Global Journal of Cancer Therapy
Synthesis of Some Aryl Ketoxime Derivatives with their in vitro Anti-microbial and Cytotoxic Activity
Oguzhan Karaosmanoglu1,2, Burcu Butun3*, Hakan Dal4, Hulya Sivas1 and Kadriye Benkli3
2Department of Biology, Kamil Özdağ Science Faculty, Karamanoglu Mehmetbey University, 70100, Karaman, Turkey
3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Bezmialem Vakif University, 34093, Istanbul, Turkey
4Department of Chemistry, Faculty of Science, Eskisehir Technical University University, 26400, Eskisehir, Turkey
Cite this as
Karaosmanoglu O, Butun B, Dal H, Sivas H, Benkli K (2019) Synthesis of Some Aryl Ketoxime Derivatives with their in vitro Anti-microbial and Cytotoxic Activity. Glob J Cancer Ther 5(1): 001-006. DOI: 10.17352/gjct.000023Benzofuwran derivatives found in several natural compounds and synthesized for various purposes. Due to their molecular structure’s electron behaviors they have several biological activities such as antitumor, cytotoxic, anticancer, antimicrobial, antifungal, ant proliferative etc. We synthesized (3-methyl-benzofuran-2-yl) ketoxime derivatives (one of them are new compound) and structure elucidation of the compounds was performed using IR, 1H-NMR, MASS spectroscopy and elemental analysis. X-Ray analysis of H2 compound was elucidated for the first time with this study in the literature. Cytotoxic activity against F2408 and HepG2 cell lines was also evaluated for the first time by MTT and NR uptake methods. Anti-microbial activity of H1, H2 and H3 was also investigated with broth micro dilution test. The results show that these ketoximes have cytotoxic and anti-microbial activity on different higher doses.
Graphical abstract
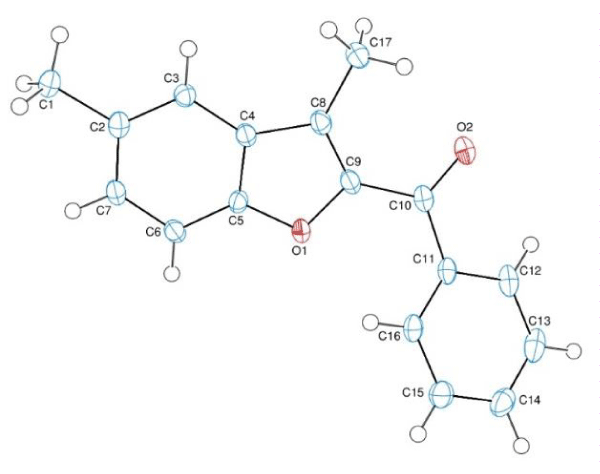
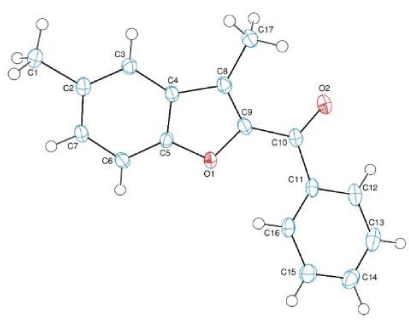
X-ray structure of compound H2: Benzofuran derivatives found in several natural compounds and synthesized for various purposes. Due to their molecular structure’s electrone behaviors they have several biological activities such as antitumor, cytotoxic, anticancer, antimicrobial, antifungal, antiproliferative etc. We synthesized (3-methyl-benzofuran-2-yl) ketoxime derivatives and elucidated their structures with several spectroscopic methods. One of the compounds which was synthesized for the first time in the literature, was given with its x-Ray analysis as shown above. We also investigated their antimicrobial activity and cytotoxicity.
