Global Journal of Cancer Therapy
Influence of Intra-ductal Carcinoma on Clinical Outcomes in Men with Prostate Cancer: Systematic Review and Meta-analysis
Jatinder Kumar*, Muhammad Umar Alam, Karthik Tanneru, Shiva Gautam, Daniel Norez, Charu Shastri, Joseph Costa, Mark Bandyk, Hariharan Palayapalayam Ganapathi, Shahriar Koochekpour, Sanjeev Shukla and KC Balaji
Cite this as
Kumar J, Alam MU, Tanneru K, Gautam S, Norez D, et al. (2020) Influence of Intra-ductal Carcinoma on Clinical Outcomes in Men with Prostate Cancer: Systematic Review and Meta-analysis. Glob J Cancer Ther 6(1): 032-037. DOI: 10.17352/2581-5407.000033Purpose: To provide a comprehensive summary of published literature regarding influence of intraductal carcinoma of prostate (IDC-P) on clinical outcomes in men with Prostate Cancer (PC).
Methods: We compared the following clinical endpoints in men with PC with or without IDC-P; Castration Resistant Free Survival (CFS) and Overall Survival (OS) for metastatic PC (Group 1), biochemical recurrence rate (BR) and/or cancer specific survival (CSS) in men undergoing radical prostatectomy (Group 2a) or radiotherapy (Group 2b). A meta-analysis was done by fixed effect model using 12 studies reporting Hazard Ratio (HR) and meeting the selection criteria.
Results: In Group 1 for men with IDC-P, the pooled HR for CFS and OS were 1.69 (CI, 1.30-2.21) and 2.00 (CI, 1.38-2.91), respectively. In group 2a BR and CSS were higher in men with IDC-P with HR 2.63 (CI, 1.99-3.49) and 2.87 (CI, 1.65-5.01) respectively. Similarly, presence of IDC-P in group 2b demonstrated a HR of 2.04 (CI, 1.10-3.78) for BR.
Conclusions: Men with IDC-P demonstrated poorer clinical outcomes including higher rate of BR following radical prostatectomy, radiation therapy either in primary and salvage settings, shorter time to CFS and poorer OS in men with metastatic disease. Our analysis and review of the literature suggest that IDC-P could be used as a novel prognostic and predictive morphological biomarker to influence clinical management in men with PC including pelvic lymph node dissection, pelvic radiotherapy or genetic testing.
Introduction
Intraductal carcinoma of prostate (IDC-P) is morphologically described as prostatic adenocarcinoma extending into and proliferating within preexisting prostatic ducts. After initial morphological description by Kovi et al in 1985, many pathological studies have been done to reach a fairly established morphology based diagnostic criteria to classify IDC-P on histopathology of surgical specimen and needle biopsy [1]. The available studies have shown that identification of IDC-P in prostate cancer tissue to be an independent variable in the prediction of pathological stage, tumor volume, Gleason score and treatment failure [2-4]. This has led to the recommendation for mandatory reporting of IDC-P by the College of American Pathology in 2017 [5]. In the PSA screening era, the incidence of IDC-P is approximately 20% [6,7], which is mostly reported in men with high volume, high grade and advanced adenocarcinoma of the prostate.
The molecular and genetic features of IDC-P are well described in the literature and there are periodic publications on clinical outcomes. However, because there is no systematic review or meta-analysis on the influence of IDC-P on clinical outcomes in men with prostate cancer, we carried out the analysis. The study demonstrates that IDC-P is a poor prognostic factor in men undergoing various treatments for prostate cancer irrespective of clinical stage. The presence of IDC-P is an adverse prognostic factor for Biochemical Recurrence (BR) in men undergoing radical prostatectomy, adjuvant or salvage prostatectomy. In patients with metastatic prostate cancer, presence of IDC-P portends earlier progression to Castration Resistant Prostate Cancer (CRPC). In men treated for CRPC, the presence of IDC-P predicts poorer Overall Survival (OS) and Cancer Specific Survival (CSS). Our study summarizes the adverse impact of IDC-P on clinical outcomes in men undergoing treatment for prostate cancer and could be potentially used as a prognostic and predictive morphological biomarker to influence management in men with prostate cancer.
