Global Journal of Biotechnology and Biomaterial Science
Mitigation with plant ethanol extracts of STZ-induced histopathological injuries in the tissues of laboratory rats
Hrachik Gasparyan1, Sona Buloyan1, Luiza Karapetyan2, Hayk Harutyunyan2, Alvard Antonyan2, Svetlana Sharoyan2 and Sona Mardanyan2
2H. Buniatian Institute of Biochemistry of Armenian NAS, Armenian
Cite this as
Gasparyan H, Buloyan S, Karapetyan L, Harutyunyan H, Mardanyan S, et al. (2020) Mitigation with plant ethanol extracts of STZ-induced histopathological injuries in the tissues of laboratory rats. Glob J Biotechnol Biomater Sci 6(1): 001-006. DOI: 10.17352/gjbbs.000011This work studied the use of several plant extracts as probable medications in the treatment of diabetes. In laboratory rats, the diabetes model was developed by injection of Streptozotocin (STZ) in a dose of 40 mg/kg of body weight. The animals, in which the blood glucose level increased 4-5 times, were considered as diabetic. The diabetic animals were treated by ethanol extracts of plants traditionally used in folk medicine and/or as food in Armenian cuisine. During four weeks, the STZ-induced diabetic rats were administered orally three times in a week with the ethanol extracts from sorrel leaves (SL, Rumex Confertus), pellicles of walnut kernel (WP, Juglans regia), grape leaves (GL, Vitis vinifera) and rose petals (RP, Rosa damascena). At the end of the experiment, certain decrease of blood glucose level was registered in these groups. An oxidative stress marker, malondialdehyde, which increased in the blood of the STZ-induced diabetic animals by 38%, decreased in the SL and WP groups to the level of the intact animals. The histopathological examination of the pancreas, kidney and liver sections from the diabetic animals, fed with the extracts from SL, RP and WP, revealed positive changes compared with the STZ-control animals. The obtained results manifest the benefit of using the studied herbal extracts in the STZ-induced diabetes model.
Introduction
Diabetes Mellitus (DM) is known to be a metabolic disorder resulting from variable interactions of hereditary and environmental factors. It is characterized by abnormalities in the insulin metabolism with subsequent distortions in carbohydrates, proteins and lipid metabolism. Chronic hyperglycemia activates different cellular pathways associated with tissue damage and development of diabetic complications [1], which are often considered as results of pathogenic effects of reactive oxygen species (ROS) [2].
Medicinal herbs contain diverse bioactive compounds [3-5]. Many of drug substances are derived or developed from plant compounds [6]. For instance, a first line drug for type 2 diabetes metformin is developed on the basis of biguanide from French lilac [7]. The efficacy of many plants as useful agents in the management of hyperglycemia and related complications is evidenced [8,9].
It is known that DM affects the pancreatic exocrine and endocrine systems. The most common cells in islets of Langerhans in endocrine system are β-cells, which produce insulin, the major hormone in regulation of carbohydrate, fat and protein metabolism. The β-cell mass in islets of Langerhans decreases during the development of DM [10,11].
One of the most widespread complications related to DM is diabetic nephropathy. Disturbances in antioxidant defense systems and ROS lead to accumulation of renal disorders, which is usually associated with kidney damage [12,13]. DM can cause chronic kidney diseases, damage both the nodular apparatus and the proximal and distal tubules. Prevention and management of diabetic nephropathy are parts of the comprehensive care of DM patients.
DM can also have serious effects on the liver. The odds of having serious liver problems at DM are almost twice as high as having cardiovascular disease. Several investigations have shown the injurious effects of hyperglycemia on liver in different in vitro and in vivo diabetic models and the amelioration of these effects by natural agents [14]. The agents, which protect the diabetic liver or decrease the severity of its injury, include flavonoids, catechins, various polyphenolic compounds, curcumin derivatives, etc [15]. Hence, it is prospective to continue studying natural compounds, plant compounds in particular, for developing new antidiabetic drugs.