Introduction
Benzofuran is a heterocyclic compound which formed by a fused benzene and furan ring. Like other heterocyclic structures, benzofurans have several pharmacological effects due to their scaffolds. Their derivatives have attracted attention in last years. They are found in various natural sources or synthesized for different purposes. Moreover compounds that contains benzofuran heterocyclic earned some features such as solubility, salt formation, absorption and bioavailability [1]. They play key role in design and synthesis of new pharmacologically active compounds. Even some medicinal plants earned pharmacological effect due to benzofuran cores. Primarily they have several biological activities such as antitumor, cytotoxic, anticancer [2], antimicrobial [3], antifungal [4], antiproliferative [5], inhibition of angiogenesis [6].
Cancer is the most dangerous life-threatening disease that cause mortality with a big proportion in all over the world [7]. Previous studies claimed that number of cancer cases will increase by 2050 and reach a peak with 16 million, so that it is very important to understand the mechanism of cancer types. They have several complex mechanisms [8]. For example, in a study two different derivative series of benzofurans were synthesized. They tried to understand relationship between the benzene, hydroxy and methhoxy fragments on 4- and 5-positions and antiproliferative activity. They wanted to search the effects of electron donating groups on antiproliferative activity. They discovered the best activity provides by the methoxy group on para position of the benzoyl moiety. Meta position was not ideal for the tubulin polimerization. In addition the best antiproliferative activity was showed by (5-hydroxy-4-phenylbenzofuran-2-yl) (4-methoxyphenyl) methanone derivative (3d), which was activate sub-micromolar concentrations against Molt/4, CEM and HeLa cancer cell lines [9].
In addition to side effects of the cancer drugs is drug resistance to cancer therapy transience [10]. Unconscious usage of antitumors and antibiotics cause to suppression of the immune system. Infection diseases are increasing with the improvement of mutagenicity due to bacteria’s resistant to drugs also [11]. This leads to the need for new antimicrobial agents that antibiotics do not resist. It is necessary to discover/design new antimicrobial agents and find practical/economical ways to synthesis bioactive heterocyclic moieties such as benzofurans. There is many research on antimicrobial potential on benzofuran derivatives which show promising results. For example in a study, researchers studied on a series of different bacteria and showed benzofuran pyrazol derivatives have high antimicrobial activity against nearly all tested organisms [12]. Antifungal studies on benzofurans also gave satisfactory results to different pathogenic fungi. A series of benzofuran-triazoles derivatives were studied against fluconazole-resistant Trichophyton rubrum and Cryptococcus neoformans and found as having in vitro antifungal activity [13]. Benzofuran ketoxime analogues were also studied with docking studies as antifungal potency. They found ketoxime moiety and at least one hydrogen bound between enzyme and molecule directly increases the activity [4].
In this study new aryl (3-methyl-benzofuran-2-yl) ketones were synthesized and identified with nuclear magnetic resonance (NMR), infrared spectroscopy (IR), mass spectroscopy (MS) and X-ray analysis. Cytotoxicity and anti-microbial potential of this benzofuran derivatives were investigated.
Materials and Methods
Experimental
Chemistry: Chemicals and solvents were obtained from Sigma–Aldrich and E. Merck (Darmstadt, Germany). The synthetic route of compounds is outlined in Scheme 1. Synthesis of arylketoximes were started with suitable 2′-hydroxyacetophenone (5 mmol), 2-bromoacetophenone (5 mmol) and potassium carbonate (6 mmol) were refluxed in acetonitrile for 4 hours. After reflux the reaction was cooled and the solvent was evaporated. The residue was washed with water and crystallized from ethanol. The reactions and synthesized molecules were monitored by thin layer chromatography (TLC) using Merck precoated silica gel plates.
Synthesis: 2′-hydroxyacetophenone (5 mmol), 2-bromoacetophenone (5 mmol) and potassium carbonate (6 mmol) used as starting materials to synthesized Aryl (3-methyl-benzofuran-2-yl) ketones at shown as Scheme 2. They were refluxed in acetonitrile for 4 hours. The reaction was controlled with thin layer chromatography. When the starter materials were run out and the product occurred, the reaction mixture was cooled. The solvent was evaporated and the raw product was filtered. The residue was washed with water and crystallized from ethanol [14-16].