Material and methods
A systematic PubMed/Medline and Cochrane Library search was conducted in July 2019. No filter for date, language or region was used for the searches. Cited references from selected studies were also retrieved and reviewed. All authors participated in the design of the search strategy and inclusion criteria. Our procedure for evaluating records identified during the literature search followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria. Separate searches were done by two independent reviewers for groups 1 and 2, and groups 2a and 2b. Search results were reviewed to assess the articles with the highest level of evidence available. The final list of included articles was selected with the consensus of all collaborating authors, verifying that they met the inclusion criteria.
We defined study eligibility using the patient population, intervention, outcome, study design approach and selected end points. Studies considered for this review were classified into two groups : Group1: patients with metastatic prostate cancer with IDC-P found on prostate biopsy, reported hazard ratio (HR) with 95% CI and reported castrate resistance free survival (CFS) or OS, Group 2a: patients with localized prostate cancer with IDC-P on biopsy treated by radical prostatectomy (RP), used HR with 95% CI and BR or CSS, and Group 2b: patients with localized prostate cancer treated by radiotherapy (RT) with IDC-P found on biopsy, used HR as point estimate with 95% CI and progression free survival (PFS) or CSS as the end points. All study designs were accepted except for case reports. In addition, review articles, meeting abstracts, editorials, and studies with ≤10 participant commentaries were excluded. If multiple studies reporting on the same or overlapping series met our inclusion criteria, the most recent study was selected.
Identification of eligible trials
Our search identified 295 manuscripts as on July 17, 2019. Of these, 16 full texts were screened for eligibility based on use of HR as point estimate. Out of these 8 articles were accepted for final analysis. Two studies reported the effect of IDC-P on the prognosis of metastatic prostate cancer (Group 1) and evaluated CFS and OS. Four studies with radical prostatectomy (group 2a) and reported BR and CSS. Two studies reported PFS/CSS as end points on patients who underwent radiotherapy (group 2b). Of note, only the studies with HR as measure of point estimate were included in this meta-analysis and systematic review. None of the studies were randomized controlled trials (RCT), two were prospective studies and all the others were retrospective analysis.
Statistical methods
In this systematic review of previously conducted studies on IDC-P, several information including numerical data from each study are collated and presented in Table 1. Even though the studies included were not uniform in their designs and were few in numbers, we attempted to pool similar information from each study to determine if they collectively provide additional insight than a single study. In order to achieve this, a meta-analytic approach was used to combine the results. These results are presented through Forest plots. Because of the inherent heterogeneity in the study designs and rather small number of studies, the results need to be interpreted with caution. However, despite these shortcomings such analysis may help draw visual and numerical conclusions to find whether all the studies are implying a similar effect. These analyses were performed using STATA statistical software (Release 15. College Station, TX: StataCorp LLC.). The results are presented along with the Forest plots. The I2 value for all the results indicated homogeneity (0-50%) and are not shown with the plots.
Results
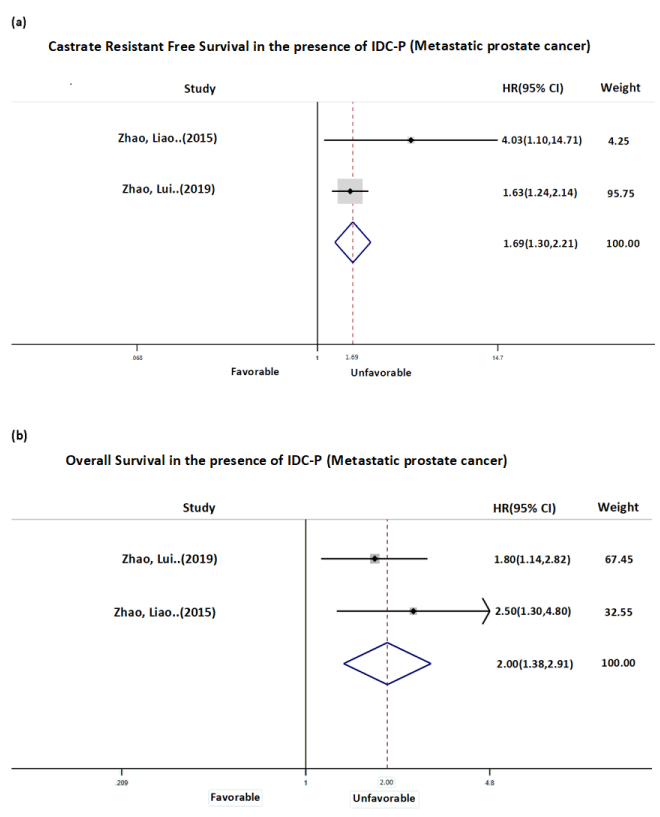
Analysis of CFS in Group 1 shows the presence of metastatic IDC-P predicts poorer prognosis. The pooled HR (Figure 1a) of the two studies with CFS as the outcome of interest was 1.69 (CI, 1.30-2.21). Obviously, this result is heavily weighted by the sample size of Zhao, et.al. (2019) study (n=644). The median time to progression to CRPC was shortened in men with IDC-P compared to men without by 13.07 and 6.8 months in each of the two studies. Overall survival is the other outcome of interest measured in Group 1 (Figure 1b). When IDCP is present, patients with metastatic disease predict shorter survival time as represented by a HR 2.00 (CI, 1.38-2.91). The decrease in median overall survival in men with IDC-P compared to men without were 28.2 and 13.95 months in each of the two studies.
