects of chronic stress on cancer growth and metastasis [49]. It was found that chronic stress applied to mice for 20 days increased the metastasis of the primary breast tumor cells to distant tissues by 38-fold versus controls. The rate constant was raised by 0.182 (1/day) (N5, Sup.), which is equivalent to the doubling time of 3.81 days (t½ = 0.693/k). Even assuming that the apparent growth constant k for the control is zero (e.g., the dormant state), this rate constant would drive cancer growth at the speed equivalent to a growth speed for 100 cancer cells to reach 1 billion in about 89 days (23 doubling cycles). While the mice model in the study cannot be directly applied to humans and the kinetic model provides only a ballpark estimate, this finding supports the point that stress can dramatically raise metastasis rates. We personally heard stories where a shock and extreme fears can inflict extreme emotional pain.
5. Physical inactivity promotes cancer growth rates: Physical inactivity is an important cause of a large number of chronic diseases [53-55]. They found: “The comprehensive evidence herein clearly establishes that lack of physical activity affects almost every cell, organ, and system in the body causing sedentary dysfunction and accelerated death.” Some cited studies show that inactivity can produce adverse impacts in as short as 3 days. Although this study does not concern cancer, the finding applies to cancer because exercise can reduce inflammation which is a central promoting factor for cancer. By making an inference, exercises can have large beneficial effects.
The magnitude of adverse impacts of lack of exercise on cancer outcomes cannot be found from cancer literature, but the beneficial impacts of exercises are well documented. Exercise is found to be an important adjunct therapy in the management of cancer [55]. In this review, a total of 100 studies were reviewed involving thousands of individual patients whose exercise behavior was assessed following the diagnosis of cancer. They concluded: “[s] pecifically, superior levels of exercise following a cancer diagnosis were associated with a 28%–44% reduced risk of cancer-specific mortality, a 21%–35% lower risk of cancer recurrence, and a 25%–48% decreased risk of all-cause mortality.” The role of exercise in reducing cancer return is outstanding.
Exercises, like any other lifestyle factors, work by altering cell division daily. They work not by killing cancer cells like medical treatments.
Naturally, they could not deliver instantaneous beneficial effects of removing the tumor, but deliver beneficial effects by influencing cell compounding speeds on a long-term basis. Each new cancer cell reduced in an early day is equivalent to killing a seed which would compound for thousands of cycles like a home mortgage loan. Thus, the benefits of exercises cannot be detected in most randomized clinical trials, but their accumulated effects are substantial. The magnitude of benefits and the scope of effects are conclusively established by a large number of studies [56-67].
Exercise affects cancer outcomes by slowing down tumor growth and stopping cancer metastasis. It reduces systemic inflammation and mitigates chronic stress, both of which are known to speed up cancer metastasis speeds. Many exercise parameters relevant to its performance were not explored in cited studies.
6. Sweet food and poor nutrition promotes cancer growth rates: Most cancer patients lose weight as a result of cancer’s natural effects.
This leads to a belief that better nutrition is necessary. Overnutrition is often seen among patients in the early stages of cancer. Since most cancer patients die while they are progressively losing weight, it is counter-intuitive to advise nutritional restriction in cancer care. Cancer cells are in an unfavorable condition to compete for nutrition because more of them need nutrition for uncontrolled cell proliferation. Cancer cells cannot grow to become more than 1-2 mm in diameter if blood vessels are not generated [68]. Obesity, junk foods (including concentrated sugars and refined flour products that impair glucose metabolism), low fiber intake, consumption of red meat, and imbalance of omega 3 and omega 6 fats all contribute to increased cancer risk. Proper diets would result in at least a 60-70 percent decrease in breast, colorectal, and prostate cancers, and even a 40-50 percent decrease in lung cancer, along with similar reductions in cancers at other sites [69]. Diets affect cancer outcomes by altering cancer growth speeds.
7. Medical treatments combination accelerates cancer growth and shortens patient lives: The four lethal factors are often associated with or aggravated by cancer treatments. When those lethal factors are combined, their total adverse impacts are expected to be extremely large.
Figure 2 shows how all medical treatments exert instantaneous impacts and long-term impacts. Surgery is extremely powerful in removing the tumor as shown in (A) in Figure 2, which shows the total burden of cancer. Cancer drugs lose their effectiveness in killing cancer cells by developing drug resistance by many mechanisms [70]. While the efficacy of killing cancer cells rapidly decreases with time, severe adverse effects are accumulated with time. Figure 2 (B) shows the damages to tissue caused by drugs. Surgery reduces organ functional capacity by removing margins tissues and organ tissues and raises systemic inflammation, and chemotherapy and radiotherapy increase the degree of damage to body cells and organ tissues with time (B). Emotional distress and chronic stress further promote cancer growth and metastasis. Lack of exercise also encourages systemic inflammation like an adverse effect. Excessive nutrition may be an additional lethal factor for some cancer patients. When all of those lethal factors work on the same patient, the tissue’s ability to resist cancer cell division is progressively reduced so that cancer daily dividing rates progressively rise with time (C). Figure 2 (C) shows that the apparent rate constant increases with time due to increased damages to organs and tissues. As a result of those impacts, medical treatments speed up cancer growth rates, cancer return rates, and metastasis rates. Surgery dramatically raises cancer growth rates by raising systemic inflammation and diminishing organ functional capacity; and chemotherapy and radiotherapy raise 2.5 to 5.0 times of original rate constants.
The adverse effects of cancer treatments could not be fully revealed in clinical trials. The side effects of cancer treatments result in cellular damage to the body. The cellular damages to vital organs influence cancer outcomes by affecting cancer cell division cycles daily, and their adverse effects are expected to have enhanced uprising exponential characteristics. The degree of cellular damage caused by drug side effects is expected to increase with time, and the tissue’s ability to resist or inhibit cancer cell division is presumed to deteriorate with time. This progressive diminishing tissue health favors more cancer cells to divide in each of the cell cycles in the patient’s lifetime.
An extra number of cancer cells on any day over a natural baseline will undergo cell division in each of later cell cycles with increasing higher chances in the future. If the patient has N cell cycles, it has N series of extra cells gains, compared with the natural reference without side effects. The total number of cancer cells which are from all those series is expected to be very large. Each series of extra cells divide at increasing rate constants as shown in (C). Even if cancer cells divide at a constant rate constant, the cancer cell growth cure exhibits exponential characteristics. However, the rate constants have uprising characteristics like slow exponential curve due to cell damage by the toxic drugs, the cancer cell growth curve exhibits nearly doubly exponential characteristics with increased uprising degree. To determine the full side effect, a clinical trial must be sufficiently long and all interfering factors must be controlled. The fast rate caused by progressively delivered side effects will nullify the benefits from the strong effects of killing cancer cells in the early times.
The effects of the side effects of medical treatments on cancer growth rates can be established by examining the role of aging. It has been established that the cancer incidence rate is proportional to the sixth power of the age [42]. This high incidence rate implies that natural aging is responsible for greatly accelerated cancer growth rates as a whole. Cancer treatments can collectively speed up a range of aging-liking changes, which include genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, chronic low-grade inflammation, and cellular senescence [45]. Aging-like cellular damages can be found in all organs and all body cells in cancer patients.
By combining aging data and kinetic characteristics, we have to find that the accelerated cellular aging in cancer patients is mainly responsible for observed rapidly reduced growth times in later stages. A normal median 15 years growth time is shortened to one-and-half a year for a second or returned cancer, and further shortened to several months for third cancer. The combined adverse impacts of all lethal factors are also reflected by a change in cancer growth rate constants by one to more orders of magnitude.
Medical treatments are driven by “killing cancer cells” shorten patient lives in several ways. In a first scenario, patients with advanced-stage cancers have lost some organ functional capacity as a result of damages of invasive cancer cells. Any additional adverse effects of cancer drugs on the patients could depress organ functional capacity below the threshold of death. In a second scenario, medical treatments raise cancer growth rates. Cancer’s natural growth time is often more than ten years, an advanced stage cancer’s growth rates level off due to resources limits. Such patients may be often attacked by adverse events, but do not lose their lives quickly. Their natural cancer courses depend on their efforts in fighting cancer. Aggressive measures that cause severe tissue loss and systemic inflammation naturally make death happen earlier.
In a third scenario, when the first tumor is removed, second cancer or return cancer appears in about one year or so. The tissue loss, systemic inflammation, and overwhelming aging-like cellular damages cause the body to raise cancer growth rate constants by one to several orders of magnitude. Even though medical treatments might have lowered the cancer burden to nearly zero, it results in a much faster cancer return. It is like that body has lost the overall capacity to contain cancer growth. We must question whether medical treatments can extend patient lives. This question can be answered only by comparing patients’ lifespans with correspondent reference lifespans that patients could achieve without using the treatments. The difference would depend on the selection of the reference. In a long history when cancer-inducing factors and influence factors were unknown, patients would do everything incorrectly to shorten lives, the aggressive medical treatments could show some benefits. This observed belief can no longer be correct. As we have known that cancer growth rates are highly sensitive to a large number of lifestyle and environmental factors, their lives can be extended by beneficially using those factors. Consistent with our theory is a large number of cancer miracles cancer can resolve [71].
In the last scenario, the primary tumor is destroyed by medical treatments, cancer patients may die from different cancer or other cause. However, the patients lose a part of their lifespans due to the severe damages caused by the side effects. Whether the side effects are fair prices depends on alternative measures for controlling cancer growth. Based on the above analysis and poor outcomes, we must conclude that medical treatments are no longer good options unless forced by the circumstances. We must rethink the wisdom against surgery before William Stewart Halsted (1908).
