Global Journal of Anesthesiology
Dexmedetomidine as an adjuvant to Nalbuphine in patient controlled analgesia for post-operative pain in Laparoscopic Cholecystectomy: A preliminary study
Nabaweya M Kamal1, Tarek A Radwan2, Ahmed A Mohamed2, Maha abdelbar3, Ahmed M Abdelaziz Hassan3, Magdy M Elsebae3* and Shady A AbdElmoneem3
2Anesthesia, Surgical ICU and Pain Management Department, Faculty of Medicine, Cairo University, Egypt
3General Surgery Department, Theodor Bilharz Research Institute, Egypt
Cite this as
Kamal NM, Radwan TA, Mohamed AA, abdelbar M, Elsebae MM, et al. (2019) Dexmedetomidine as an adjuvant to Nalbuphine in patient controlled analgesia for post-operative pain in Laparoscopic Cholecystectomy: A preliminary study. Glob J Anesth 6(1): 012-018. DOI: 10.17352/2455-3476.000047Introduction
Postoperative pain control is an important factor affecting patient recovery, return to normal bowel movement, ambulation and daily activity. Intravenous patient-controlled analgesia (IV-PCA) which allows the patient to administer his own pain relief is considered as an efficient tool to control postoperative pain. Safety of IV-PCA relies on the concept of negative feedback control system so that the patient will become too sedated to physically push the button to receive more opioid before reaching a critical point of severe respiratory depression [1].
Although morphine is the most common opioid used for this purpose in PCA due to its short action duration and strong analgesic effect; however, it may induce many adverse events, such as, postoperative pruritus, nausea, vomiting, constipation, decreased blood pressure, respiratory depression, and drowsiness and urinary retention [2-4]. Nalbuphine is an opioid has a ceiling effect in respiratory depression hence, it is considered to be safer than morphine, being mu antagonist and kappa agonist, it has lower incidence of adverse effects in comparison with morphine [5]. Dexmedetomidine is a potent and highly selective α-2 adrenoceptor agonist with sympatholytic, sedative, amnesiac, and analgesic properties. It provides unique “conscious sedation” analgesia, without respiratory depression. It decreases central nervous system (CNS) sympathetic outflow in a dose- dependent manner and has analgesic effects best described as opioid-sparing. There is increasing evidence of its organ protective effects against ischemic and hypoxic injury [6-8].This study aimed at assessing the role of Dexmedetomidine as an adjuvant to Nalbuphine in patient controlled analgesia for post-operative pain in laparoscopic cholecystectomy.
Patients and Methods
Study design
This study included forty patients scheduled for elective laparoscopic cholecystectomy. It is designed to explore the ability of usage of Dexmedetomidine as an adjuvant to Nalbuphine for post-operative laparoscopic pain. The study was conducted in Theodor Bilharz Research Institute after ethics committee approval of the study protocol. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants at the beginning of the study.
Inclusion criteria involved18-65 years old patients with ASA score I and II and BMI < 30 Kg / m2. Exclusion criteria involved patients older than 65 years or younger than 18 years, history of psychiatric/neurological illness, cardiovascular disease, hypertension, uncontrolled diabetes, morbid obesity, known allergic reaction to any of the study medication, pregnant and nursing women, recent use of sedatives or analgesics, significant laboratory abnormalities and patient’s refusal.
Patients were allocated into two equal groups D and P (20 patients each). Both groups received PCA (Accufuser plus M2015M Woo Young Medical CO.LTD) containing 20 mg Nalbuphine (Nalufin –Nalbuphine HCl 20mg / 1ml- Amoun Pharmaceutical Co. SAE.) to 100 ml with normal saline over 24 hours. The PCA infuser unit infused at rate of 2 ml.h-1 with lock out time 15 min & bolus of 1 ml per time. Patients in group D received Dexmedetomidine (Precedex – Dexmedetomidine HCl 200 µg/2ml- Hospira, Inc, Lke Forest, IL 60045 USA) (0.5 µg.kg-1 over 10 min followed by 0.2 µg.kg.-1h-1) prepared with 50 ml normal saline as intravenous infusion over 24 hours post-operative. Patients in group P received 50 ml of normal saline as intravenous infusion over 24 hours. Primary outcome measure was Nalbuphine consumption. Secondary outcome measures were visual analogue scale (VAS) for pain control, patient satisfaction and side effects.