Biology
Cell Culture: A human hepatocellular carcinoma cell line, HepG2 (ATCC® 77400™) and a rat embryo fibroblast cell line, F2408 (JRCB) were used in the study. The cells were maintained in DMEM supplemented with 10% FBS (Sigma) and 1% penicillin-streptomycin (PAA) at 37 0C under 5% CO2. Cells were harvested and passaged using 0.025 % trypsin/EDTA.
The neutral red uptake assay: F2408 and HepG2 cells were seeded 5,000 cells/well and 10,000 cells/well in 96-well plates respectively. After grown for 24 hours, treated with certain concentrations of the H1, H2 and H3 compounds. The stock solutions, 100 mM, were prepared by dissolving the compounds with sterile distilled water. In order to obtain 10-fold decreasing concentrations (1000-0,001 µM) of the test compounds proper dilutions were applied with DMEM. A positive control which was treated with any test agent and a negative control in which F2408 cells treated with 10 µM and HepG2 cells was treated with 100 µM cisplatin was maintained. The neutral red uptake (NRU) assay was performed as previously described [17]. The NR stock solution was prepared in sterile distilled water with the concentration of 3, 3 mg/ml and was filtered. At the end of the treatment periods (24 h, 48 h and 72 h) NR working solution with the concentration of % 1 was prepared with DMEM, 250 µl working solution was added to each well. After incubation period which is 3 h at 37 0C 100 µl desorb solution (glacial acetic acid: ethanol: distiled water 1:49:50) was added to each well. After 15 min incubation, 96-well plate was read by ELISA reader (Biotech ELx 808 Ultra microplate reader) at 540 nm wavelength. By that way, the cell viability was determined in terms of absorbance values. Then it was converted to % viability with the following formula:
% viability = (test-blank) / (negative control-blank)*100.
Three independent experiments was done by that way.
The MTT assay
F2408 and HepG2 cells were seeded 5,000 cells/well and 10,000 cells/well in 96-well plates respectively. After grown for 24 hours, treated with certain concentrations of the H1, H2 and H3 compounds. The stock solutions, 100 mM, were prepared by dissolving the compounds with sterile distile water. In order to obtain 10-fold decreasing concentrations (1000-0,001 µM) of the test compounds proper dilutions were applied with DMEM. A positive control which was treated with any test agent and a negative control in which F2408 cells treated with 10 µM and HepG2 cells was treated with 100 µM cisplatin was maintained. The MTT viability assay was performed as previously described [18], with little changes. The MTT stock solution was prepared in PBS with the concentration of 5 mg/ml and was filtered. At the end of the treatment periods (24 h, 48 h and 72 h) MTT working solution with the concentration of 1 mg/ml was prepared with the DMEM, 125 µl of working solution was added to each well. After incubation period which is 3 h at 37 0C 100 µl DMSO was added to each well. After 15 min incubation, 96-well plate was read by ELISA reader (Biotech ELx 808 Ultramicroplate reader) at 570 nm wavelength. By that way, the cell viability was determined in terms of absorbance values. Then it was converted to % viability with the following formula.
% viability = (test-blank) / (negative control-blank)*100.
Three independent experiments were done by that way.
Anti-microbial activity
Anti-microbial activity of the compounds was further determined by Broth Micro dilution (BM) method as previously described [19]. Briefly, stock solutions of H1, H2 and H3 were diluted to achieve serial decreasing dilutions ranging from 500 to 0.8 µg/ml and transferred to 96-well microtitre plates. Seven microorganisms, Bacillus subtilis (NRS 744), Pseudomonas aeroginosa (LMG 6395), Staphylococcus aureus (NRRL B-767), Salmonella typhimurium (NRRL B-4420), Escherichia coli (ATCC 25922), Saccharomyces cerevisiae (NRRL Y-12632), and Candida utilis (NRRL Y-900) were grown overnight. A 100 ml of each microorganism suspension was transferred into the wells containing media and sterile distilled water. Inoculum was used for positive control and chloramphenicol for reference negative control. The minimal inhibitory concentration (MIC) values were determined after incubation at 37 0C for 18-24 h. The MIC values were determined as the lowest compound concentration where absence of growth was recorded. Each test was repeated at least twice with triplicate for all microorganisms.