Clinical trials are unable to detect slowly accumulated side effects due to a large number of interfering factors [72], buffering effects of vital organ reserves [73-75], and short follow-up time. The accumulated adverse impacts may be revealed only in long-term studies. Lifespans of cancer survivors are cut shorter by an estimated 30% [44,45] for a certain type of cancer.
Whether or not medical treatments extend patient lives should be based on human inherent potential ability to survive. That ability is abundantly reflected in a large number of cancer miracles, where cancer resolves or heals naturally (N6, Sup.). Some patients would do whatever they can to survive, true merits of medical treatments for such patients should be established by using their whole program as a reference.
C. Non-medical measures can control cancer growth rates
1. A large number of non-medical factors can slow down cancer growth rates: A body of evidence acquired after 1980 shows that cancer is highly sensitive to hundreds of factors. Emotional distress, chronic stress, lack of exercise, and inactivity have been discussed above. Other factors include omega-3 fatty [47], pollutants and toxins [69], unhealthy diets and nutritional imbalance [76], inflammation causing factors [40], chemical carcinogens [77], other chronic diseases such diabetes [78], natural products and natural apoptosis-inducing compounds [79-81], etc.
Those and other similar measures are referred to as non-medical measures. They include exercises, emotional management, diets, and nutrition, changing lifestyles, natural anti-cancer products, etc. They can influence apparent rate constants for cancer growth. They can be used in a beneficial way to slow down cancer growth rates.
2. Accumulated benefits of non-medical measures are very large: The current medical research model is capable of detecting strong and fast treatment effects, but unable to detect any effects that are realized slowly. Wu and Zha found that randomized clinical trials are inherently biased in studying weak and slow treatment benefits [72] (N7, Sup.). For the same reason, the adverse effects of each medical treatment cannot be accurately determined because the adverse effects are interfered with by other factors. Thus, the medical research model is biased in favor of hiding adverse effects and against finding true benefits of non-medical measures. Past findings from clinical trials exaggerate the merit of medical treatments, underestimate the adverse effects of medical treatments, and underrate the true benefits of non-medical measures. This three-way of biases makes randomized clinical trials findings inaccurate.
Figure 3 below shows that the beneficial effects are accumulated over time and thus bring down cancer cell dividing rates progressively over time with a potential to reach negative values. Negative rate constants mean that cancer will have negative growth or become smaller and smaller with time.
The beneficial effects of non-medical measures cannot be appreciated without understanding the compounding effects. A reduction in the daily growth rate on any day will result in a small reduction of cancer cells on that given day. The reduction is like removing a few “seeds” which could compound in more than a thousand cycles in the person’s lifetime. When the apparent rate constant is negative, the cancer is in a process of healing as cancer self resolution cases [71]. A presumed cure for cancer is “a negative rate constant.” Considering rate constant’s daily fluctuations, a presumed cure for cancer is to reach “overall negative rate constants.”
Different effects of different rate constants caused by medical treatments and non-medical measures are shown in Figure 4. The cancer burden is at the joint point at the time zero. If the net rate constant is zero, the cancer size will not change as shown in line (A). If cancer grows naturally (B), the total cancer cell number exponentially increases due to first-order characteristics. Due to resource limits, the growth curve will actually level off. If the cancer is treated by medical treatments (C), the cancer burden is rapidly reduced in the early time; but cancer cells repopulate as a result of increasingly enlarged k values. Surgery can instantly get rid of the whole tumor or most cancer cells, but cancer can repopulate much faster. Because medical treatments promote cancer spread and thus generate more tumor sites, resource limits can no longer effectively control the growth of widespread tumors. This is why cancer spreading is nearly always deadly. If the cancer is controlled by non-medical measures only (D), cancer cell number continues increasing for some time particularly in the early stage.
However, the apparent rate constants gradually go down if the patient can deliver sufficient measures for slowing down cancer growth. By improving organ reserve functions and tissue health, the body will improve its anti-tumor immunity and cause the rate constant to become smaller and smaller by each day. Thus, the cancer growth curve shows a leveling-off point followed by a downward trend, which is also a double-exponential decay. Whether medical treatments can extend lives over the natural growth curve depends on cancer types, patient health, and his ability to use non-medical measures. If the patient attempts to use non-medical measures, it is also possible that the cancer burden hits the threshold of death if the measures are insufficient to slow down cancer growth in the early time. Based on cancer fight stories, we noted that the chance of success depends on personal willpower and the use of the right measures.
Figure 4 shows the cumulative effects of medical treatments and non-medical measures. (A) shows the growth curve for cancer in a dormant state; (B) shows how a naturally growing cancer may kill the person because the burden is beyond what the vital organ can sustain. If medical treatment is used, its beneficial effect is delivered quickly (C). Medical treatments are more powerful than non-medical measures in destroying cancer cells (A). However, the side-effects of the treatment is accumulated slowly, and the slowly realized side effects will gradually nullify its beneficial effects in the long run. The performances of each drug or treatment will follow the similar pattern. C shows that chemotherapy can kill cancer cells and reduce their number, but the site will generate more cancer cells due to increased growth constants. C also shows how surgery and chemo can rapidly reduce the cancer burden to near zero. However, both surgery and chemotherapy will dramatically raise the rate constant, resulting in a doubly exponential curve. The curve may hit the death threshold earlier. If a treatment is applied to second cancer or third cancer, accumulated net effects will become progressively worse. The adverse effects such as lost tissues, damaged tissue cells, increased systemic inflammation, etc. raise cancer growth rate constants and slowly bring down the beneficial effects to zero or negative values in a long run. For the reason found by Wu and Zha [72], the weak beneficial effects can be nullified by adverse effects of side effects of medical treatments.
Figure 5 (A) shows the medical treatment has strong instant benefits but also large accumulated side effects. Thus, its net benefit is marginal or negative. As shown in above figure (B), non-medical measures do not have inherent side effects when they are correctly used to match patients’ conditions. They produce a small amount of often-undetectable beneficial effects in each day. Since no adverse side effects are accumulated, small beneficial effects are added up to exhibit larger and larger final benefits. Their instantaneous daily effect can cause the tissue to reduce cancer cells each day, which has the effects of removing “seeds” for later cancer cell division. The accumulated beneficial effects will become larger and larger with time, and thus have more power to slow down cancer cell division rates on later days. All of those effects can change cancer cell numbers by altering compounding effects (e.g. a downward bending curve). Their net accumulated beneficial effects are much more powerful than medical treatments in a long run.
Non-medical measures can alter cancer outcomes not by destroying the tumor and killing cancer cells but by altering the balance of the rate between cancer cell division rate and cancer cell death rate. Cancer will be stabilized or cured if the apparent rate constant is reduced to zero or negative. Final cancer cell numbers are very sensitive to rate constants. Based on latent times of cancer, rate constants expressed as a percent of cancer cells are rather small. This overall slow growth process is the basis that non-medical measures can be cures to cancer as long as they are used properly to right patients.
3. Exercises can dramatically slow down cancer growth rates: Some factors such as exercise, emotion management, diets and nutrients, body temperature, physical activity levels, etc. have universal impacts on all patients of all types of cancer, they could be used reliably to fight all types of fully developed cancer. The impacts of lifestyle factors on cancer growth rates are extremely large when viewed in a long run. A significantly lower risk of cancer recurrence was observed for patients with higher exercise levels in studies [79-82]. Both exercise intensity and duration are important parameters. Three MET-hours is equivalent to walking at an average pace of 2 to 2.9 mph for 1 hour.
Compared with women who engaged in less than 3 MET-hours per week of physical activity, the adjusted relative risk (RR) of death from breast cancer was 0.80 for 3 to 8.9 MET-hours exercise per week, 0.50 for 9 to 14.9 MET-hours exercise per week, 0.56 for 15 to 23.9 MET-hours per week, and 0.60 for 24 or more MET-hours per week [82]. Compared with patients engaged in less than three metabolic equivalent task (MET)-hours per week of physical activity, the adjusted hazard ratio for disease-free survival was 0.51 for 18 to 26.9 MET-hours per week and 0.55 for 27 or more MET-hours per week [83]. Men who walked briskly for 3 h/wk or more had a 57% lower rate of progression than men who walked at an easy pace for less than 3 h/wk. Walking pace was associated with decreased risk of progression. There was a suggestive inverse association between the risk of progression and intensity of activity. The author also noted that exercise intensity is an important factor for eradicating actively expanding moles (N8, Sup.).
Cancer cells have a poor ability to tolerate moderately raised temperature [86] and thus exercises can slow down the cancer growth rate by raising body temperature. Exercise also increases the degree of mechanical vibrations, which can inhibit cell division by disrupting cell division apparatuses [87]. Exercise causes working muscles to deplete glucose levels in the blood and thus makes less glucose available to cancer cells. Exercises, diets, and lifestyle factors affect the vascular system, the renal system, the respiratory system, and Central Nervous System, the body’s systemic inflammation level, and the body’s physical conditions on a daily basis.
Non-medical factors include any lifestyle factors that would influence cancer growth rates. They even include eating habits, working habits, thinking habits, and activity patterns [71]. Among causal factors, risk factors, and influencing factors, only some of the factors may be relevant to a specific patient. While the scope of applicability of the factors depends on patients’ lifestyles, potentially, a large number of sub-sets of known factors may be relevant to the patient. The effects of the factors are additive in an unknown manner. When a lifestyle factor can reduce cancer relapse incidence by 50%, it can be viewed as causing relapse incidences to fall in wider time windows so that half of the incidences are not observed within the trial follow-up times. The factor actually slows down the cancer growth rate dramatically. Exercise alone can have enough power to alter cancer outcomes for a large portion of cancer patients. If several, tens, hundreds of relevant factors are used in combination, they can alter cancer outcomes reliably.