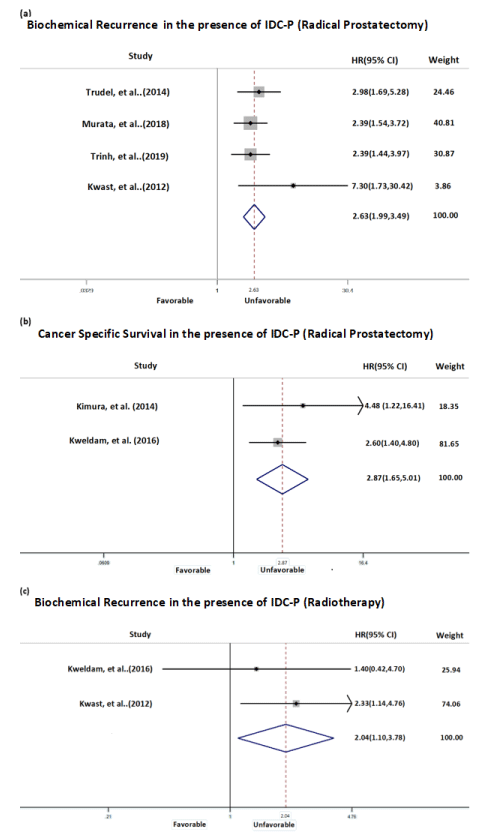
In Group 2a consisting of men with localized prostate cancer with IDC-P in biopsy and undergoing RP, the BR and CSS were used. Each study reporting BR predicted shorter time to biochemical failure among those with or without IDC-P resulting in a HR of 2.63 (CI, 1.99-3.49) (Figure 2a). Furthermore, all studies demonstrate a similar magnitude of effect size represented by plots on the same side of the no difference line. The second outcome of interest, CSS in Group 2a, which showed the presence of IDC-P predicted shorter survival time represented by HR 2.87 (CI, 1.65-5.01) (Figure 2b). The Group 2b consists of localized prostate cancer with IDC-P found on prostate biopsy and treated with radiotherapy. One study reported PFS as endpoint with HR of 2.33 (CI, 1.14-4.76) and the second study reported CSS as end point with HR of 1.4 (CI, 0.42-4.7). The pooled HR of these outcomes was 2.04 (CI, 1.10-3.78) suggesting the poorer outcome for those with IDC-P present (Figure 2c).
Discussion
As the adverse clinical outcomes based on the presence of IDC-P in primary prostate cancer is established, the revised guidelines on pathology reporting recommend standard reporting of IDC-P [8-11]. Pathologists broadly refer to two biologically distinct diseases. Whereas the rarely encountered pure IDC-P is a precursor of prostate cancer, IDC-P associated with invasive carcinoma (IDC-P-inv) generally represents a growth pattern of invasive prostatic adenocarcinoma [12,13]. While the prognostic significance of IDC-P-inv is apparent, pure IDC-P may be a precursor for cancer and further clarification is needed regarding re-biopsy of these men [14].
Reporting of IDC-P in a biopsy report could directly impact clinical decision making. A study retrospectively reviewed 110 men with pelvic lymph node metastasis identified either IDC-P or cribriform pattern in the prostate in 94% of men with > 1 pelvic lymph node metastasis. Another study evaluating copy-number alteration analysis on 34 morphologically distinct tissue areas in one prostatectomy specimen that the lymph node metastasis in the same patient was from a foci of IDC-P, further confirming that IDC-P may be a sentinel event contributing to metastatic disease [15]. Fewer patients are undergoing routine pelvic lymph node dissection at the time of radical prostatectomy because lymph nodal metastases at the time of radical prostatectomy are infrequent, extended lymph node dissection significantly increases perioperative complications and overall survival benefit is not well established [16-19]. Because there is consensus that IDC-P-inv is an established poor prognostic and these patients are more likely to harbor metastatic disease, a lymph node dissection at the time of radical prostatectomy or inclusion of pelvic lymph nodes at radiotherapy planning may be considered.
