Forensic Science Today
Unbalanced intake of methyl donor supplements, may lead to DNA methylation and cause breast cancer in offspring
Arefhosseini Seyed Rafie, Alijani Sepideh* and Mohammadi Sumaieh
Cite this as
afie AS, Sepideh A, Sumaieh M (2020) Unbalanced intake of methyl donor supplements, may lead to DNA methylation and cause breast cancer in offspring. Forensic Sci Today 6(1): 020-023. DOI: 10.17352/fst.000018Statistical data in Iran reflects a worrying trend for an increased incidence of breast cancer. recent national data on average annual crude incidence for primary breast cancer indicated 22.6 (95%CI 22.1-23.1) per 100,000. It seems that genome encoding with no changes in the sequence of DNA contributes to Lamarck’s theory as a probable causative factor for the deterioration of this disorder by supplementation. Unconscious methylation of DNA by environmental factors make genetic modifications through epigenetic process in nucleotides that create new make up for DNA and DNMTs, expedite one of the most prevalent health disorders. Uterus environment, youth up to a retired and postmenopausal age are periods for changes in genome assembly that include tiny alterations in CpG islands. A review article had been performed by the electronic search for the manuscripts that had been published among current databases and declared facts about the produced results and the main proves had ascertained from the present articles. They were included by the Med Line and PubMed database and Iran’s vital statistics. As a result consumption of selected nutrients has the ability for methylation by group donors and causes an actual transfer of a methyl to the C5 carbon of cytosine for making 5-methylcytosine. Characteristic of a living organism referred to the Genetic and Epigenetic information and unbalanced intake of selected nutrients with dual function like folic acid persuades the creation of a genetic foundation for breast cancer in offspring.
Introduction
Indeed, understanding the complex interactions between diet and physiology is not easy [1]. The most important clue for creation of cancer is the instability of the cell library. DNA is not destiny [2] and the present situation approached the point that we would be able to discuss it for creation of the growing prevalence of the developed malignancy in the breast tissues. This paper discusses the effect of receiving methyl donor supplements on the increased risk of breast cancer through epigenetic processes. The creation of an ongoing dilemma in the world is discussed in this article and summarized in Figure 1.
Nutrition in the first view seems to have particular attention to the dietary substances against developing problems in their deficient status, however critical important molecular functions also would produce in their optimum balance and cause important contribution in the genome as the essential sector of the cell [4].
These changes could be inherited and make a reasonable base to detect genetic modification for alteration in this assembly such as mutations, translocation, insertions, and deletions that introduce the creation of the alterations in the cell library (Glier, et al. 2014). Discuss the encoding without changes in the DNA sequence is the actual reason for creation of big health problems and diet as the most detectable parameter could contribute to the main reason in methylation of the genome [4]. It is the connecting issue to join a theory that had been introduced by la Marck two centuries ago: The contribution of environmental factors for changes in phenotypic parameters that presented by Jean-Baptiste Chevalierde la Marck, 1744 –1829, is the first point that French scientist had announced about this phenomenon that contributes to physiologic function and the process of inheritance [5]. Modifications in phenotypic characteristics change the offspring’s genetic constituents, however recently it was joined to the recent Nobel Prize owner’s great discovery (MicroRNA in the cell, while some of the miRNA known as circulating miRNA and in the extracellular environment, like different physiologic media) had modified the understanding about genome and inheritance. It was revealed that approximately this discovery, sets 90% of the protein encoder genes, as well as the mainstream at various biological levels [6,7]. Victor R. Amberous (1993) from the University of Massachusetts Medical School in the Department of Program in Molecular Medicine discovered the miniature changes in genetic structure and established that methylation or Ubiquinonization of the genome after down-regulation, causes alterations in the genetic structure and consequently phenotypic entity of the produced proteins for offspring in the consequent stage is impressed and develops a new characteristic by the impressed gene [8]. In the real situation, genes experience no shift in the sequence of nucleotides, however, by this group’s discovery the alterations in the programming of its genetic information revealed the transform of original genome to a new makeup and creates some misinterpretations such as the development of new phenotypic character, like malignancy in the breast tissues [9,10].