Earlier, inhibition of activities of DM-relating enzymes Dipeptidyl peptidase IV and Adenosine deaminase by aqueous extracts from Armenian Highland plants, was demonstrated [16]. The in vitro and in vivo effects of ethanol extracts from several plants and their constituents on the activities of these enzymes were described [17]. The transmission and scanning electron microscopy study proved the ability of the ethanol extract from rose petals and of its phenole glycoside fraction to hinder the aggregation of the pancreas peptide hormone, amylin, which is one of the causes of developing DM [18]. The in vitro protection of islet β-cells in the presence of cytotoxic aggregated amylin and decrease of its aggregation with the ethanol extracts and fractions from several plants, were demonstrated [19-21]. These results prove the usefulness of herbal preparations in prevention and treatment of DM.
The present work describes the beneficial influence of several plant extracts on the STZ-induced histopathological disturbances in the pancreas, kidneys and liver of rats. The work studied the plants, which are traditionally used in folk medicine and/or as food in Armenian cuisine, but are not studied widely.
Materials and methods
Plants
The leaves of grape (GL, Vitis vinifera) and sorrel (SL, Rumex Confertus), the rose petals (RP, Rosa damascena) and the walnut kernel pellicles (WP, Juglans regia) were collected in the Armenian Highland and dried in shade. A voucher specimen has been deposited in the herbarium of the Botanical Department of Yerevan State University (Dr. Narine Zaqaryan). The ethanol extract preparation and characterization by chemical analyses, thin layer chromatography and UV-Vis absorbance were described earlier [22].
Animals
The male white rats of 150-200 g body weight were taken from vivarium of the Institute of Biochemistry. The rats were housed in one room, on an identical diet, in separate cages, 3-5 animals in each, under normal day and night cycles, at temperature of 23±4°C. They were given free access to standard food and tap water.
Ethical approval
Care and treatment of animals, and the experiments were approved by the Ethics Committee on Animal Use at the Institute of Biochemistry (Protocol number: 8, 25.05.2016), based on the main provisions of the “Appendix A of the European Convention for the protection of Vertebrate animals used for experimental and other scientific purposes (ETS N 123)”.
Creation of diabetes model in laboratory rats
The diabetes model was developed by intraperitoneal injection of streptozotocin (STZ, AppliChem GmbH, Germany, dissolved in citrate buffer, pH 4.5, in a dose of 40 mg/kg of animal) to 150-200 g starving rats. The animals were considered as STZ-induced diabetics, if blood glucose index (measured by glucometer) in 3 days raised from 4.8 ± 0.2 (87 mg/L) to 19.6 ± 1.7 (356 mg/L) – 2 6.5±1.9 (481 mg/L).
Histological investigations
During four weeks, the STZ-induced diabetic rats were given ethanol extracts of the chosen plants three times a week. At the end of the experiment the animals were subjected to instant euthanasia, samples of the pancreas, kidney and liver were fixed in 10% buffered formalin and embedded in paraffin; 3-5 μm sections were prepared, stained with H&E [23] and examined/photographed with Zeiss Jeneval light microscope.
The average surface area of islets of Langerhans was determined using the Axio Vision LE 4.8.2.SP3 software. Digital data were expressed as mean ± standard deviation (s.d.).
Measurement of malondialdehide
The oxidative stress marker, malondialdehyde (MDA) assay mixture in 1.1 ml contained: 100 µl of blood plasma, 750 µl of 2% H2PO4, 250 µl of 0.8% thiobarbituric acid. It was incubated in boiling water bath for 45 min, and the absorbance at 535 nm was registered after cooling [24].
Evaluation of protein carbonylation
The protein carbonylation assay mixture in 400 µl contained: 20 µl of blood plasma, 280 µl H2O, 100 µl of 10 mM 2,4-dinitrophenyl hydrazine in 2 M HCl. After shaking for 15 min, 100 µl of 6 M NaOH was added, the shaking was continued for additional 15 min to form 2,4-dinitrophenyl hydrazone derivatives, and samples were centrifuged at 6000 g for 5 min. Then the absorbance at 450 nm was registered [25].
Data were analyzed using the statistical software InStat, version 3 for Windows (GraphPad Software, Inc., SanDiego, CA, USA). Specific differences were examined using Student’s two–tailed t–test. The data showing P<0.05 were considered as statistically authentic. Results were expressed as mean ± s.e.m.