4. Feasibility of using non-medical measures to slow down cancer growth rates: Some cancer experts suggest that any non-approved methods other than the legalized few cannot cure cancer. Their belief is based on the assumption that destroying the tumor is the only right approach. It should be rejected now.
We have shown that clinical trials have triple biases and cannot produce correct results. They are not the only sources of biases. Most studies use a five-year (few with ten years) follow-up time. Both adverse effects of medical treatments and beneficial effects of lifestyle factors (such as exercises and changed diets) are realized by long-term effects. Their true effects cannot be realized in short times. A short time window allows surgery and drugs to realize their effects of killing cancer cells, but also effectively hide their side effects. When patients are still healthy in the early years, their side effects are unable to depress the organ’s functional capacity below the threshold of death. However, the side effects are accumulated with time; and start affecting a patient only when the cancer burden has depressed the organ functional capacity to near the threshold of death. If the trial lasts sufficiently long, the adverse effects of surgery and cancer drugs also influence the cancer growth curve by altering rate constants. They affect cell division in each cell cycle. The short follow-up time is also a reason for underrating the beneficial benefits of lifestyle factors.
Risk factors, lifestyle factors, and environmental factors affect cancer outcomes by influencing cancer growth rates. Cancer initiation and growth take place at varying speeds. If a factor is found to reduce cancer incidence rate, the factor slows down cancer initiation and growth speeds so that the detection times of the tumor will shift to later times. Thus, tumor detection times for some patients fall outside follow-up times and thus exhibit reduced cancer incidence rates. A significant reduction in the incidence rate means a slower cancer growth rate. Nearly all factors discovered after 1980 speed up or slow down cancer initiation and growth speeds. They can be used in a beneficial way to cure cancer.
The feasibility of using lifestyle factors to slow down cancer growth or metastasis rates can be seen from the high sensitivity of changing rate constants on growth rates. Tiny or small changes in growth rate constants significantly reduce the final tumor sizes in a long run (N9, Sup.). If the rate constant is reduced by 10% from 0.01 to 0.009 (1/day), the total tumor size would be only 2.6% of the reference tumor in ten years. The tumor size would differ by 38 times. Assuming that a tumor of 1 billion cells grows at the rate of 0, 0.001, or 0.1%, we will see very different results. If the tumor is held in check at 0, the tumor will be dormant. If in one day, the body’s temporary condition allows the tumor to produce a million new cancer cells, those extra cancer cells would become 1.4, 3.0, and 6.2 million in 1, 3, and 5 years if they grew at the same rate. Any extra cancer cells in any day continue dividing by the same fraction for perhaps a thousand cycles. This is the basis for why multiple slow-working non-medical factors can alter cancer outcomes. Those examples explain why correct exercise can reduce cancer morbidity by as high as 50%. It also signifies fighting cancer is a daily task and the successors belong to those who can fight tirelessly. It also signifies that excessive cancer cells produced in one day or some health condition must be addressed by subsequent activities as soon as possible.
The predicted feasibility of using lifestyle factors does not guarantee success. Failure can be attributed to a patient’s failure to understand cancer growth kinetics. Cancer compounding is similar to loan compounding except that cancer has the fastest compounding pace and variable daily rates. In paying a loan, when the loan situation is out of control, it would be very hard to reverse and often ends up with bankruptcy. In contrast, when a debtor is able to manage the payment, it would become progressively easier with each payment. In fighting cancer, a good strategy is to use sufficient measures with sufficient firepower to hold daily cell division in check. If the measures are insufficient, cancer will progress and expand. Fighting cancer must be aimed to change the tissue ecosystem each day.
When the body is in intense exercise, the tissue ecosystem is unfavorable to cancer cell division and holds cancer cells division in check. When the patient stops doing exercise, the tissue ecosystem will slowly go back to the condition that favors cancer cell division. Therefore, one important criterion is the time-averaged MET value per day must be sufficiently high. Reasonably intense exercises are performed in three to six sections each day. Most cancer patients do not see the need to stick to strict disciplines. Simulations can show that three-day exercises and two-day breaks will achieve very little. This can be explained by loan payment: a debtor can not pay off a loan by paying two payments and skipping one.
5. The notion against using non-medical measures to cure cancer is a product of using a flawed research model: Our findings refute the notion that non-medical measures cannot be used to cure cancer. Medicine confines its treatment options to the very few options that clash with evolution. FDA outlaws doctors from suggesting or prescribing vitamins, supplements, herbs, and super-foods, and legally endorses surgery and approved “treatments”. American Cancer Society and FDA often made statements to preclude true cures in a long history. Medicine frequently criticizes alternative options for fighting cancer [88]. The public is taught to discredit non-medical measures as unproven and disapprove of cancer treatments. A common statement is like: “no evidence supports claims that X is effective in preventing or treating cancer» [88,89]. Some of them are clearly the best cancer-fighting measures if they are used correctly to right patients. One article states: “Some alternative therapies are harmful, and their promoters may be fraudulent.” It makes a wrong finding because it improperly relied on evidence of controlled trials. Clinical trials produce wrong results because it is for detecting strong effects that can be delivered in short times.
The medical system creates a catch-22 for non-medical treatments. It never looks into options as cures beyond surgery, chemotherapy, radiotherapy, etc, but discourages the public from exploring non-medical options. By using randomized clinical trials, medicine favors fast-acting and strong measures. Patent law bars patenting on anything that is from nature and made of nature. Tax law and medicare provide a legal basis for discouraging the public from exploring non-medical measures. Under such a legal framework, nobody would study the true slow-delivering benefits of non-medical options. Then, medicine discredits any non-medical measures for “lack of evidence.”
The flaws in relying on clinical trials ruin population health wisdom, prevent researchers from finding cures for cancer and make cancer much worse than it really is. Influenced by such propagation in several decades, a vast majority of cancer patients have not realized the importance of lifestyle factors and the super-strong combination effects of non-medical measures. Believing nothing can kill cell cancer cells, cancer patients choose invasive surgeries, accept toxic drugs, harmful radiotherapy, etc. to do more violence to organs than cancer. Cancer patients are willing to get onto deadly palliative tracks. When patients are treated by medical treatments, cancer patients survive only by miracles or survive by withstanding increased cancer growth rates or by overcoming severe adverse side effects.
6. Multiple factors optimization can dramatically decrease cancer growth rates: Figure 6 shows the importance of using multiple factors in fighting cancer. In a randomized trial, beneficial effects on some subjects are negated by adverse effects on other patients due to statistical averaging. Based on an assumption that a factor works on about 10% of the patient population, an optimization mean it should not be used on the 90% mismatched patients. If a single factor is used in an optimization trial, its negating effects that normally exist in a randomized trial can be avoided. Assuming that one factor would deliver 10% benefits in a randomized trial if 10 similar-strength factors are used on different persons, the combined effects would be raised by nearly 100 times, and hypothesis statistics for affirming true treatment benefits will be raised by about 320 times relative to a randomized trail focusing on a single factor (with the other 9 factors be treated as interfering factors). Also, multiple lifestyle factors may be used based on patient personal situations to reach the highest response rate.
D. Adverse effects of early diagnosis of cancer
When predictive cures cannot be found, it is believed by a supermajority of cancer experts that the best strategy is early diagnosis. However, we question its validity. It was estimated that among 70-79-year-old people, more than one-third of Caucasian men and half of African American men have indolent prostate cancer that would not cause harm if not diagnosed and untreated [87]. The detection of indolent prostate cancer has obvious adverse consequences [90]. It has been estimated that 42-66% of diagnosed prostate cancers would have caused no clinical harm had they remained undetected [91]. One study estimated the magnitude of over-diagnosis from randomized trials: about 25% of mammographically detected breast cancers, 50% of chest x-ray and/or sputum-detected lung cancers, and 60% of prostate-specific antigen-detected prostate cancers [92].
We believe that early diagnosis is a wrong strategy for several reasons.
First, the latent times of naturally occurring cancers can be from 5 to 70 years. Growth from a large adenoma to cancer was estimated to require about 17 years, and generally, the same mutations are present in primary tumors and their metastases [93-95]. The time scale implies that cancer could be easily controlled by any of a large number of non-medical measures. Second, it is a well-known fact that many cancers are dormant and inactive and can remain in that state for patient lives [96]. Histologically advanced microscopic tumors are detected in many tissues of adult humans [97,98], but appear to be mostly held in check by unknown mechanisms. This line of evidence together with cancer self-healing cases [71,99] shows that cancer could be cured or held in check by using non-medical measures. A recent review extensively discusses cancer spontaneous-resolution which was recognized as early as the 12th century. Spontaneous resolution of cancer has been found for nearly all types of cancer. While it is hard to find mysterious driving forces, cancer spontaneous resolution cases imply that cancer can be contained by controlling cell net growth rate. We believe that using multiple lifestyle factors to optimize the immune system would be a viable approach. One advantage of this approach is that cancer research has identified a large number of influence factors in the last fifty years but many more factors are yet to be investigated.
An early cancer diagnosis will have overwhelming adverse impacts on patients. The biggest adverse effect of the strategy is a shift of cancer diagnostic ages from old ages or post-death “ages” to younger ages. The strategy could have the effect of labeling more people with cancer at the ages of 50, 60, 70, etc. rather finding cancer after their deaths or having the undetected tumors self resolved. A diagnosis of cancer always triggers the on-set of the adverse effects of three or four lethal factors. Early detection of cancer means starting to affect patients’ lives at earlier ages. In addition, early diagnosis also inflicts routine emotional distress. Annual screening using embarrassing procedures such as colonoscopy can inflict great pains and suffering. Each time when growth, a polyp, bleeding, or whatever is found, the person will be tormented for a few days until a biopsy can rule out malignancy.