Anesthetic technique
Premedication: All patients received Midazolam 0.05 mg kg-1, 4mg Ondansetron and 50 mg Ranitidine intravenously before induction of anesthesia.
Monitoring: Five-lead ECG monitor, NIBP monitor, pulse oximetry, peripheral nerve stimulator, end tidal carbon dioxide (ETCO2) estimation (Capnography) and esophageal thermometer.
Induction of anesthesia: Both D and P groups received (Ringer Acetate) infusion at a rate of 3–6mlkg-1h-1 for supplying fluid maintenance and deficit. After pre-oxygenation, anesthesia was induced with 1.5–2mg kg-1 Propofol and 2µg kg-1 Fentanyl. Neuromuscular blockade was achieved with 0.5mg kg-1 Atracurium followed by tracheal intubation. Anesthesia was maintained with Isoflurane administered in fresh gas flow oxygen /air 40% at a rate of 1L.min1. Neuromuscular blockade was achieved with intermittent doses of Atracurium 10mg when Train of Four (TOF) ratio reached 25% and ventilation was adjusted to obtain end-tidal carbon dioxide of 30-35 mm Hg. The mean arterial blood pressure was maintained within 20% above or below pre-anesthetic level. Atropine IV 0.5 mg increments was used to control bradycardia (<50beat min-1) while hypotension (less than 20% of pre-anesthetic level) was managed by increasing fluid infusion rate or incremental IV 5mg doses of ephedrine. In case of hypertension and tachycardia increment dose of 50 µg Fentanyl was given. Reversal of neuromuscular blocker was achieved by intravenous administration of Neostigmine 0.05 mg kg-1 and atropine 0.02 mg kg-1 guided by TOF guard 4:1 ratio 0.9.Normothermia was maintained by means of a warm blanket, humidifier, and warm intravenous fluids. At the end of the procedure,2 mg Nalbuphine was injected IV for all patients as loading analgesic dose in post-operative care unit followed by repeated boluses of 1 mg each aiming to reach VAS score 40mm Then all patients monitored for 24 hours:
Post-operative pain was evaluated every 6 hours using Visual Analogue Scale (VAS): no pain (0–4mm), mild pain (5–44mm), moderate pain (45–74 mm), and severe pain (75– 100 mm) [9].
Nausea and vomiting was recorded every 6 hours on a four-point scale: (0 = no nausea; 1 = mild nausea; 2= severe nausea requiring antiemetic; and 3 = retching and/or vomiting) [10].
Sedation score was recorded every 6 hours on a four-point scale: (0 = fully awake; 1 = drowsy, closed eyes; 2 = asleep, easily aroused with light tactile stimulation or a simple verbal command; 3 = asleep, arousal only by strong physical stimulation; and 4 = un-arousal) [11].
Vital signs including heart rate , blood pressure , peripheral capillary oxygen saturation (SpO2)&respiratory rate were recorded every 5 min for 30 min then every 30 min for 3 hours then every 2 hours till the end of the 24 hours.
Patient satisfaction score: was assessed at the end of 24 hours about their satisfaction concerning pain control using pain treatment satisfaction scale (PTSS, 0= no satisfaction to 10= complete satisfaction) [12].
Nalbuphine consumption: Nalbuphine consumed by the accufuser was calculated and recorded at the end of 24 hours for all patients.
Sample size
As no previous study evaluating the effect of Dexmedetomidine on Nalbuphine consumption in patient controlled analgesia for post-operative pain of laparoscopic surgeries was available to calculate number of participants included, we considered this research as a pilot study and 20 patients in each group were suitable.