Results
Treatment of diabetic rats with plant extracts
Usually, in each of 5 independent in vivo experiments, a group of 3-5 of 30 laboratory rats was kept as intact control group. In the other animals, after a 12 hour fast, STZ was injected intraperitoneally in a dose of 40 mg/kg of body weight. The rats were given 5% glucose for the next 24 hours to avoid hypoglycemia. After three days, the development of diabetes was confirmed by measuring glucose in blood samples obtained by cardiopuncture with a heparinized syringe. The STZ-induced diabetic animals, which blood glucose level increased 4-5 times as compared with the intact animals, were divided to groups of 3-5 rats in each. One of these groups was kept as STZ-control. During four weeks, the animals in the rest groups were fed orally three times a week with water suspension of dried ethanol extract from walnut kernel pellicles (WP), Rose petals (RP), leaves of grape (GL) and Sorrel (SL), in a dose of 400 mg/kg of body weight. This dose was chosen as the most suitable after preliminary testing of doses between 150-500 mg/kg.
At the end of four weeks, in the rats with STZ-induced diabetes fed with ethanol extracts from RP, GL, WP and SL, the average values of blood glucose, summarized by all independent experiments (number of animals 8-15) was lower than the value in the STZ-control group by: 7.5%, 18.9%, 27.5%, and 29.8%, respectively.
The mortality of diabetic animals during the experimental period decreased in the order: STZ-control > WP > SL> RP > GL (44%, 30%, 17%, 7.7% and 0% of total number of animals, respectively). The numbers of recovered animals in percentage to the total number of diabetics in these groups were: 5%, 35%, 28%, 15% and 25%, respectively.
Lipid peroxidation and protein carbonylation
Lipid peroxidation is implicated in many diseases, including diabetes. Malondialdehyde (MDA) is recognized as a marker of lipid peroxidation. In our experiments, the level of MDA in the STZ-control group was higher as compared with the intact control group by 38 % ± 1.2. At the end of the experiment, in the groups, fed with extracts from WP and SL, the MDA differed from the STZ-control significantly (p<0.05), approaching to the level in the intact animal group, 110% ± 8.1 and 94% ± 6.6, respectively.
The level of protein carbonylation is one of the indexes of protein damage caused by oxidative stress [26]. In our experiments, contrary to the lipid peroxidation, the difference in the protein carbonylation was not registered in any of experimental groups. In the groups of the STZ-control and those receiving ethanol extracts from SL and WP for four weeks, the level of protein carbonylation was around 90% of the level in the intact control. Presumably, the expression of this damage demands more longitude following the diabetic conditions.
Histopathology of tissues in diabetic rats
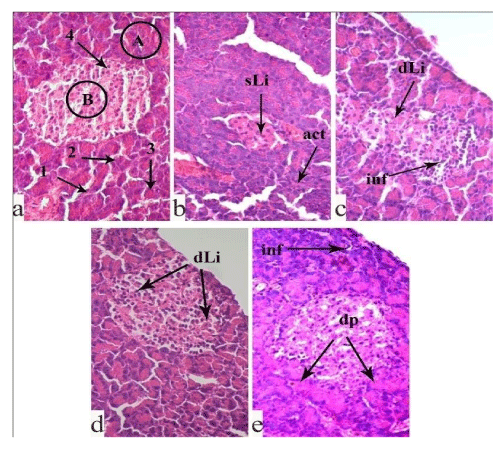
Histopathological characteristics of pancreas in the experimental animals: Normally, the pancreas consists of the exocrine and endocrine parts, of which the former includes pancreatic acinus and excretory ducts, while the latter includes round or oval pale stained islets of Langerhans, interspersed between exocrine acinus. In the islets, the endocrine cells create irregular, branched anastomosing chords, separated by blood capillaries. Interlobular connective tissue septa are clearly defined (Figure 1a).