Magnetic Resonance Imaging (MRI) is being increasingly used in diagnosis, staging and management of men with prostate cancer. While 20% of clinically significant prostate cancer are thought to be invisible on MRI, the remaining visible tumors are enriched in hallmarks of nimbosus, an aggressive pathological, molecular, and micro-environmental phenomenon in prostate cancer, which includes IDC-P [20,21]. MRI screening for prostate cancer has demonstrated a higher detection rate of clinically significant and high grade prostate cancer [22]. It is conceivable that several of the men with clinically significant disease harbor IDC-P, which could have contributed to the detection of prostate cancer.
In the era of precision medicine, identification of germline and somatic mutations can influence treatment decisions in management of men with prostate cancer. Of several genetic dysregulations associated with prostate cancer, mutations in DNA mismatch repair genes, which is found in > 10% of men with metastatic prostate cancer is currently used to identify men to be treated with poly ADP ribose polymerase (PARP) inhibitors [23]. Among the DNA damage repair genes, BRCA 2 mutations is the most frequent and patients who harbor germline BRCA 2 mutations have worse clinical outcomes than non-carriers when treated with surgery or radiotherapy [24]. Interestingly, BRCA2-mutant tumors commonly show the concurrent presence of IDC-P and subclonal analyses demonstrate that IDC-P and invasive adenocarcinoma in BRCA2-mutant tumors can arise from the same ancestral clone [24]. Genomic and methylomic profiling of localized prostate cancer from 14 carriers of deleterious germline BRCA2 mutations demonstrated dysregulation of the MED12L/MED12 axis, which is frequently dysregulated in metastatic castration-resistant prostate cancer [25]. This dysregulation was enriched in BRCA2-mutant prostate cancer harboring IDC-P. Microdissection and sequencing of IDC and juxtaposed adjacent non-IDC invasive carcinoma in 10 patients demonstrates a common ancestor to both histopathology [25]. The data suggests that IDC-P may signify field disease and therefore patients with IDC-P may benefit from definitive whole gland therapy. As a corollary, the presence of IDC-P could be used as an indication for genetic testing.
Pathology studies have shown that IDC-P persists in localized prostate cancers after Androgen Deprivation Therapy (ADT) and/or chemotherapy, maintaining its characteristic morphological features and, in some cases, increasing in prevalence. This has led to speculation that IDC-P may be inherently resistant to current therapies. The incidence and extent of IDC-P is however difficult to assess in matched pre-and post-treatment specimens because of difficulties in precisely resampling the same tumor region. Therefore, it is unclear whether existing IDC-P lesions persist after treatment or are selected by treatment [26-28]. A study that generated patient derived xenograft mouse models from seven patients with IDC-P demonstrated that castration led to persistence of IDC-P cells in the tumor compared to acinar cancer cells, which suggests that IDC-P cells could be inherently castration resistant [29]. The finding provides a plausible biological basis for shorter progression time to CRPC in men with metastatic prostate cancer and IDC-P present.
During the literature analysis for this study, we identified certain limitations. There are no randomized or prospective studies evaluating the role of IDC-P in management of men with prostate cancer. Several of the studies included in the analysis have small sample sizes and may be underpowered to assess the actual effect sizes. Nevertheless, the findings that IDC-P is a poor prognostic marker in men undergoing treatment for prostate cancer is consistent across all studies, which strongly supports the conclusions.
Conclusions
IDC-P is an established poor prognostic pathological feature in men diagnosed with prostate cancer. There is a comparatively significantly higher treatment failure rates for localized and metastatic disease in men with IDC-P. IDC-P could be used as an indication of active intervention in patients who may otherwise be considered for surveillance. In addition, presence of IDC-P may be used as an indication for pelvic lymph node dissection at the time of radical prostatectomy, inclusion of pelvic field during prostate radiotherapy or prompt genetic testing in men with high risks or metastatic prostate cancer. In fact, IDC-P could be used as a novel prognostic and predictive morphological biomarker to influence management in men with prostate cancer.