Statistical analysis
Results were expressed as mean ± standard deviation (SD) or number (percent). Comparison between categorical data [number (%)] was performed using Chi square test. According to test of normality, comparison between different variables in the two groups was performed using either unpaired t test or Mann-Whitney U test whenever it was appropriate. In normality distributed data, pair-wise comparison (baseline versus different times of measurements] for the same variable was performed using repeated measure ANOVA followed by Bonferroni test if significant results was recorded with corrected p value= 0.401. In not normality distributed data, pair-wise comparison was performed using Friedman ANOVA followed by Wilcox on Signed Ranks test. Statistical Package for Social Sciences (SPSS) computer program (version 19 windows) was used for data analysis. P value ≤ 0.05 was considered significant.
Results
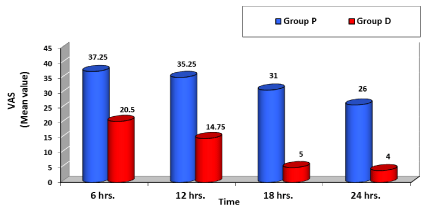
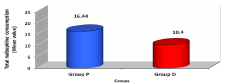
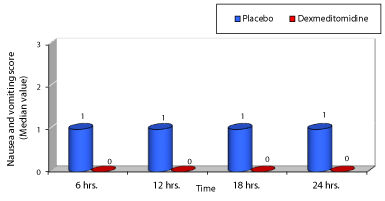
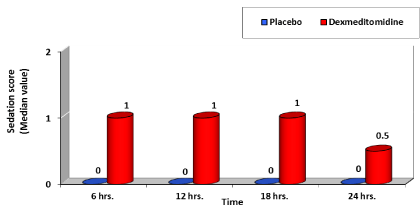
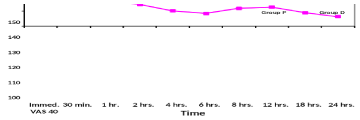
The age, gender, height and weight of patients were comparable in both studied groups with no significant difference (Table 1). Post-operative pain as evaluated using Visual Analogue Scale (VAS); group D showed significant low VAS value compared to group P during all times selected (Table 2, Figure 1) . Nalbuphine consumption in the two studied groups showed significant decrease in group D compared to group P by 36.74 % while patient satisfaction score over 24 hours showed significant increase in group D compared to group P by 51.24 % (Table 3, Figures 2, 3) . As regard nausea and vomiting score at different selected times in the two studied groups showed significant decrease in group D compared to group P (Table 4,5 & Figure 4). Sedation score at different selected times in the two studied groups showed significant increase in group D compared to group P (Table 6,7 & Figure 5).
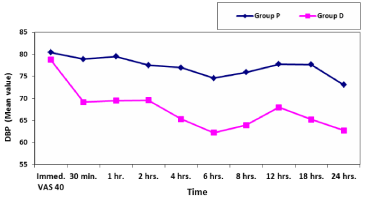
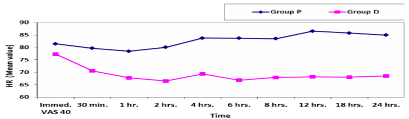
As regard hemodynamic parameters; mean values of systolic and diastolic blood pressure at different selected times in the two studied groups showed significant decrease in group D compared to group P (Table 8,9). The mean blood pressure at different selected times in the two studied groups showed significant decrease in group D compared to group P (Table 10, Figure 6). Mean values of heart rate at different selected times in the two studied groups showed significant decrease in group D compared to group P (Table 11, Figure 7). However, mean values of peripheral capillary oxygen saturation (SpO2) at different selected times in the two studied groups showed no significant difference between both groups (Figure 8).