Results
Chemistry
Melting points were determined by using an Electrothermal 9100 digital melting point apparatus and were uncorrected. Spectroscopic data were recorded on the following instrument, IR: Schimadzu 435 IR spectrophotometer. 1H-NMR: Bruker DPX 400 NMR spectrometer in DMSO-d6 using TMS as internal standard. MS: VG Platform Mass spectrometer. Analysis for C, H, N were within 0.4% of the theoretical values.
Structure elucidation
As expected, the presence of the derivatives was confirmed by a thin layer chromatography and NMR spectral data. In the IR spectra C=C and C=N stretching bands, characteristic for all the compounds were obtained at 1510–1616 cm−1 region. Ketone’s C=O bands were observed at 1638–1647 regions. All the protons resonated as expected in the NMR spectra. Aliphatic protons resonated in two groups for methyl 2.12 and 2.15, methoxy 3.77 and 3.80 and methylene 5.28 and 5.42 ppm regions, respectively.
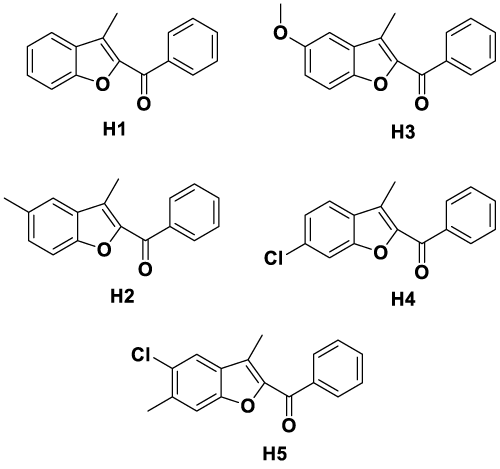
H1 (3-methylbenzofuran-2-yl) (phenyl) methanone: M.p. 236.30 0C. IR (KBr) υmax (cm−1): 1645 (C=O), 1647–1564 (C=C).
1H-NMR (400 MHz) (DMSO-d6) δ (ppm): 2.58 (3H, s, CH3), 7.39–7.88 (7H, m, Ar-H), 8.01–8.04 (2H, m, Ar-H). ES-MS: m/z: 237 (M+1).
H2 (3,5-dimethylbenzofuran-2-yl) (phenyl) methanone: M.p. 250.30 0C. IR (KBr) υmax (cm−1): 1643 (C=O), 1640–1552 (C=C).
1H-NMR (400 MHz) (DMSO-d6) δ (ppm): 2.45 (3H, s, CH3), 2.54 (3H, s, Ar-CH3), 7.37–7.69 (6H, m, Ar-H), 7.96–7.99 (2H, m, Ar-H). ES-MS: m/z: 251 (M+1).
H3 (5-methoxy-3-methylbenzofuran-2-yl) (phenyl) methanone: M.p. 266.45 0C. IR (KBr) υmax (cm−1): 1647 (C=O), 1651–1550 (C=C).
1H-NMR (400 MHz) (DMSO-d6) δ (ppm): 2.45 (3H, s, CH3), 3.84 (3H, s, Ar-OCH3), 7.55–8.23 (8H, m, Ar-H). ES-MS: m/z: 267 (M+1).
H4 (6-chloro-3-methylbenzofuran-2-yl) (phenyl) methanone: M.p. 270.95 0C. IR (KBr) υmax (cm−1): 1645 (C=O), 1648–1565 (C=C).
1H-NMR (400 MHz) (DMSO-d6) δ (ppm): 2.65 (3H, s, CH3), 7.49–8.16 (8H, m, Ar-H). ES-MS: m/z: 271 (M+1).
H5 (5-chloro-3, 6-dimethylbenzofuran-2-yl) (phenyl) methanone: M.p. 330.40 0C. IR (KBr) υmax (cm−1): 1641 (C=O), 1638–1550 (C=C).
1H-NMR (400 MHz) (DMSO-d6) δ (ppm): 2.45 (3H, s, CH3), 2.54 (3H, s, Ar-CH3), 7.47–8.09 (7H, m, Ar-H). ES-MS: m/z: 285 (M+1).