Ethical committee
This research involves already published data. The UF health ethics committee has confirmed that no ethical approval is required.
- Kovi J, Jackson MA, Heshmat MY (1985) Ductal spread in prostatic carcinoma. Cancer 56: 1566-1573. Link: https://bit.ly/3lUtqxI
- Cohen RJ, McNeal JE, Baillie T (2000) Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate 43: 11–19. Link: https://bit.ly/3k3CEYj
- Rubin MA, de La Taille A, Bagiella E, Olsson CA, O'Toole KM (1998) Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol 22: 840–848. Link: https://bit.ly/2SVWkBg
- Dawkins HJ, Selner LN, Turbett GR, Thompson CA, Redmond SL, et al. (2000) Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate 44: 265-270. Link: https://bit.ly/3nYFRKM
- Srigley JR, Zhou M, Allan R (2017) Protocol for the examination of specimens from patients with carcinoma of the prostate gland v4.0.0.0. College of American Pathologists.
- Humphrey PA (2015) Intraductal carcinoma of the prostate. J Urol 194: 1434-1435.
- Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM (1998) Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol 29: 1119-1123. Link: https://bit.ly/2H9uA9O
- Kench JG, Judge M, Delahunt B, Humphrey PA, Kristiansen G, et al. (2019) Dataset for the reporting of prostate carcinoma in radical prostatectomy specimens: updated recommendations from the International Collaboration on Cancer Reporting. Virchows Arch 475: 263-277. Link: https://bit.ly/37bxzcM
- Kato M, Hirakawa A, Kobayashi YM, Yamamoto A, Ishida R, et al. (2019) The influence of the presence of intraductal carcinoma of the prostate on the grade group system's prognostic performance. Prostate 79: 1065-1070. Link: https://bit.ly/3j1nDon
- Sakamoto N, Ueda S, Mizoguchi H, Kawahara I, Kobayashi T, et al. (2017) Significance of Intraductal Carcinoma of the Prostate in Post-Operative Biochemical Recurrence. Nihon Hinyokika Gakkai Zasshi 108: 5-11. Link: https://bit.ly/2T17apA
- Dinerman BF, Khani F, Golan R, Bernstein AN, Cosiano MF, et al. (2017) Population-based study of the incidence and survival for intraductal carcinoma of the prostate. Urol Oncol 35: 673 e679-673 e614. Link: https://bit.ly/350pRQ1
- Varma M, Delahunt B, Egevad L, Samaratunga H, Kristiansen G (2019) Intraductal carcinoma of the prostate: a critical re-appraisal. Virchows Arch 474: 525-534. Link: https://bit.ly/2T5pC0d
- Epstein JI (2009) Precursor lesions to prostatic adenocarcinoma. Virchows Arch 454: 1-16. Link: https://bit.ly/2T1p0cd
- Downes MR, Xu B, van der Kwast TH (2019) Gleason grade patterns in nodal metastasis and corresponding prostatectomy specimens: impact on patient outcome. Histopathology 73: 715-722. Link: https://bit.ly/2IBVxUj
- Lindberg J, Kristiansen A, Wiklund P, Gronberg H, Egevad L (2015) Tracking the origin of metastatic prostate cancer. Eur Urol 67: 819-822. Link: https://bit.ly/31dP4Fw
- Dillioglugil JF, Leibman BD, Leibman NS, Kattan MW, Rosas AL, et al. (1997) Risk factors for complications and morbidity after radical retropubic prostatectomy. J Urol 157: 1760-1767. Link: https://bit.ly/3j59TsC
- Meng MV, Carroll PR (2000) When is pelvic lymph node dissection necessary before radical prostatectomy? A decision analysis. J Urol 164: 1235-1240. Link: https://bit.ly/3lO6mRt
- El-Galley RES, Keane TE, Petros JA, Clarke HS, Cotsonis GA, et al. (1998) Evaluation of staging lymphadenectomy in prostate cancer. Urology 52: 663-667. Link: https://bit.ly/342ToJB
- Salomon L, Hoznek A, Lefrere-Belda MA, Chopin DK, Abbou CC (2000) Non dissection of pelvic lymph nodes does not influence the results of perineal radical prostatectomy in selected patients. Eur Urol 37: 297-300. Link: https://bit.ly/2IsOc9k
- Houlahan KE, Salmasi A, Sadun TY, Pooli A, Felker ER, et al. (2019) Molecular Hallmarks of Multiparametric Magnetic Resonance Imaging Visibility in Prostate Cancer. Eur Urol 76: 18-23. Link: https://bit.ly/318K9FB