Discussion
Few randomized clinical trials compared the efficacy of Dexmedetomidine as an adjuvant to different opioids (e.g. morphine & Fentanyl) in minimizing their consumption and side effects. However, there was no single study evaluating such effect as regarding Nalbuphine which is the drug of choice in this study. Lin et al. [13], studied whether Dexmedetomidine added to intravenous PCA morphine could improve analgesia while reducing opioid related side-effects. They found that patients in Dexmedetomidine and morphine group required significantly less PCA morphine than those from morphine alone group at all times in the study. During the 0–24 h postoperative period, cumulative PCA morphine use was 29% less in Dexmedetomidine group than in morphine group [23.3 ±10 vs. 32.8 ±12.4 mg, P<0.01]. Their results were in consistence with the results of the current study in reduction of cumulative PCA Nalbuphine usage by 36.74 % in group D in comparison with group P in which we use Nalbuphine alone without Dexmedetomidine [10.4 ±0.44 vs. 16.44 ±1.44 mg, P<0.05]. Also, Kim et al. [12], studied the efficacy of Dexmedetomidine in the reduction of Fentanyl consumption and opioid related side effects during intravenous PCA during post-procedure 24 h. They found that patients in Dexmedetomidine group required less Fentanyl during first 6 h after procedure (P < 0.001), but Fentanyl consumption was not significantly different between the two groups after 6 h. The cumulative Fentanyl consumption at 24 h was 28% less in Dexmedetomidine group compared with placebo group (729 ± 342 µg vs1017 ± 363 µg, P = 0.006).This was similar to our study as mentioned before as regard Nalbuphine consumption with significant P value in our study <0.05, but the difference was we found decrease in Nalbuphine consumption all through the 24 hours.
As regard pain control; Lin et al. [13], found that pain was significantly lower in Dexmedetomidine and morphine group compared to morphine group from the 2nd postoperative hour onwards and throughout the study. In addition, two patients in morphine group reported insufficient analgesia and received adjunctive analgesics. In our study, pain was significantly decreased in group D compared to group P with [P<0.05] at 6,12,18,24 hours postoperatively.
As regard sedation; Kim et al. [14], found that the level of sedation was higher in Dexmedetomidine group compared with placebo group (P < 0.001). Also in this point there was similarity with our study as we found that sedation was higher in group D compared to group P with significant P value <0.05 at 6. 12, 18 & 24 hour’s post-operative. This confirm that Dexmedetomidine had a clear sedative effect on all patients all through the 24 hours post-operative with no or minimal affection on their oxygen saturation which was compared between the two group and showed no significant difference between both groups with P value >0.05. Degree of Sedation had a great role in assessment of patient satisfaction at the end of 24 hours. Group D showed significant increase in patients satisfaction score compared to group P with P value <0.05 with mean value 9.15 ±0.67 for group D compared to mean value 6.05 ±1.19 for group P.
As regard nausea and vomiting Lin et al. [13], found that the incidence of nausea during the 4–24 h period was significantly lower in Dexmedetomidine group compared to morphine group (34% vs. 56.3%, P<0.05). Furthermore, the overall incidence of severe nausea was significantly lower in Dexmedetomidine group than in morphine group (6% vs. 20.8%, P<0.05). However, the incidence of vomiting during 4–24 h (18% vs. 33%, P=0.106) and the overall incidence of severe vomiting (6% vs. 17%, P=0.117) were lower in Dexmedetomidine group than in morphine group, but the differences were not significant. In contrast to our study which showed significant decrease in nausea and vomiting in group D compared to group P with P value <0.05 at all different selected times (6, 12, 18 & 24 h) post-operatively. There was no incidence of nausea or vomiting all through 24 hours in group D while in group P at 6h postoperatively 35 % of patients had mild nausea , 20% had severe nausea required anti-emetics and 15% had vomiting. Furthermore, at the end of 24 hours 60% of patients in group P experienced mild nausea.