It is known that STZ is cytotoxic for insulin-producing cells of the islets of Langerhans, for β-cells in particular. In the pancreas of the non-treated, STZ-control animals (Figure 1b), the islets of Langerhans undergo destructive and dystrophic changes. The insulocytes are necrotic, nuclear pyknosis and karyolysis are observed, the cytoplasm is eosinophilic. Some insulocytes and exocrine cells are vacuolated. The interlobular connective tissue septum is almost absent. The acini and lumen of excretory ducts in the exocrine part are narrowed.
In the pancreas of the STZ-diabetic animals fed with SL, vacuolization, pyknosis, and karyolysis of insulocytes are seen (Figure 1c). However, these pathological destructive changes are less prominent compared to those in the STZ-control group.
A relatively different histological picture is observed in the pancreas of the STZ-diabetic animals fed with the ethanol extract from RP (Figure 1d). The histostructure of parenchymal elements and islets of Langerhans are restored. The destructive and dystrophic changes are moderate. The interlobular connective tissue septum is well detected. The lumen of pancreatic acini and excretory ducts is normal. Yet, in the tissue of this group, cells with vacuolated cytoplasm, dark and pyknotic nuclei are seen.
An identical morphological picture is seen in the pancreas of the STZ-diabetic animals fed with the ethanol extract from WP (Figure 1e).The destructive changes are less prominent as compared to the STZ-control group. In the exocrine part of the gland, the histological structure of the pancreatic acini and excretory ducts are mostly preserved. In the endocrine part, the dark pyknotic nuclei are fewer in number, however, insulocytes with vacuolated cytoplasm are registered.
In the pancreas of the non-treated, STZ-control animals, the morphometric analysis showed significant decrease of the area of islets of Langerhans due to the shrinkage of the size and number of the islets (Figure 2).
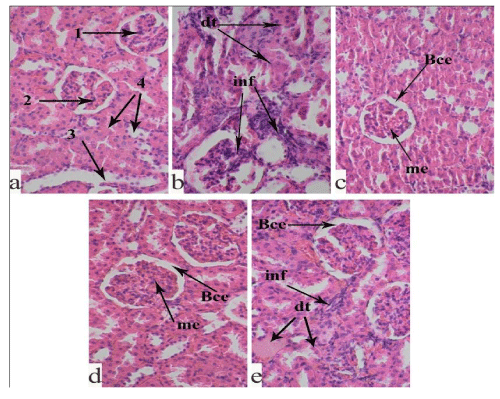
Histopathological characteristics of kidney in the experimental animals: Our histological examination showed normal microscopic structure of the cortex and medulla in the kidney of the intact group. There are no degenerative and inflammatory processes in the proximal and distal tubules (Figure 3a).
In the renal tissue of the STZ-control rats, nephropathy structural changes, characteristic for diabetes, are seen (Figure 3b). In the microscopic fields, many necrotic glomeruli with Bowman’s capsule expansion, as well as thickening in the basement membrane of some glomeruli are observed. Degeneration processes of the proximal and distal tubules with prominent inflammatory infiltration are registered. The end of the distal tubules is markedly expanded. The collecting duct system has an irregular arrangement.
The histological study showed that the abovementioned pathological changes were less in the kidney of the SL fed animals (Figure 3c) compared with the STZ-control group. The renal corpuscles are mostly preserved; the proximal and distal tubules are without visible pathological changes, although in some places, there are inflammatory foci and destructive processes in the parenchyma.
The analysis of the histological picture of the kidney of the RP fed STZ-diabetic animals (Figure 3d) showed relatively less severity of pathological changes compared with the STZ-control group. The histostructure of renal corpuscles and capillary glomeruli are mostly preserved. The destructive and dystrophic processes in the proximal and distal tubules are moderate. The collecting ducts are well-shaped. However, along with this, the epithelial cells are vacuolated in some places. Mesangial expansion of some glomeruli, as well as thickening of the basal membranes are seen.
In the kidney of the STZ-diabetic rats fed with the ethanol extract from WP (Figure 3e), the observed nephropathic changes are moderate. The renal capsules are preserved, but there are foci of destructive processes in the capillary glomeruli. Mesangial expansion of the glomeruli, as well as thickening of the basal membrane are observed. Lymphohistiocytic infiltrations are found in all the histological sections.