Early diagnosis will generate a big cancer patient population. Cancer statistical data shows that maximum cancer occurring ages are above 70 years (1 in 3) and 85 above (nearly a unit). Now, men have a 39.66% probability, or approximately one in three risks, of developing cancer in their lifetime. Men have a 22.05% lifetime risk of dying from cancer, while the risk for women is around 18.75%. Cancer in a good portion of old people is not diagnosed [100]. The prevalence rate is close to 50% among US White and European men aged 80 or above. If this prevalence rate is added with the clinically diagnosed prevalence rate, one would expect to see a unity for those of 85 or above. Projected based on the age and racial distribution, life expectancy, and total U.S. population in 2015, these data suggest roughly 45 million cases of potentially detectable prostate cancer in the U.S [101].
The above data is about only one type of cancer. If all types of dormant and micro tumors were diagnosed and their incidence rates are added together for elderly people, the total chances could be 90% to 100% of the people who have lived above 80. Medicine will never solve the cancer problem by cutting off tumors and killing cancer cells. Early diagnosis and treatment of indolent, small, and/or slowly developing cancer have adverse impacts on patients, society, and nation.
Even for highly malignant cancer, the incidental benefits brought by changes to lifestyles in cancer treatment are not enough to neutralize the adverse effects of the four lethal factors. The early diagnosis will deprive chances for tumors to self-resolve and invite unnecessary emotional pains against dormant, harmless tumors or tumors. Early diagnosis may be good for only extremely aggressive rare cancers that medical treatments can control while non-medical measures cannot.
Implied from the cancer spontaneous resolution is that cancer must come and go or grow and shrink, consistent with the host person’s lifestyle, emotional state, and daily activities. It is beyond dispute that the immune system is influenced by a large number of lifestyle factors, and tumor development direction and speed can vary on a daily basis.
The perceived benefits of cancer early diagnosis are most probably incorrect.
The reduced incidence rate for cancer is mainly attributed to a reduced population of smokers in the population, a big reduction in lung cancer cases, and indirect benefits from anti-cancer efforts such as a healthy diet, lifestyles, and exercise. Moreover, improved cancer survival rates among early diagnosed cancer patients are inaccurate because the 5-year survival rate is an improper measure of the survivals for early diagnosed cancer. The perceived increase in five years survival rate is actually transferred from long latent cancer development time to five-year survival. Making a diagnosis by 10 years earlier but losing the life 7 years later is not a winning strategy. Also, some patients might die in the same time window if they had not been diagnosed with cancer earlier. In addition, some patients would heal their cancers naturally if they had not been inflicted with the four lethal factors. The perceived benefits of early diagnosis are a temporary trend seen for some types of cancer, and the true picture will appear only when a large number of those early diagnosed patients start dying.
The most apparent benefits for cancer patients cannot be attributed to the medical treatments. If cancer is cured while the patient accepts medical treatments (e.g. “type A miracle”), the true cures cannot be attributed to drugs, surgery, and radiotherapy. Current medical treatments cannot permanently restore altered biochemical and cellular process patterns. Cancer is not like a lodged bullet, poison, traumatic injuries, and bacteria that can be removed. What actually cure cancer are things that are used in parallel to medical treatments.
Based on above reasons, medicine should explore a wiser strategy that is to delay slow down cancer growth speed and delay detection times to post-death and encourage people to use cancer-risk reduction programs as proactive preventive measures to stop cancer from growing without being named as characterizing the patients as cancer patients.
E. Adverse Impacts of over treatments of cancer care
In 2019, there are an estimated 1,762,450 new cancer cases diagnosed and 606,880 cancer deaths in the U.S. We use a 33% overdiagnosis rate, about 200,000 annual deaths in the U.S. could be attributed to unnecessary treatments. The number of cancer survivors in the U.S. is between 15 to 20 million now. Those people will lose lifespans by large margins. In China, there are 4.51 million cases and 3.04 million deaths for 2020. We estimate that medical treatments may cause at least 1 million annual deaths in China. In the world, about 9 million people are dying from cancer. A large number of deaths are accelerated by medical treatments while the remaining survivors lose considerable parts of their lifespans or die from future cancer.
To save a life from terminal diseases, patients naturally want to accept various treatments. Patients’ trust in medicine, doctors’ financial incentive to earn medical service revenues, and doctors’ desire to avoid malpractice lawsuits for failure to diagnose or treat cancer become a concordant driving force for creating the over-treatment landscape. When all interests are aligned to promote over treatments, over treatments become the hallmarks of the cancer care industry.
Patients’ trust in medicine becomes a negative factor in the area where medicine has no real cure. Medicine is accepted as the only science-based medicine, and its performance in treating acute diseases is not questioned. Even in treating cancer, patients still depend on medicine in treating emergency problems such as bleeding, blockages, fracture, stroke, heart attack, organ failure, etc. The patient’s trust has impaired their judgment in cancer care. Patient stories reflect a common understanding that best care is equivalent to more drugs, newest drugs, more treatments, more hospital stays, etc., and most patients do not appreciate the magnitudes of harmful risks of misused medical treatments. It is well known that, unlike normal people, cancer patients are more willing to use treatments with small benefits and major toxicity [102].
Over treatments are in part caused by conflicting findings in cancer research. The population-based medicine has molded a popular belief that every disease could be cured by the same treatment protocol. However, cancer research has generated a massive number of conflicting findings. In selecting treatment options, doctors are often not in a position to make a final decision and thus have to let patients make final calls. Medical science has produced a large number of complex issues that few patients cannot understand. They are unable to understand complex cancer knowledge and could not evaluate statistical analysis and experimental designs. We note that most patients cannot tell differences between a 2% reduction in a hazard ratio at p=0.001 and a 20% reduction in the death rate at p=0.09. When they are in doubt, they often err on the side of getting more treatments.
When patients’ desire for getting over treatments is consonant with doctors’ desire to avoid liability from withholding treatments, overtreatment is very common. Patients tend to accept over treatments with the unrealistic expectation that a tiny good chance like 1% will happen to them but major risks like 60% will not.
Palliative care studies reflect that patients hope that “something will be done, a wonder drug will be available”, a patient “….struggles on and fights because he/she clings to a hope which is probably 99% unrealistic,” and patients “still maintain their expectations despite all evidence to the contrary” [103]. Patients often are on chemo even just a few days before their deaths. Over treatments are clearly driven by patients. The only way to stop such tragedies is by educating patients with the right knowledge.
Studies show that a cancer drug may extend life by a few months at a highly significant level but also has any combination of around 30 to 50 specific side effects. Cancer drugs can often damage nerves, liver, kidneys, ears, heart, etc, and can cause nausea, vomiting, hair loss, cognitive dysfunction, fatigue, changes in sexual functioning, and reduced quality of life. Most studies underestimate true side effects. Medicine has a convention to characterize drug side effects as localized symptoms, but not as systemic damages. They do not study lost functional capacity of vital organs. Some damages are revealed in obvious changes in patient’s intellectual capacity, darkened blood vessels, impaired nerve functions, etc, but are neglected in studies. The aging-like adverse effects can be found only in long-term studies [45].
Over treatments are also driven by the belief that a cure to cancer is killing “every cancer cell.” If patients want to achieve zero levels, doctors could meet patients’ demands. Since cancer adverse outcomes happen at high chances, a refusal to meet a patient’s demand may be a ground for a malpractice suit if the patient later dies, but shortening the patient’s life by medical treatments will not. Honoring the patients’ demands is consistent with established treatment protocols, liability law, and doctors’ financial gains. From published diseased patients’ stories on blogs, one can see the same pattern that patients are driving for over treatments.
From discussions with cancer patients and online case reports, we found that a good patient population cannot understand the real purpose of palliative care, the magnitudes of the risks of drugs, and the precluding effects of medical treatments for future success.
Palliative care, which is always accompanied by three to four lethal factors, shortens patients’ lives. The final outcomes of palliative care are well understood in cancer literature. The use of this option is based on a presumption that absolutely no other option can save a life. However, there is no basis to assert that none of the tens of thousands of non-medical options can save lives. Any assertion that cancer cannot be cured cannot stand in front of a large number of cancer miracles. Thus, “terminal” is factually incorrect; and patients’ consents to palliative care may be acquired with a legal defect.
Leaving the “incurable” notion aside, patients are not properly informed of the nature of palliative care. It is found that one-third of patients being treated palliatively thought that their therapy was curative [104]. In another study of 149 patients with incurable cancer, 45 (31%) believed their cancer was incurable, 61 (42%) were uncertain, and 39 (27%) believed their cancer was curable [105]. We estimate that a supermajority of patients never think that cancer drugs can potentially preclude future cures. Medicine has not considered and has not studied methods of using the right combination of lifestyle factors to slow down cancer growth rates or reverse cancer progression direction as a better strategy for curing cancer.
Most patients cannot conduct risk-and-benefit analysis in the context of palliative care. They do not understand the long-lasting adverse impacts of cancer treatments. Most patients hope that medical treatments can save their lives for a few years, with wishful thinking to further extend life. They never understand palliative care most probably set the maximum limit on their survival times: when they get on this track, they give up the best path and accept the worst outcomes which are known in the literature.
Another problem is that cancer patients are exposed to regular risks from medical treatments such as surgeries, drugs, and radiotherapy and from diagnostic procedures such as CT scans and invasive sampling procedures. The risks from CT scans are known [106-108]. If the risks from all sources are added up, some of them may hit 100%, and some are exposed to different categories of risks with each being close to unity. They may get secondary cancer by certainty, ruin their kidneys by certainty, destroy the liver by certainty, and cripple their immune systems by certainty. However, since each risk cannot be materialized without a time course, they appear to be well. So, they keep taking more and more risks. If all risks are viewed on a long term basis, they would die in one of several ways.