X-ray crystallography
The red coloured crystals of the title compound was crystallized from chloroform at room temperature. The crystallographic data are given in table 1 and the selected bond lengths and angles are listed in table 2. Crystallographic data were recorded on a Bruker Kappa APEXII CCD area-detector diffractometer using Mo Kα radiation (λ=0.71073 Å) at T=108(2) K. Absorption correction by multi-scan was applied. Structure was solved by direct methods and refined by full-matrix least squares against F2 using all data [20]. All non-H atoms were refined anisotropically. The C-bound H-atoms were positioned geometrically with C---H = 0.93 and 0.96 Å for aromatic and methyl H-atoms, respectively, and constrained to ride on their parent atoms, with Uiso (H) = k x Ueq(C), where k = 1.5 for methyl H-atoms and k = 1.2 for aromatic H-atoms.
Crystal structure
In the molecule of the title compound (Scheme 3), the bond lengths and angles (Table 2) are generally within normal ranges. The compound contains one benzofuran [A (O1/C2-C9)] and one benzene [B (C11-C16)] rings, where ring A is approximately planar with a maximum deviation of -0.018(2) Å (for atom C8). Its mean plane is oriented with respect to ring at a dihedral angle of 41.22(6)°. Atoms C1, C10 and C17 are 0.0015[24], -0.0237[24] and -0.0346(25) Å away from the benzofuran ring plane, respectively [20,21].
Biology
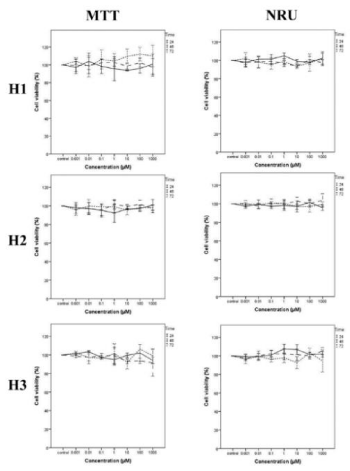
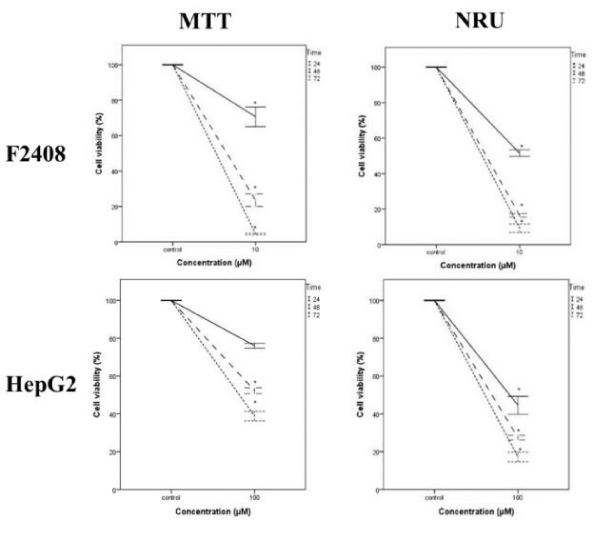
A serial concentration of H1, H2 and H3 compounds was tested against seven standard microorganisms and they were not exhibited antimicrobial activities up to 500 µg/ml concentration whereas chloramphenicol as a positive control inhibited the microbial growth. MTT and NRU cytotoxicity assays showed that H1, H2 and H3 compounds were not cytotoxic on both cell lines as normal fibroblast F2408 cells (Scheme 4) and hepatocarcinoma HepG2 cells (Scheme 5) up to 1000 µM tested. On the other hand, cisplatin [22], which is an anticancer drug used as a positive control [23], showed very strong cytotoxicity on the both cell types (Scheme 6) at 100 µM concentration and for all periods of time tested.
Discussion
In this work, we have synthesized molecules named as H1, H2 and H3, completely purified, elucidated and subjected to X-ray analysis. Further the molecules were tested for their bioactivity using anti-microbial and cytotoxicity assays. As a results of activity tests, H1, H2 and H3 compounds was not able to inhibit the microbial growth even at 500 ug/ml concentration whereas chloramphenicol inhibited the microbial growth. Consistent with the anti-microbial activity, H1, H2 and H3 did not exhibited any cytotoxic activity for mammalian cell lines even at higher doses.