Histopathological characteristics of liver in the experimental animals: Along with the increase of the risk of kidney disease, nerve damage, blood vessel damage, infections, blindness and heart disease, diabetes can also have profound effects on the liver. Various pathological changes in the liver can occur, in particular, fatty degeneration, leading to the steatosis and probability of developing liver cirrhosis.
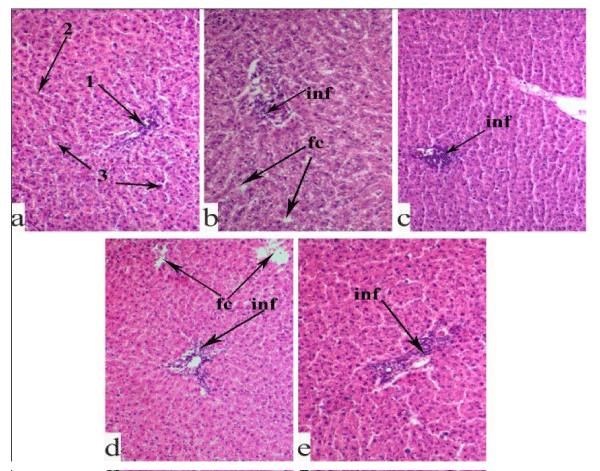
In intact rats, we observe a normal histological structure of the liver tissue (Figure 4a); around the central vein, hepatocytes are arranged in cords. There are no pathological abnormalities in the portal tracts and periportal zones. No inflammatory processes are identified; the sinusoidal capillaries are normal.
In the histological structure of the liver tissue of the STZ-induced diabetic rats (Figure 4b), fatty degeneration, destructive processes of parenchymal elements, pyknosis and karyolysis of hepatocytes are revealed. In the portal triad, a phenomenon of hyperplasia and perivascular infiltration and, in some places, tissue fibrosis are seen.
In the liver tissue of the STZ-diabetic animals, fed with the extract from SL (Figure 4c), the destructive and dystrophic changes are less than in the STZ control animals. The cords of hepatocytes are mostly preserved; there are much fewer foci of fatty changes and pyknosis of hepatocyte. At the same time, in the parenchyma, the sinusoidal capillaries are dilated; hyperplasia of the bile ducts and perivascular infiltration in the portal triads are seen.
A similar morphological picture is recorded in the liver of the RP fed STZ-diabetic animals (Figure 4d). Moreover, along with the above pathological changes, the parenchyma of the liver lobules is destroyed; in the intercellular spaces of hepatocytes, bile appears.
In WP fed animals (Figure 4e), dilation of sinusoidal capillaries was observed. In the portal triads, hyperplasia, lymphohistocytic infiltrations and early fibrosis are registered.
Discussion
In the present work we performed the in vivo study on STZ-induced diabetes model of laboratory rats. Feeding the diabetic animals with extracts from SL, WP, GL and RP decreased the blood glucose level only by 7%-30% of the STZ-controls, remaining essentially higher than the level in the intact controls. It is noteworthy that a higher rate of glucose improvement was registered in the animals of the WP and SL groups. Worth noting, that at the end of the experiment, higher percentages of animals (35% and 28% of the number in each group, respectively) recovered in the same groups. Moreover, feeding of the diabetic animals with ethanol extracts from SL and WP during one month decreased the MDA level down to the level in the intact animals.
Anyhow, in the pancreas, kidney and liver of the STZ-diabetic animals fed with ethanol extracts from SL, RP and WP, the histopathological study revealed positive changes compared with the STZ-control animals.
• In the SL fed animals: in the pancreas, the degenerative phenomena are less prominent, the number and size of insulin islets increases; in kidney, fewer nephrotic changes and necrotized glomeruli are seen; in liver, there is no fatty degeneration, the cords are preserved, although processes of pyknosis and cytolysis of hepatocytes are observed.
• In the WP fed animals: in the pancreas, the size and area of the islets of Langerhans are larger; in kidney, the pyknosis, degenerative and nephritic changes are less prominent.