They do not know that abusive treatments and procedures forever cut their lives short and nothing can help except miracles.
Discussions
A Summary of flaws in the medical research model
All cancer treatments driven by the notion of destroying the tumor or killing cancer cells have been poor did not work, and will never work. After repeated failure to find predictable cures for cancer, we have a common interest to explore powerful alternatives. The right strategy is to beneficially use multiple factors to slow down cancer growth rates. The presumed cure for cancer is “achieving overall negative cancer growth constants.” This strategy requires a completely different analysis of available options and measures.
Cancer cell daily gain or loss depends on cancer dividing rate and total death rate, and the final cancer cell number is the sum of net gains or losses of cancer cells over the entire patient lifespan. The cancer cell death rates depend on cancer cell necrosis, natural death, cell programmed death or apoptosis, cell destruction caused by immune responses, and possibly cell reformation (like stem cells change their differentiation behaviors). Cancer net growth rate constants are generally very small, and adverse cancer outcomes are due to the unique compounding effects of cell division cycles. Both the cancer cell division rates and cancer cell death rates are highly sensitive to a large number of lifestyle factors, personal activities, and environmental factors. A viable approach to fighting cancer is to slow down cancer net growth rates. Cancer could be cured by beneficially using any combination of non-medical measures to reverse cancer growth direction. This approach does not depend on molecular specificity although activation of anti-tumor immunity by luck may rapidly shorten the entire healing process.
The inability to find curative benefits of non-medical measures are attributed to (1) selecting improper controls by precluding all non-medical measures, (2) grossly underestimating the role of tissue loss and cell damages caused by medical treatments, (3) the use of insufficient follow-up times in clinical trials, (4) the averaging effects of between treatment and interfering factors in randomized trials, (5) failing to use multiple factors approach, and (6) failing to understand the compounding effects of cancer cells division. The research model with those flaws exaggerates strong treatment effects, but consistently undermines weak treatment effects and slowly damaging side effects.
The research model has triple biases in favor of confirming the strong effects of medical treatments. The biases collectively reduce the treatment effects of non-medical measures by one or more orders of magnitude. Moreover, under the current legal framework, factually wrong propagation has discouraged cancer patients from using best, safest, and most powerful cures which are built-in human genes or can be readily found from nature. The terminal and incurable labels can severely affect a patient’s ability to fight cancer. All of the biased effects of the medical treatments are shown in the following table.
Adverse effects of medical treatments
Claimed benefits of medical treatments for cancer are refuted for all of the reasons listed in the following Table 2.
All medical treatments were developed with the notion to remove the tumor or kill cancer cells. This notion was formed long before 1846, was based on obsolete cancer theories, and clashes with the latest discoveries of the changes in biochemical and cellular processes and the latest evolutionary cancer theory. The latest knowledge and cancer theories imply that cancer cannot be cured by cutting, radiating, and drugging. Cancer incidence rate found in an autopsy, evolutionary cancer theory, a massive number of cause-relating studies, and our kinetic simulations collectively show that a real cure for cancer is to slow down cancer growth rates or reverse its course. The use of the rate balance approach will become the most powerful approach to ending the cancer pandemic.
The options of medical treatments were severely limited by the flawed legal framework. All performance data of medical treatments are acquired by making chain comparisons among surgeries, drugs, and radiotherapy, all of which are similarly ineffective and harmful. Each of the medical treatments may clash with other compounds or cell apparatuses in the human body because they were not exposed to the human body in evolution. Medicine did not explore how a comprehensive program comprising multiple lifestyle factors would perform. Thus, medicine does not know how medical treatments perform on an absolute scale, as compared with best references which would be based on any combinations of ten of thousands of lifestyle factors.
All medical treatments are associated with three to four deadly lethal factors. The surgery increases cancer apparent growth rate constants by as much as 10 times, and chemotherapy and radiotherapy can raise cancer growth rate constants by 2.5 to 5 or more times. Emotional distress and chronic stress could increase cancer growth rate constants for metastasis by adding 0.182 (1/day) to correspondent values. Surgery, chronic stress, and physical inactivity can jointly promote cancer metastasis which had the effect of removing resource limits.
When adverse impacts from surgery, chemotherapy, radiotherapy, and emotional distress are added up, medical treatments cannot deliver benefits in a conceivable way. Surgery shortens lives by reducing the vital organ’s functional capacity. The complete response rates of 7.4% and overall performance of chemotherapy reflect the limits of “the tallest dwarfs” selected from a narrow choice of options. Those facts explain why cancer growth times rapidly reduce from about 15 years to several months or shorter.
Clinical trials are biased in favor of detecting strong effects but are incapable of detecting slowly-working beneficial effects and slowly-damaging drug side effects. In a randomized trial, treatment is indiscriminately used on patients, thus some beneficial effects and some adverse effects are evened out by statistical averaging. Also, the beneficial effects of a single factor are too small when multiple other interfering factors affect the same measured health properties like the treatment. Such a randomized trial reduces the statistical mean of the treatment and raises error variance, thus resulting in failure to affirm the true treatment effect. Due to interference of other factors and short follow-up times, clinical trials are unable to detect the slow-delivering side effects that are detectable in later times. Compared with a health optimization trial, the treatment effect is underestimated by one to several orders of magnitudes, depending on the number of interfering factors and trial duration.
Some studies found that cancer’s global survival rate is steadily improved over the years and have given credit to the use of surgery, drugs, and early diagnosis of cancer. The real reason for the improvement in the survival data is the increased use of cancer-fighting measures by cancer patients. Cancer patients know the importance to improve diets, adjusting lifestyles, do more exercise as a result of influences by studies published after 1980. When those lifestyle factors are used beneficially by a substantial portion of patients, overall death cancer rates are reduced, and more cancer miracles naturally happen. However, no single study has proven how a diminished organ functional capacity, raised systemic inflammation, and damaged cells and tissues can improve cancer survival rates.
The one-time tumor destruction cannot explain any success. Adverse effects of surgeries, drugs, and radiation may nullify whatever benefits alternative non-medical treatments may offer. Any cure based on the notion of killing cancer cells clashes with the presumed cure of slowing down cancer growth rates.
True curative benefits of non-medical measures
Hundreds of well-documented cases and estimated millions of undocumented cancer miracles conclusively prove that cancer can self resolve or heal naturally [99], with the fastest time scale from 1 month to 6 months. The incurable notion is factually incorrect. Cancer self-healing becomes miracles (we call type B miracles) because medicine does not explore the causes of self-healing and has not explored as cures exercises, diets and nutrition, natural products (containing any of tens of thousands of anti-cancer compounds), and other lifestyle factors. Those factors were never used as comparisons in evaluating medical treatments. Thus, “lack of scientific valid” evidence is a result of limitations of clinical trials.
Cancer self-healing is not a miracle. Behind cancer, miracles are thousands of basic medical discoveries, which could explain the mysteries of each cancer miracle. The difference between “cancer patients” and “normal people” is cancer growth rates. A body of evidence shows that the potential benefits of exercises are one to several magnitudes larger than medical treatments if their respective effects are evaluated for a long term. Well-designed and well-executed exercise programs can be cures for most types of naturally occurring cancers. Some cancer miracles happened when the tumor was inoperable or patients did not accept medical treatments. We attribute the miracles in the main part to avoidance of three or four lethal effects, and avoidance of raised apparent growth rate constants.
Some cancer miracles can be attributed to improvements in an emotional state. Since emotional distress, chronic stress, and emotional state have huge impacts on metastasis processes, successful control of emotional problems and abasement of chronic stress could be enough to change cancer outcomes in some cases. Right dietary adjustments and nutritional programs can alter cancer outcomes by reducing cancer growth rate constants. Any other lifestyle factors or natural anti-cancer compounds from natural products may be able to alter cancer outcomes by slowing down tumor growth rates. We estimate that a good cancer-fighting program is one to several magnitudes more powerful than any of radical medical treatments.
Medical researchers are not provided with incentives to study weak treatment effects of lifestyle and environmental factors. A change in future research direction requires abandoning the old strategy and using the optimization method for using weak and slow delivering effects.
Adverse effects of early cancer diagnosis
Early diagnosis of cancer is a wrong strategy because cancer is always a part of human life and somatic evolution. Early diagnosis is accompanied by three to four lethal factors and the total destruction of life hopes. Incidental benefits from early diagnosis is marginal. Declined cancer death rates are an “artifact” caused by the flawed short survival measure and cannot be attributed to early diagnosis. The improved 5-year survival is transferred from the long latent time of most types of cancer. Cancer screening torments fragile people by inflicting serious emotional pains. A better strategy is to use cancer risk reduction programs to slow down cancer growth or reverse cancer development direction without labeling patients with “incurable” cancer.
Issues in palliative cancer care
Given the fact that cancer can resolve by itself and naturally heal under the influences of a large number of lifestyle factors, the incurable notion is untrue. Patients are generally not informed of one or more severe adverse impacts of medical treatments, nor the four associated lethal factors. They generally are not told how cancer drugs raise future cancer growth rates and dramatically cut short their lifespans. Research articles cannot predict drug side effects. Most patients are unable to appraise accumulated risks from surgical operations, drugs, radiation, CT scans, invasive tissue sampling, etc. Most patients are not informed that medical treatments have precluding effects on future cures. Few patients are told that the use of such drugs may completely diminish the body’s ability to fight cancer in the future. Thus, palliative treatments are often used without getting informed consent. If patients understand all medical treatment problems, insufficiently disclosed risks, and numerous flaws in the research model, most patients could not accept palliative treatments. Also, if a cure exists, few patients would accept palliative care.