It has been reported that some benzofuran (bearing 2-methylimidazole or 2-ethylimidazole ring and substitution of the imidazolyl-3-position with a naphthylacyl or methoxyphenacyl group) and benzofuran-2-carboxamide derivatives (especially bearing benzo [b] furan, in particular, 2-imidazolynyl substituted compound) were found to have cytotoxic activity in vitro against a panel of human tumor cell lines [24,25,4]. Telvekar et al. [4], have been performed a 3-D QSAR analysis and docking studies on synthesized benzofuran ketoxime analogues by Benkli et al. [16], and provided useful information about designing of new benzofuran for their bioactivities such as antifungal agent. They suggested that the hydrogen bonding between the hydroxyl group of ketoxime of benzofuran, hydrophobic interaction between phenyl of benzofuran core, and phenyl ring attached to carbon of ketoxime are important to bind amino acids. Therefore, the reason our molecules being ineffective may be due to the absence of these type of functional groups in the molecules that allows interaction with amino acids or other macromolecules.
In our next studies, different derivatives of our compounds such as oxime esters, acetyl and benzoyl compounds will be synthesized and tested. They are thought to have key role to discover more potent molecules.
We thank to Prof. Dr. Mustafa Yamac Eskisehir from Osmangazi University who kindly provided us test microorganisms and facilities in his laboratory. We also thank to Anadolu University Faculty of Pharmacy and Bezmialem Vakıf University Faculty of Pharmacy for their support. We are grateful to Scientific Research Projects Commission of Anadolu University, Eskisehir, Turkey for the funding grant 1507F563 (to H.S and O.K.). The authors gratefully acknowledge a financial support of this work by TUBITAK (the Scientific and Technological Research Council of Turkey), carried out in the frame of a project (Project No:113Z694).
- Leeson PD, Springthorpe B (2007) The influence of drug-like concepts on decision-making in medicinal chemistry. Nature Reviews Drug Discovery 6: 881-890. Link: https://goo.gl/dPjyFC
- Abd El-Karim SS, Anwar MM, Mohamed NA, Nasr T, Elseginy SA (2015) Design, synthesis, biological evaluation and molecular docking studies of novel benzofuran-pyrazole derivatives as anticancer agents. Bioorganic Chemistry 63: 1-12. Link: https://goo.gl/z9Jsef
- Kossakowski J, Ostrowska K, Struga M, Stefanska J (2009) Synthesis of new derivatives of 2,2-dimethyl-2,3-dihydro-7-benzo[b]furanol with potential antimicrobial activity. Medicinal Chemistry Research 18: 555-565. Link: https://goo.gl/UuJks1
- Telvekar VN, Kundaikar HS, Patel KN, Chaudhari HK (2008) 3-D QSAR and Molecular Docking Studies on Aryl Benzofuran-2-yl Ketoxime Derivatives as Candida albicans N-myristoyl transferase Inhibitors. Qsar Comb Sci 27: 1193-1203. Link: https://goo.gl/gfqAqR
- Yang L, Lei H, Mi CG, Liu H, Zhou T, et al. (2011) Synthesis, antiproliferative activities and in vitro biological evaluation of novel benzofuransulfonamide derivatives. Bioorg Med Chem Lett 21: 5389-5392. Link: https://goo.gl/3hjm77
- Chen Y, Chen SP, Lu X, Cheng H, Oua YY, et al. (2009) Synthesis, discovery and preliminary SAR study of benzofuran derivatives as angiogenesis inhibitors. Bioorg Med Chem Lett 19: 1851-1854. Link: https://goo.gl/16KReX