• In the RP fed animals: in the pancreas, fewer destructive and dystrophic changes in both the exocrine and endocrine parts are seen, the average surface area of islets of Langerhans is larger compared to the other experimental groups (except the intact); in the kidney, the morphological changes are moderate, but necrosis of glomeruli and destruction of tubules are not so prominent; in liver, karyopyknosis and karyolysis of hepatocytes are moderate.
While carrying out our previous and the present investigations, special attention was given to sorrel leaves (Rumex Confertus), historically used in Eastern, particularly, in Armenian cuisine. Previously, relatively low antioxidant activity of the ethanol extract from sorrel leaves has been registered [22]. Nevertheless, this extract was surprisingly very effective in protecting pancreas β-cells from toxic action of aggregated amylin (IC50 as low as 0.03 ± 0.002 μg/ml) [20] and had a significant toxic effect on cancer cells (particularly, IC50 in inhibition of growth of Ehrlich ascites carcinoma cells was as low as 0.1 ± 0.04 ng/ml, the lowest among the studied plant extracts) [22]. Interestingly, the ethanol extract from SL can also serve as a source for a low-cost production of the known anticancer agent emodin, with additional anti-diabetic and anti-amyloidogenic features [27].
Actually, in the present work, the ethanol extract from SL proved to be most effective in improving the blood glucose level and the histopathological distortions in the pancreas, kidney and liver of the STZ-induced diabetic rats. This observation is contrary to our expectations, since the efficacy of the SL in inhibiting both the ADA and the DPPIV enzymes is rather low (IC50=0.68 ± 0.06 mg/ml and >100 mg/ml, respectively) [17]. It has to be noted, that many reserchers consider inhibition of these enzymes, especially the DPPIV enzyme, as highly beneficial in the treatment of hyperglicemia [28].
Based on the described results, which manifest the benefits of using the studied herbal extracts in the STZ-induced model of diabetes, we recommend a well-designed clinical trial to confirm the positive effects of these extracts (particularly of SL) on diabetic patients.
Funding source
This work was supported by the State Committee of Science of the ES Ministry of Republic of Armenia in the frame of the research project № 15T-1F164.
- Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107: 1058-1070. Link: https://bit.ly/364681G
- Anwar MM, Meki AR (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comparative Biochemistry and Physiology. Part A: Molecular Integrative Physiology 135: 539-547. Link: https://bit.ly/369iYMs
- Matheka DM, Alkizim FO (2012) Complementary and alternative medicine for type 2 diabetes mellitus: Role of medicinal herbs. J Diabetes Endocrinol 3: 44-56. Link: https://bit.ly/3dR6Ja2
- Malviya N, Jain S, Malviya S (2010) Antidiabetic potential of medicinal plants. Acta Pol Pharm 67: 113-118. Link: https://bit.ly/2Awv72d
- Ríos JL, Francini F, Schinella GR (2015) Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med 81: 975–994. Link: https://bit.ly/3bxrBkL
- Maridass M, de Britto AJ (2008) Origins of plant derived medicines. Ethnobotanical Leaflets 12: 373-387. Link: https://bit.ly/2AwDKd8
- Oubr´e AY, Carlson TJ, King SR, Reaven GM (1997) From plant to patient: an ethnomedical approach to the identification of new drugs for the treatment of NIDDM. Diabetologia 40: 614–617. Link: https://bit.ly/3cBL59t
- Ezuruiken UF, Prieto JM (2014) The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J Ethnopharmacol 155: 857-924. Link: https://bit.ly/3dHJ1N8
- Almohaimeed HM, Amin HA, El-Aziz GSA, Saleh HA (2019) Arabic gum Acacia in combination with metformin and Vitamin B12 improves diabetic peripheral neuropathy in rats: Ultrastructural histopathological study. J Interdiscip Histopathol7: 1-9. Link: https://bit.ly/2yQj6Er