In our study we found that all patients in group D experienced bradycardia which was within the clinically accepted range (>50 BPM) as there was significant decrease in HR in group D compared to group P at all different selected times allover 24 hours post-operatively with [P<0.05] all through these selected times with the least mean value 66.5 ±8.64 at 2 hours post-operative. Systolic, diastolic and mean blood pressure showed significant decrease in group D compared to group P. As well as, peripheral capillary oxygen saturation (SpO2) at different selected times in the two studied groups showed no significant difference between both groups. Thus, hemodynamic parameters was within the clinically accepted range with no harm on patients
Studies of effect Dexmedetomidine alone for intravenous PCA in comparison with Fentanyl and not as an adjuvant ; nausea found that the incidence of the incidence of postoperative and vomiting was less than Fentanyl while the VAS scores and sedation scale were not significantly different [11,15]. This study was similar to our study in illustration that Dexmedetomidine alone can minimize incidence of nausea and vomiting when compared to opioids (. However, its effect as a sole agent in the previous study can’t cause more pain control or more sedation compared to Fentanyl. Thus, it differs from our study as they used Dexmedetomidine alone in comparison with Fentanyl and not as an adjuvant. Dexmedetomidine offers a unique ability of providing both sedation and analgesia without respiratory depression. It is a new agent with a wide safety margin, excellent sedative capacity and moderate analgesic properties [16-18]. Our study demonstrated that usage of Dexmedetomidine as an adjuvant to Nalbuphine was effective as evidenced by decreased total Nalbuphine consumption and its side effects. Dexmedetomidine also improved pain control as assessed by VAS and patient satisfaction.
From surgical point of view; Although LC results in less pain than open cholecystectomy; it is not a pain free procedure. Many methods of analgesia for pain after laparoscopy have been evaluated involving non-steroidal anti-inflammatory drugs, wound and intraperitoneal local anesthetics, intraperitoneal saline, low-pressure gas and nitrous oxide pneumo-peritoneum have been shown to reduce pain after LC however, The clinical significance of this pain reduction is questionable. Pain after LC is multifactorial. Studies demonstrate that visceral pain accounts for most of the pain experienced after LC in the postoperative first day which is causes more discomfort than abdominal wall pain and pain referring to shoulder. It was linked to operative time and extent of tissue trauma caused by surgery [19,20]. Also, the intensity of pain after LC depends on pneumoperitoneum pressure, speed of insufflation and the residual gas volume [21]. In order to eliminate all these variables the procedure was performed by the same surgical team in similar durations in this study. After laparoscopic surgery was introduced to many surgical fields, IV-PCA became the standard modality for postoperative pain control. The advantages of reduced pain intensity, lower need for analgesics and reduced occurrence of nausea and vomiting must be weighed against some disadvantages such as an increased rate of urinary retention and restriction of mobility.
Patients were allocated into two equal groups D and P (20 patients each) using sealed envelope technique at the beginning of the study and personnel included in the postoperative pain management did not know in which study group D or P patient belong. Age, gender and CPT class distribution was comparable in both groups of the study. LC procedure was performed by the same surgical team with almost similar operative time and pneumoperitoneum pressure in this study. However, the randomization was biased by the fear of addition of Dexmedetomidine in some of the cases against the closed envelope choice if there is a worry about over sedation. So, this study became a prospective cohort analysis. Age, gender and CPT class distribution was comparable in both groups of the study. LC procedure was performed by the same surgical team with almost similar operative time and pneumoperitoneum pressure in this study. This was the main limitation of the study.
Conclusion
Dexmedetomidine could be used as a good adjuvant to Nalbuphine decreasing its consumption, improving its analgesic effect, providing good sedation and good patient satisfaction in patient controlled analgesia for post-operative pain in laparoscopic cholecystectomy. Although there was a significant decrease in BP and HR with usage of Dexmedetomidine, yet it was within the clinically accepted range. There was no significant difference in oxygen saturation by its usage.
- Grass JA (2005) Patient-controlled analgesia. Anesthesia & Analgesia 101: S44-S61. Link: https://tinyurl.com/yxt9zyqu
- Yeh YC, Lin TF, Lin FS, Wang YP, Lin CJ, et al. (2008) Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain. Br J Anaesth 101: 542–548. Link: https://tinyurl.com/y6da8w39
- Kim SH, Shin YS, Oh YJ, Lee JR, Chung SC, et al. (2013) Risk assessment of postoperative nausea and vomiting in the intravenous patient-controlled analgesia environment: predictive values of the Apfel's simplified risk score for identification of high-risk patients. Yonsei Med J 54: 1273–1281. Link: https://tinyurl.com/y2x5kqo4
- Rosen M (2009) The genesis, development and current usage of patient-controlled analgesia (PCA). Anaesth News 267: 9–11.