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, CA Cancer J Clin 64: 9-29. Link: https://goo.gl/6L8n1r
- Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2: 533-543. Link: https://goo.gl/eHhgWw
- Romagnoli R, Baraldi PG, Sarkar T, Cara CL, Lopez OC, et al. (2008) Synthesis and Biological Evaluation of 2-aroyl-4-phenyl-5-hydroxybenzofurans as a New Class of Antitubulin Agents. Med Chem 4: 558-564. Link: https://goo.gl/sU36jQ
- Radadiya A, Shah A (2015) Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. Eur J Med Chem 97: 356-376. Link: https://goo.gl/WDjixu
- He Y, Wu BG, Yang J, Robinson D, Risen L, et al. (2003) 2-Piperidin-4-yl-benzimidazoles with broad spectrum antibacterial activities. Bioorg Med Chem Lett 13: 3253-3256. Link: https://goo.gl/GfwLU2
- Abdel-Wahab BF, Abdel-Latif E, Mohamed HA, Awad GEA (2012) Design and synthesis of new 4-pyrazolin-3-yl-1,2,3-triazoles and 1,2,3-triazol-4-yl-pyrazolin-1-ylthiazoles as potential antimicrobial agents. Eur J Med Chem 52: 263-268. Link: https://goo.gl/Lqab4E
- Liang Z, Xu H, Tian Y, Guo MB, Su X, et al. (2016) Design, Synthesis and Antifungal Activity of Novel Benzofuran-Triazole Hybrids. Molecules 21. Link: https://goo.gl/XrRuKc
- Serrano-Wu MH, St Laurent DR, Mazzucco CE, Stickle TM, Barrett JF, et al. (2002) Oxime derivatives of sordaricin as potent antifungal agents. Bioorg Med Chem Lett 12: 943-946. Link: https://goo.gl/iLS1op
- Demirayak S, Ucucu U, Benkli K, Gundogdu-Karaburun N, Karaburun AC, et al. (2002) Synthesis and antifungal activities of some aryl(benzofuran-2-yl) ketoximes. Farmaco 57: 609-612. Link: https://goo.gl/Ty6Yr2
- Benkli K, Gundogdu-Karaburun N, Karaburun AC, Ucucu U, Demirayak S (2003) Synthesis and antifungal activities of some aryl (3-methyl-benzofuran-2-yl) ketoximes. Arch Pharm Res 26: 202-206. Link: https://goo.gl/tU1SrM
- Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3: 1125-1131. Link: https://goo.gl/v4hP5F
- Mosmann T (1983) Rapid Colorimetric Assay for Cellular Growth and Survival - Application to Proliferation and Cyto-Toxicity Assays. J Immunol Methods 65: 55-63. Link: https://goo.gl/TLMYYT
- Karci F, Sener N, Yamac M, Sener I, Demircali A (2009) The synthesis, antimicrobial activity and absorption characteristics of some novel heterocyclic disazo dyes. Dyes Pigments 80: 47-52. Link: https://goo.gl/z13UJc
- Bruker A (2012) Inc: Madison. Wisconsin, USA.
- Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64: 112-122. Link: https://goo.gl/5YxPMN
- Durgo K, Kostic S, Gradiski K, Komes D, Osmak M, et al. (2011) Genotoxıc effects of green tea extract on human laryngeal carcınoma cells in vıtro. Arh Hig Rada Toksikol 62: 139-146. Link: https://goo.gl/S3Vy4T
- Zhang R, Niu YJ, Zhou YK (2010) Increase the cisplatin cytotoxicity and cisplatin-induced DNA damage in HepG2 cells by XRCC1 abrogation related mechanisms. Toxicol Lett 192: 108-114. Link: https://goo.gl/YiJHno
- Wan WC, Chen W, Liu LX, Li Y, Yang LJ, et al. (2014) Synthesis and cytotoxic activity of novel hybrid compounds between 2-alkylbenzofuran and imidazole, Med Chem Res 23: 1599-1611. Link: https://goo.gl/rjBHht
- Hranjec M, Sovic I, Ratkaj I, Pavlovic G, Ilic N, et al. (2013) Antiproliferative potency of novel benzofuran-2- carboxamides on tumour cell lines: cell death mechanisms and determination of crystal structure, Eur. J Med Chem 59: 111-119. Link: https://goo.gl/e962KL

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