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102-110. Link: https://bit.ly/364oUWI
- Hanley SC, Austin E, Assouline-Thomas B, Kapeluto J, Blaichman J, et al. (2010) {beta}-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology 151: 1462-1472. Link: https://bit.ly/2y5N0nJ
- Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J 24: 547-553. Link: https://bit.ly/2ZdnMi6
- Hahr AJ, Molitch ME (2015) Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol 1: 2. Link: https://bit.ly/2Z7AizK
- Erukainure OL, T Ebuehi OA, Adeboyejo FO, Oladunmoye OO, Aliyu M, et al. (2015) Short-Term Feeding of Fibre-Enriched Biscuits: Protective Effect against Hepatotoxicity in Diabetic Rats. Biochem Res Int 2015: 868937. Link: https://bit.ly/2WzxTw8
- Dey A, Lakshmanan J (2013) The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct 4: 1148-1184. Link: https://bit.ly/2Z1GKsa
- Mardanyan S, Sharoyan S, Antonyan A, Zakaryan N (2011) Dipeptidyl peptidase IV and adenosine deaminase inhibition by Armenian plants and antidiabetic drugs. Int J Diabetes and Metabolism 19: 69-74. Link: https://bit.ly/2Z5px0R
- Karapetyan LG, Harutyunyan HA, Antonyan AA, Sharoyan SG (2018) In vitro and in vivo influence of plant preparations on dipeptidyl peptidase IV and adenosine deaminase asctivity. Biol J Arm 70: 52-59. Link: https://bit.ly/2Z7E10s
- Hovnanyan K, Sharoyan S, Antonyan A, Hovnanyan N, Mardanyan S (2017) Effects of Extract and Phenol Glycoside from Rose Petals on the Amylin Fibrils. Open Access Library Journal 4: e3343. Link: https://bit.ly/2Ls4YUm
- Sharoyan S, Antonyan A, Mardanyan S, Harutyunyan H (2014) Inhibition of fibrillogenesis of amylin by the extracts of medicinal plants and their fractions. Biological Journal of Armenia 66: 13-19.
- Sharoyan SG, Antonyan AA, Harutyunyan HA, Mardanyan SS (2015) Inhibition of Amylin Fibril Formation and Protection of Islet β-Cells by Medicinal Plants. International Journal of Pharmacognosy 2: 234-241. Link: https://bit.ly/2Z6ZVAX
- Sharoyan SG, Antonyan AA, Harutyunyan HA, Mardanyan SS (2015) Medicinal plants support the amylin-suppressed viability of islet β-cells. International Journal of Pharmacognosy 2: 448-453.
- Antonyan A, Sharoyan S, Harutyunyan H, Movsisyan N, Sargisova Y, et al. (2013) Cytotoxicity of some edible plants toward Ehrlich ascites carcinoma cells. Research Journal of Medicinal Plant 8: 20-31.
- Spector DL, Goldman RD (2006) In: Basic Methods in Microscopy. Protocols and Concepts from Cells: A Laboratory Manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA.
- Zeb A, Ullah F (2016) A Simple Spectrophotometric Method for the Determination of Thiobarbituric Acid Reactive Substances in Fried Fast Foods. J Anal Methods Chem 2016: 9412767. Link: https://bit.ly/2y59LrT
- Colombo G, Clerici M, Garavaglia ME, Giustarini D, Rossi R, et al. (2016) A step-by-step protocol for assaying protein carbonylation in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 1019: 178-190. Link: https://bit.ly/3cvB11D
- Muñoz S, Méndez L, Dasilva G, Torres JL, Ramos-Romero S, et al. (2018) Targeting Hepatic Protein Carbonylation and Oxidative Stress Occurring on Diet-Induced Metabolic Diseases through the Supplementation with Fish Oils. Mar Drugs 16: E353. Link: https://bit.ly/2WC6Ct7
- Antonyan A, Sharoyan S, Harutyunyan H, Barboni L, Lupidi G, et al. (2016) Protection of hippocampal and islet beta cells in vitro by emodin from leaves of Rumex Confertus. International Journal of Pharmacognosy 3: 437-444. Link: https://bit.ly/3cDvbv2
- Karasik A, Aschner P, Katzeff H, Davies MJ, Stein PP (2008) Sitagliptin, a DPP-4 inhibitor for the treatment of patients with type 2 diabetes: a review of recent clinical trials. Curr Med Res Opin 24: 489-496. Link: https://bit.ly/2Z7Drj2

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