- Minai FN, Khan FA (2003) A comparison of morphine and nalbuphine for intraoperative and postoperative analgesia. J Pak Med Assoc 53: 391-396. Link: https://tinyurl.com/y5yqa2ra
- Chrysostomou C, Schmitt CG (2008) Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol 4: 619-627. Link: https://tinyurl.com/y64fjr55
- Penttilä J, Helminen A, Anttila M (2004) Cardiovascular and parasympathetic effects of dexmedetomidine in healthy subjects. Can J Physiol Pharmacol 82: 359-362. Link: https://tinyurl.com/yy8lne59
- Haselman MA (2008) Dexmedetomidine: a useful adjunct to consider in some high-risk situation. AANA J 76: 335-339. Link: https://tinyurl.com/yyz2yvuu
- Jensen MP, Chen C, Brugger AM (2003) Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 4: 407–414. Link: https://tinyurl.com/yyb8fng7
- Yi MS, Kang H, Kim MK, Choi GJ, Park YH, et al. (2018) Relationship between the incidence and risk factors of postoperative nausea and vomiting in patients with intravenous patient-controlled analgesia. Asian J Surg 41: 301-306. Link: https://tinyurl.com/y626k4ud
- Chen XH, Wang ZJ, Xiang QM, Zheng JW (2017) Effect of dexmedetomidine alone for postoperative analgesia after laparoscopic cholecystectomy. Zhonghua 97: 295-299. Link: https://tinyurl.com/y3esp2gp
- Evans CJ, Trudeau E, Mertzanis P (2004) Development and validation of the pain treatment satisfaction scale (PTSS): a patient satisfaction questionnaire for use in patients with chronic or acute pain. Pain 112: 254–266. Link: https://tinyurl.com/y4lxzcpy
- Lin TF, Yeh C, Lin S (2009) Effect of combining dexmede-tomidine and morphine for intravenous patient-controlled analgesia. Br J Anaest 102: 117-122. Link: https://tinyurl.com/y52odq56
- Kim SY, Chang CH, Lee JS (2013) Comparison of the efficacy of dexmedetomidine plus fentanyl patient-controlled analgesia with fentanyl patient-controlled analgesia for pain control in uterine artery embolization for symptomatic fibroid tumors or adenomyosis: a prospective, randomized study. Journal of Vascular and Interventional Radiology 24: 779-786. Link: https://tinyurl.com/y3bqh5pq
- Wang X, Liu W, Xu Z (2016) Effect of Dexmedetomidine Alone for Intravenous Patient-Controlled Analgesia After Gynecological Laparoscopic Surgery: A Consort-Prospective, Randomized, Controlled Trial. Medicine 95: e3639. Link: https://tinyurl.com/y3wd7tzb
- .Arcangeli A, D'Alò C, Gaspari R (2009) Dexmedetomidine uses in general anesthesia. Curr Drug Targets 10: 687-695. Link: https://tinyurl.com/y64rfts4
- Grosu I, Lavand’homme P (2010) Use of dexmedetomidine for pain control. F1000 Med Rep 2: 90. Link: https://tinyurl.com/y5jjbzpg
- Afonso, Joana, Reis F (2012) Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol 62: 125-133. Link: https://tinyurl.com/yxw23ryg
- Kum CK, Wong CW, Goh PM, Ti TK (1994) Comparative study of pain level and analgesic requirement after laparoscopic and open cholecystectomy. Surg Laparosc Endosc 4: 139-141. Link: https://tinyurl.com/y6pdwatu
- Wills VL, Hunt DR (2000) Pain after laparoscopic cholecystectomy. Br J Surg 87: 273-284. Link: https://tinyurl.com/y38ldemw
- Joris J, Thiry E, Paris P, Weerts J, Lamy M (1995) Pain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaine. Anesth Analg 81: 379-384. Link: https://tinyurl.com/y3tueu6y

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