Limitations of This Study
Due to the exploratory nature, some evidence is approximate. However, the validity of the findings does not depend on data accuracy because the conclusion is not based on percent differences. Most findings are based on orders of magnitude or consistent patterns that have been observed in different settings. The gain from using an optimization method over the randomized trial would be one to more orders of magnitude. Most studies are backed up by multiple reliable findings in cancer research. It is understood that the kinetic data have little utility in population medicine, and cannot be used to make a comparison between one person and another, but is used merely to predict changes within the same person in personalized medicine. Simulation data are used to show growth trends, treatments’ effect patterns, and relative tumor sizes. It is irrefutable that a huge number of factors affect cancer growth rates, and can be used in practice to alter cancer outcomes. Cancer miracles provide further evidence in support of our findings. Since the analysis of research model problems and cancer treatment strategical problems require a new analytic framework, we must explore references outside medical literature. We interpret clinical trials data differently in some use situations.
Conflicts of Interest
None. This study is exploring the limitations in current cancer treatments relative to the rate-based health optimization approach for fighting cancer. It does not promote what can be owned or patented. Upon disclosing this approach, it is for anyone to use. All underlying data are acquired by respective cancer researchers.
- Ashdown ML, Robinson AP, Yatomi-Clarke SL, Ashdown ML, Allison A, et al. (2015) Chemotherapy for Late-Stage Cancer Patients: Meta-Analysis of Complete Response Rates. F1000Res 4: 232.Link: https://bit.ly/3ucAJ9R
- Albero A, Lopéz JE, Torres A, de la Cruz L, Martín T (2016) Effectiveness of chemotherapy in advanced di fferentiated thyroid cancer: a systematic review. Endocr Relat Cancer. 23: R71- R84. Link: https://bit.ly/3CUFXex
- Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, et al. (2017) Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ 359: j4530. Link: https://bit.ly/3wpv2rY
- Morgan G, Ward R, Barton M. (2004) The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin. Oncol. (R. Coll. Radiol.) 16: 549-560. Link: https://bit.ly/3ucBbVB
- Harv Letter. (2008) A second look at beta blockers and blood pressure. A beta blocker alone isn’t usually the best choice for simple, uncomplicated high blood pressure. Harv Heart Lett 18: 4-5.
- Na Z, Qiao X, Hao X, Fan L, Xiao Y, et al. (2018) The effects of beta-blocker use on cancer prognosis: a meta-analysis based on 319,006 patients. Onco Targets Ther 11: 4913-4944. Link: https://bit.ly/3JsSfNH
- Yap A, Lopez-Olivo MA, Dubowitz J, Pratt G, Hiller J, et al. (2018) Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth. 121: 45-57. Link: https://bit.ly/3imcHDR
- Bai ZG, Zhang ZT. (2018) A systematic review and meta-analysis on the effect of angiogenesis blockade for the treatment of gastric cancer. Onco Targets Ther. 11: 7077-7087. Link: https://bit.ly/3qlJnSk
- Li Y, Zhou Y, Hong Y, He M, Wei S,et al. (2019) The Efficacy of Different Chemotherapy Regimens for Advanced Biliary Tract Cancer: A Systematic Review and Network Meta-Analysis. Front Oncol 9: 441. Link: https://bit.ly/3IyPNnG
- Dos Santos MA, Pignon JP, Blanchard P, Lefeuvre D, Levy A, et al. (2015) Systematic review and meta-analysis of phase I/II targeted therapy combined with radiotherapy in patients with glioblastom a multiforme: quality of report, toxicity, and survival. J Neurooncol 123: 307-314. Link: https://bit.ly/3ttdzNA
- Su J, Cai M, Li W, Hou B, He H, et al. (2016) Molecularly Targeted Drugs Plus Radiotherapy and Temozolomide Treatment for Newly Diagnosed Glioblastoma: A Meta-Analysis and Systematic Review. Oncol Res 24: 117-128. Link: https://bit.ly/3CYSJbJ
- Maeda H, Khatami M (2018) Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs, Clin Transl Med 7: 11. Link: https://bit.ly/3CWuV8D
- Wikipedia, cancer (2019).
- American Cancer Society (2019). Early Theories about Cancer Causes.
- Brücher B, Jamall IS (2016) Somatic Mutation Theory - Why it’s Wrong for Most Cancers. Cell Physiol Biochem 38: 1663-1680. Link: https://bit.ly/3JIQVXf
- Nowell PC (1976) The clonal evolution of tumor cell populations. Science. 194: 23-28. Link: https://bit.ly/3tshUAD
- Attolini CS, Michor F (2009) Evolutionary Theory of Cancer. Ann N Y Acad Sci 1168: 23-51. Link: https://bit.ly/3L036ij
- Hanahan D, Weinberg R (2000) The Hallmarks of Cancer. Cell 100: 57-70. Link: https://bit.ly/3wnQbTf
- Hochberg ME, Noble RJ (2017) A framework for how environment contributes to cancer risk. Ecol Lett 20:117-134. Link: https://bit.ly/3ioPCR4
- Lange MM, Rutten HJ, van de Velde CJ (2009) One hundred years of curative surgery for rectal cancer: 1908-2008. Eur J Surg Oncol 35: 456-463. Link: https://bit.ly/3L3PuD0
- Twyman RA. (2009) A brief history of clinical trials. The Human Genome. 2004.
- Collier R (2009) Legumes, lemons and streptomycin: A short history of the clinical trial. CMAJ 180: 23-24. Link: https://bit.ly/3CW2asu
- Au DH, Castro M, Krishnan JA (2007) Selection of Controls in Clinical Trials, Introduction and Conference Summary. Proc Am Thorac Soc 4: 567-569. Link: https://bit.ly/3qpMqJh
- Tournigand C, André T, Achille E, Lledo G, Flesh M, et al. (2004) FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J Clin Oncol 22: 229-237. Link: https://bit.ly/3iq1cLE
- DeVita VTJr, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68: 8643-8653. Link: https://bit.ly/3troMOx
- Chang SC, Liu KH, Hung CY, Tsai CY, Hsu JT, et al. (2018) Adjuvant Chemotherapy Improves Survival in Stage III Gastric Cancer after D2 Surgery. J Cancer 9: 81-91. Link: https://bit.ly/3N4zkLl
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, et al. (2018) Adjuvant chemotherapy guided by a 21- gene expression assay in breast cancer. N Engl J Med 379: 111- 121. Link: https://bit.ly/3JtOYxA
- Gee AW, Balogh E, Patlak M, Nass SJ (reportors) (2017) The Drug Development Paradigm in Oncology: Proceedings of a Workshop. National Academies of Sciences; National Cancer Policy Forum. Washington (DC): National Academies Press (US). Link: https://bit.ly/3N4zuCr
- Verkooijen HM, Fioretta GM, Rapiti E, Bonnefoi H, Vlastos G, et al. (2005) Patients’ Refusal of Surgery Strongly Impairs Breast Cancer Survival. Ann Surg 242: 276-280. Link: https://bit.ly/3wpOCo8
- Tubiana M (1988) Repopulation in human tumors. A biological background for fractionation in radiotherapy. Acta Oncol 27: 83-88. Link:
https://bit.ly/3CWw5Rx - Tubiana M (1989) Tumor Cell Proliferation Kinetics and Tumor Growth Rate. Acta Oncol 28: 113-121. Link: https://bit.ly/3N6cz9K
- Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P (2007) Specific Growth Rate versus Doubling Time for Quantitative Characterization of Tumor Growth Rate. Cancer Res 67: 3970-3975. Link: https://bit.ly/3tqQk6E
- Baum M, Demicheli R, Hrushesky W, Retsky M. (2005) Does surgery unfavourably perturb the natural history of early breast cancer by accelerating the appearance of distant metastases? European J Cancer 41: 508- 515. Link: https://bit.ly/37Rf7sp
- Ahles TA, Root JC, Ryan EL. (2012) Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol 30: 3675-3686. Link: https://bit.ly/3L3oh2W
- Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, et al. (2016) Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J Clin Oncol 34: 927-935. Link: https://bit.ly/3CXOSf3
- Cheng L, Swartz MD, Zhao H, Kapadia AS, Lai D, et al. (2012) Hazard of recurrence among women after primary breast cancer treatment-A 10-year follow-up using data from SEER-medicare. Cancer Epidemiol. Biomarkers Prev 21: 800-809. Link: https://bit.ly/3ipvFtq
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, et al. (2008) Systemic spread is an early step in breast cancer. Cancer Cell 13: 58-68. Link: https://bit.ly/3N9YRTq
- Krall JA, Ferenc R, Mercury OA, Pattabiraman DR, Brooks MW, et al. (2018) The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med 10: eaan3464. Link: https://bit.ly/369swvp
- Demicheli R, Retsky MW, Hrushesky WJ, Baum M (2007) Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat Clin Pract Oncol 4: 699-710. Link: https://bit.ly/3DcljH3
- Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P (2010) Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev 29: 243-248. Link: https://bit.ly/3N5jyQd
- Tohme S, Simmons RL, Tsung A (2017) Surgery for Cancer: a trigger for metastases. Cancer Res 77: 1548-1552. Link: https://bit.ly/352XcxO
- Armitage P, Doll R (1954) The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer 8: 1-12. Link: https://bit.ly/3uh6IWu
- Chen CP, Weinberg VK, Jahan TM, Jablons DM, Yom SS, et al. (2011) Implications of Delayed Initiation of Radiotherapy Accelerated Repopulation after Induction Chemotherapy for Stage III Non-small Cell Lung Cancer. J Thorac Oncol 6: 1857-1864. Link: https://bit.ly/3CWfX2c
- Drewinko B, Alexanian R, Boyer H, Barlogie B, Rubinow SI, et al. (1981) The growth fraction of human myeloma cells. Blood 57: 333-338. Link: https://bit.ly/3Jyjg2b
- Cupit-Link MC, Kirkland JL, Ness KK, Armstrong GT, Tchkonia T, et al. (2017) Biology of premature ageing in survivors of cancer. ESMO Open. Link: https://bit.ly/3NaTP91
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153: 1194-1217. Link: https://bit.ly/3ubWwP7
- Navarro SL, Kantor ED, Song X, Milne GL, Lampe JW, et al. (2016) Factors associated with multiple biomarkers of systemic inflammation. Cancer Epidemiol Biomarkers Prev 25: 521-531. Link: https://bit.ly/3L1SqA0
- Segerstrom SC, Miller GE (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130: 601-630. Link: https://bit.ly/37RgNSJ
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, et al. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70: 7042-7052. Link: https://bit.ly/3ipx7Mo
- Moreno-Smith M, Lutgendorf SK, Sood AK (2010) Impact of stress on cancer metastasis. Future Oncol 6: 1863-1881. Link: https://bit.ly/3trDdlO
- Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, et al. (2005) Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol 23: 7105-7113. Link: https://bit.ly/3tqMPNx
- Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, et al. (2011) Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun 25: 250-255. Link: https://bit.ly/3qo7boC
- Booth FW, Roberts CK, Laye MJ (2012) Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143-1211. Link: https://bit.ly/3N6HEdt
- Carlsson S, Andersson T, Lichtenstein P, Michaëlsson K, Ahlbom A, (2007) Physical activity and mortality: is the association explained by genetic selection? Am J Epidemiol 166: 255-259. Link: https://bit.ly/3Jumyn0
- Cormie P, Zopf EM, Zhang X, Schmitz KH (2017) The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev 39: 71-92. Link: https://bit.ly/3JtJK4Y
- Des Guetz G, Uzzan B, Bouillet T, Nicolas P, Chouahnia K, et al. (2013) Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract 340851. Link: https://bit.ly/3Js9Uox
- Friedenreich CM, Neilson HK, Farris MS, Courneya KS (2016) Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res 22: 4766-4775. Link: https://bit.ly/350g8NE
- Ibrahim EM, Al-Homaidh A (2011) Physical activity and survival after breast cancer dia gnosis: meta-analysis of published studies. Med Oncol 28: 753-765. Link: https://bit.ly/37MsLwN
- Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. (2013) Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer 133: 1905-1913. Link: https://bit.ly/34Wq1vB
- Lahart IM, Metsios GS, Nevill AM, Carmichael AR (2015) Physical activity, risk of death and recurr SCR (JW), v1.04, revision 45 A preprint 1520 1525 1530 1535 1540 1545 ence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol 54: 635-654. Link: https://bit.ly/3Is0kRh
- Li T, Wei S, Shi Y, Pang S, Qin Q, et al. (2016) The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 50: 339-345. Link: https://bit.ly/3N7DbqY
- O’Keefe JH, Vogel R, Lavie CJ, Cordain L (2010) Achieving Hunter-gatherer Fitness in the 21(st) Century: Back to the Future. Am J Med 123: 1082-1086. Link: https://bit.ly/3KZOL5t
- Otto SJ, Korfage IJ, Polinder S, van der Heide A, de Vries E, et al. (2015) Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Support Care Cancer 23: 1237-1250. Link: https://bit.ly/3L1fP4i
- Schmid D, Leitzmann MF (2014) Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 25: 1293-1311. Link: https://bit.ly/3qmZK0V
- Tipton CM (2014) The history of “Exercise Is Medicine” in ancient civilizations. Adv Physiol Educ 38: 109-117. Link: https://bit.ly/3Is0ojZ
- Wu W, Guo F, Ye J, Li Y, Shi D, et al. (2016) Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: a systematic review and meta-analysis. Oncotarget 7: 52095-52103. Link: https://bit.ly/3JzApZy
- Zhong S, Jiang T, Ma T, Zhang X, Tang J, et al. (2014) Association between physical activity and mortality in breast cancer: a meta-analysis of cohort studies. Eur J Epidemiol 29: 391-404. Link: https://bit.ly/3KYd6bY
- Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J (1972) Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 136: 261-276. Link: https://bit.ly/36B45H2
- Donaldson MS (2004) Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr J 3: 19. Link: https://bit.ly/3JumCTM
- Housman G, Byler S, Heerboth SH, Lapinska K, Longacre M (2014) Drug Resistance in Cancer: An Overview. Cancers 6: 1769-1792. Link:
https://bit.ly/3qlBPyS - Wu J, Zha P (2021) Immune Functional Capability Dynamics Determine Disease Outcomes as in Cancer, Heart Diseases, COVID-19, Etc. Link: https://bit.ly/3L3Lz8T
- Wu J, Zha P. (2019) Randomized Clinical Trial Is Biased and Invalid In Studying Chronic Diseases, Compared with Multiple Factors Optimization Trial. Link: https://bit.ly/3JyiceH
- Bortz WMT, Bortz WM 2nd (1996) How fast do we age? Exercise performance over time as a biomarker. J Gerontol A Biol Sci Med Sci 51: M223-M225. Link: https://bit.ly/3qn07Zn
- Goldspink DF (2005) Ageing and activity: Their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics 48: 1334-1351. Link: https://bit.ly/3wkxuA0
- Sehl ME, Yates FE (2001) Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci 56: B198-B208. Link: https://bit.ly/3wlrTJN
- Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, et al. (2017) Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev 75:405-419. Link: https://bit.ly/3qlv1RW
- Stratton MR (2011) Exploring the genomes of cancer cells: progress and promise. Science 331: 1553-1558. Link: https://bit.ly/3JuBS3d
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care 33: 1674- 1685. Link: https://bit.ly/369c2Dx
- Bailon-Moscoso N, Cevallos-Solorzano G, Romero-Benavides JC, Orellana MIR (2017) Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr Genomics 18: 106-131. Link: https://bit.ly/3JuCu90
- Sagar SM, Yance D, Wong RK (2006) Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1, Current Oncol 13:14-26. Link: https://bit.ly/36cX4w8
- Millimouno FM, Dong J, Yang L, Li J, Li X (2014) Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev Res 7: 1081-107. Link: https://bit.ly/3CVM6aa
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293: 2479-2486. Link: https://bit.ly/3D1vKwL
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, et al. (2006) Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 24: 3535-3541. Link: https://bit.ly/3N7DfHe
- Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, et al. (2011) Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res 71: 3889- 3895. Link: https://bit.ly/3IkT6yA
- Sternfeld B, Weltzien E, Quesenberry CP, Castillo AL, Kwan M, et al. (2009) Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE Study. Cancer Epidemiol Biomarkers Prev 18: 87-95. Link: https://bit.ly/3JmkLjX
- Levine EM, Robins EB (1970) Differential temperature sensitivity of normal and cancer cells in culture. J Cellular Physiology 76: 373-379. Link: https://bit.ly/3qpxlHF
- Yeung PK, Wong JT (2003) Inhibition of cell proliferation by mechanical agitation involves transient cell cycle arrest at G1 phase in dinoflagellates. Protoplasma 220: 173-178. Link: https://bit.ly/3qiNa32
- Wikipedia, List of unproven and disproven cancer treatments. Link: https://bit.ly/36y4rya
- Brigden ML (1995) Unproven (questionable) cancer therapies. West J Med 63: 463-469. Link: https://bit.ly/3L3LeTF
- Hoffman KE, Niu J, Shen Y, Jiang J, Davis JW, et al. (2014) Physician Variation in Management of Low-Risk Prostate Cancer: A Population-Based Cohort Study. JAMA Intern Med 174: 1450-14599. Link: https://bit.ly/3tpGSAk
- Draisma G, Etzioni R, Tsodikov A, Wever E, Mariotto A, et al. (2009) Lead time and overdiagnosis in prostatespecific antigen screening: importance of methods and context. J National Cancer Institute 101: 374-383. Link: https://bit.ly/3wnApI1
- Welch HG, Black WC (2010) Overdiagnosis in cancer. J Natl Cancer Inst 102: 605-613. Link: https://bit.ly/3KZfvDd
- Wang TL, Rago C, Silliman N, Ptak J, Markowitz S, et al. (2002) Prevalence of somatic alterations in the colo rectal cancer cell genome. Proc Natl Acad Sci USA 99: 3076-3080. Link: https://bit.ly/3N4F5IR
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T ,et al. (2007) The genomic landscapes of human breast and colorectal cancers. Science 318: 1108-1113. Link: https://bit.ly/3CZ0T43
- Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, et al. (2008) Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA 105: 4283-4288. Link: https://bit.ly/3wlruaf
- Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dorman SCR (JW), v1.04, revision 48 A preprint 1595 1600 1605 1610 1615 cy. Nat Rev Cancer 7: 834-846. Link: https://bit.ly/3L1Aohi
- Greaves M, Maley CC (2012) Clonal evolution in cancer. Nature 481: 306-313. Link: https://bit.ly/3KUOC3a
- Naumov GN, Akslen LA, Folkman J (2006) Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5: 1779-1787. Link: https://bit.ly/37NaVK4
- Radha G, Lopus M (2021) The spontaneous remission of cancer: Current insights and therapeutic significance. Transl Oncol 14: 101166. Link: https://bit.ly/350vLog
- Stemmermann GN (1982) Unsuspected cancer in elderly Hawaiian Japanese: an auto psy study. Hum Pathol 13: 1039-1044. Link:
https://bit.ly/3IlqyVL - Jahn JL, Giovannucci EL, Stampfer MJ (2015) The high prevalence of undiagnosed pro state cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int J Cancer 137: 2795-2802. Link: https://bit.ly/3JrsLjO
- Matsuyama R, Reddy S, Smith TJ (2006) Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 24: 3490-3496. Link: https://bit.ly/34WsAhe
- Pfeil TA, Laryionava K, Reiter-Theil S, Hiddemann W, Winkler EC (2015) What Keeps Oncologists From Addres sing Palliative Care Early on With Incurable Cancer Patients? An Active Stance Seems Key. Oncologist 20: 56-61. Link: https://bit.ly/36fF7wX
- Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS (1988) Cancer patients’ perceptions of their disease and its treatment. Br J Cancer 58: 355-358. Link: https://bit.ly/3KYdrvg
- Beadle GF, Yates PM, Najman JM, Clavarino A, Thomson D, et al. (2004) Beliefs and practices of patients with advanced cancer: implications for communication. Br J Cancer 91: 254- 257. Link: https://bit.ly/3D0ezLX
- Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK,et al. (2013) Cancer risk in 680000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346: f2360. Link: https://bit.ly/36claXK
- Zondervan RL, Hahn PF, Sadow CA, Liu B, Lee SI, et al. (2013) Body CT scanning in young adults: examination indications, patient outcomes, and risk of radiation-induced cancer. Radiology 267: 460-469. Link: https://bit.ly/352EqGQ
- Zhou Y, Li C, Huijbregts MAJ, Mumtaz MM (2015) Carcinogenic Air Toxics Exposure and Their Cancer-Related Health Impacts in the United States. PLoS One 10: e014003. Link: https://bit.ly/3qlXcQZ

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
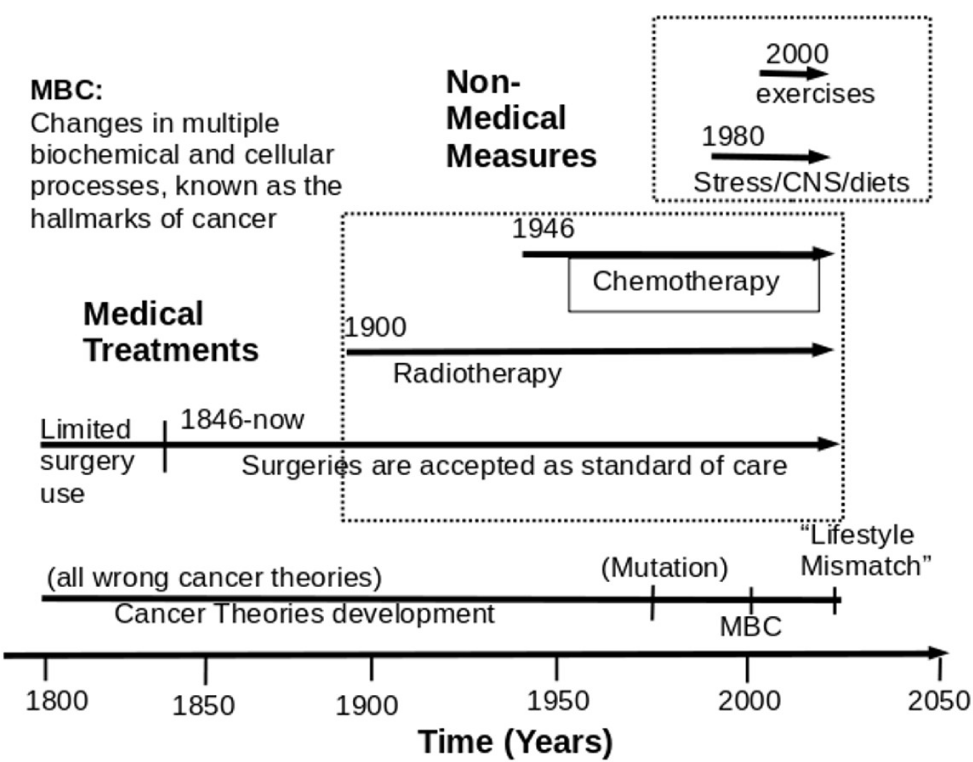
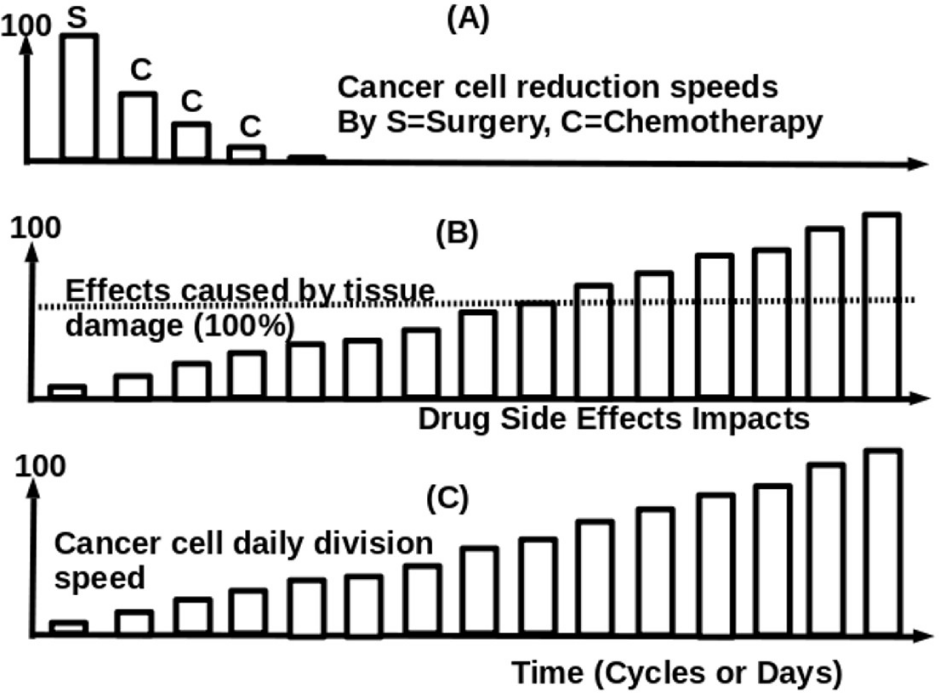

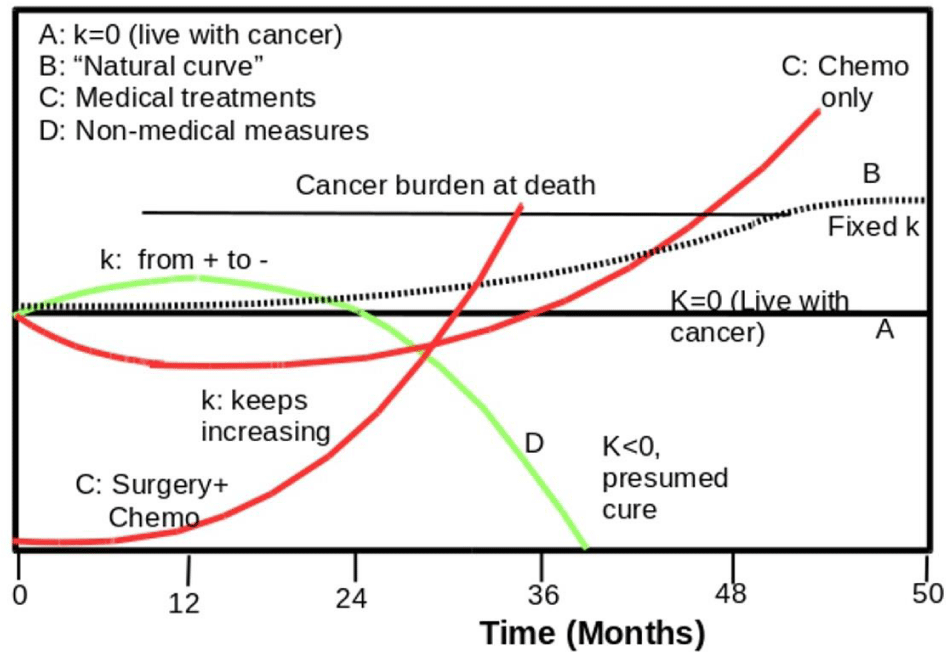
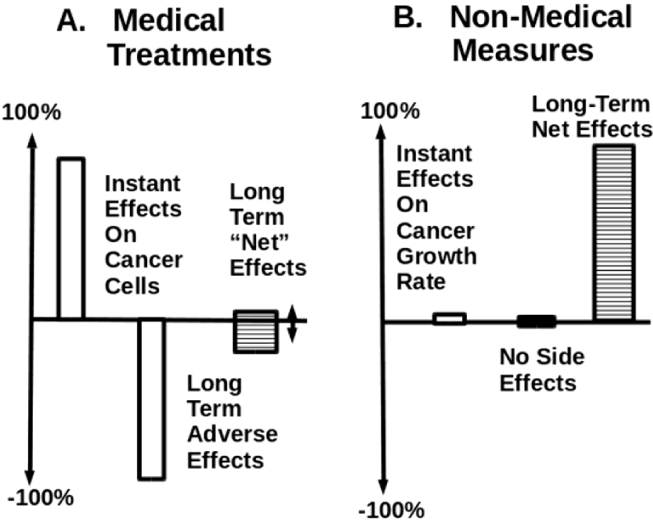
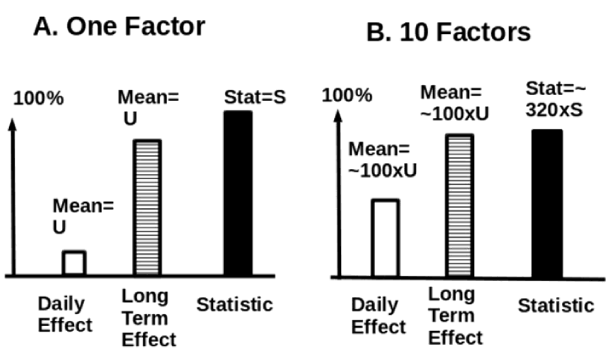
 Save to Mendeley
Save to Mendeley