- Chua MLK, Lo W, Pintilie M, Murgic J, Lalonde E, et al. (2017) A Prostate Cancer "Nimbosus": Genomic Instability and SChLAP1 Dysregulation Underpin Aggression of Intraductal and Cribriform Subpathologies. Eur Urol 72: 665-674. Link: https://bit.ly/2H6O0fN
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, et al. (2018) MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. NEJM 378: 1767-1777. Link: https://bit.ly/2FwlBii
- Mateo J, Cheng HH, Beltran H, Dolling D, Xu W, et al. (2018) Clinical Outcome of Prostate Cancer Patients with Germline DNA Repair Mutations: Retrospective Analysis from an International Study. Eur Urol 73: 687-693. Link: https://bit.ly/3j3MiZF
- Taylor RA, Fraser M, Rebello RJ, Boutros PC, Murphy DG, et al. (2019) The influence of BRCA2 mutation on localized prostate cancer. Nat Rev Urol 16: 281-290. Link: https://go.nature.com/3dvALB2
- Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, et al. (2017) Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nature Communications 8: 13671. Link: https://go.nature.com/37bb9Iz
- Chen Z, Chen N, Shen P, Gong J, Li X, et al. (2015) The presence and clinical implication of intraductal carcinoma of prostate in metastatic castration resistant prostate cancer. Prostate 75: 1247-1254. Link: https://bit.ly/3dI3h2F
- Efstathiou E, Abrahams NA, Tibbs RF, Wang X, Pettaway CA, et al. (2010) Morphologic characterization of preoperatively treated prostate cancer: toward a post‐therapy histologic classification. Eur Urol 57: 1030-1038. Link: https://bit.ly/3dD1xHJ
- O'Brien C, True LD, Higano CS, Rademacher BLS, Garzotto M, et al. (2010) Histologic changes associated with neoadjuvant chemotherapy are predictive of nodal metastases in patients with high‐risk prostate cancer. Am J Clin Pathol 133: 654-661. Link: https://bit.ly/3lPdmgU
- Porter LH, Hashimoto K, Lawrence MG, Pezaro C, Clouston D, et al. (2018) Intraductal carcinoma of the prostate can evade androgen-deprivation,with emergence of castrate tolerant cells. BJU Int 121: 971-978. Link: https://bit.ly/3k72xGv
- Zhao T, Liao B, Yao J, Liu J, Huang R, et al. (2015) Is there any prognostic impact of intraductal carcinoma of prostate in initial diagnosed aggressively metastatic prostate cancer? Prostate 75: 225-232. Link: https://bit.ly/3dxR8x0
- Zhao J, Liu J, Sun G, Zhang M, Chen J, et al. (2019) The Prognostic Value of the Proportion and Architectural Patterns of Intraductal Carcinoma of the Prostate in Patients with De Novo Metastatic Prostate Cancer. J Urol 201: 759-768. Link: https://bit.ly/3dD0ibx
- Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, et al. (2014) Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer 50: 1610-1616. Link: https://bit.ly/3o6sOr0
- Murata Y, Tatsugami K, Yoshikawa M, Hamaguchi M, Yamada S, et al. (2018) Predictive factors of biochemical recurrence after radical prostatectomy for high-risk prostate cancer. Int J Urol 25: 284-289. Link: https://bit.ly/2IEmAyp
- Trinh VQ, Benzerdjeb N, Chagnon-Monarque S, Dionne N, Delouya G, et al. (2019) Retrospective study on the benefit of adjuvant radiotherapy in men with intraductal carcinoma of prostate. Radiat Oncol 14: 60. Link: https://bit.ly/31c03z0
- Van der Kwast T, Al Daoud N, Collette L, Thoms J, Sykes J, et al. (2012) Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer 48: 1318-1325. Link: https://bit.ly/37cGcnu
- Kimura K, Tsuzuki T, Kato M, Saito AM, Sassa N, et al. (2014) Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate 74: 680-687. Link: https://bit.ly/2SYMdeZ
- Kweldam CF, Kümmerlin IP, Nieboer D, Verhoef EI, Incrocci L, et al. (2016) Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma. Eur J Cancer 66: 26-33. Link: https://bit.ly/318HpYP

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