There is an important fact about the Dual Functions of some selected nutrients, as if they are essential for sustaining normal metabolism and growth, in higher doses or in combination with other nutrients in new metabolic circumstances they could develop particularly new effects in the down-regulation process to evoke or silence some specific genes. A decrease in biosynthesis of a component in a cell,like aspecific protein and their RNA in response to any external parameter is called the down-regulation process and vice versa is up-regulation that they accompanied with genetic modification, by experience the DNA Methyltransferase (DNMT)is activateed [11,12], this family of enzymes serve as the methyl donor for unbalanced methylation [13].
An review article had been performed by the electronic search for the manuscripts that had been published among current databases and declared facts about the produced results and the main proves had ascertained from the present articles. They were included by the Med Line and PubMed database and Iran’s vital statistics.
Sum up
The diversified expression of a gene is mediated by epigenetic process and they involved in methylation of DNA, that include the other molecules addition and superimposing of molecular groups such as Methyl and Ubiquinone to the histone could be performed, as they are real and present in the inheritor materials of cell. Based on the biochemical reactions, the process of methylation causes repression and ultimately makes silencing of the gene, and inverted acetylation causes them to be expressed (Kalish, et al. 2003). Expression of genes is done by engagement of proteins binding of transcription factors to DNA [14] and the indicated modifications prevailed the following changes in three different sectors and the results could be retrieved in:
A. Mothers Uterus, is the new born’s first environment that emerges the defined program. They recognize this phenomenon by realizing the phenomena that occur in this environment, some of the nutrient molecules are received in a dual role. The new identity may cause offspring to carry the specified new genome who imply specific characteristics,
B. Spermatozoid, to confirm the father’s pivotal role in its contribution to the offspring’s inherited genome.
C. Environment and Nutrient as the most detectable factor, including Methyl donors [11].
Hypermethylation of DNA, occurs in mammary tumors and the increase in methylation in CpG islands promotes its development.
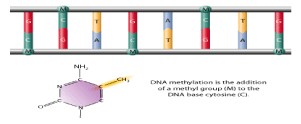
MiRNAs are small non-coding RNA molecules consisting of 18–24 nucleotides, which function in mRNA silencing and post-transcriptional regulation of gene expression .MiRNA expression is changed in many human cancers including leukemia, and this is regarded as an essential event in pathogenesis of these diseases. In the transfer of a methyl group to the C5 position of the cytosine to form 5-methylcytosine, the methyl Transferase family catalyzes the methylation process, which transmits a methyl group through the effect of DNA methyltransferase (DNMT) and Figure 2 illustrates Gene modification for this issue. DNA cytosine methylation and its molecular design indicates the function for methylation of DNA that catalyzed by DNA methyltransferases (Dnmts). Meanwhile, a Methyl group from S-adenyl methionine (SAM) transferred to the fifth carbon of cytosine residue to form 5-methylcytosine (5mC) [16]. Somatically heritable states are epigenetic changes for gene expression without alterations in the sequence of DNA and histone methylation [3].
Discussion
Implementation of environmental factors demonstrates their effects on organisms and genetic structure, express their modifications. Consequently, the newborn inherits them through three different ways that modified by MicroRNA, as a result of the environmental interaction to their own genetic constituents, develops ′Epigenetic process’ and their specifications of the acquired alterations would be described by changes in living organisms and selected micronutrients with epigenetic characteristics plays the main scenario for the occurred changes in the genetic structure: Respect to the process of down-regulation, some dietary factors (Methyl donor supplements) induce the detectable variables that attribute the alteration in the genome stability by this “intermediate process″.
DNA-binding proteins attach to the DNA and contribute to its effects by attracting or evacuating various DNA-binding proteins to regulate the final effects by expression of its information in its stricter or the modified Histones [11]. Down-regulation by environmental factors (Some Nutrients as the most manageable factors), contributes to the unchanged sequence of DNA, that results to different phenotype, and it makes new manifestation according to the new sequence of the new modified DNA, moreover, various living biomolecules would be created in a variety of ways and introduce the different effects for living organisms. It produces those effects on the impressed living organism and they yield the new dictation for genes that would be manifested for the creation of a new character. In order to introduce any support for this idea, it had been shown that changes in DNA methylation play important roles in breast cancer development [17]. This is accomplished by pairing to the end of the ‘3’ associated base with mRNA (3’UTR), and in this environment, some of the nutrient molecules reveal its biologic roll in a double manner that had been declared by Ameros. These new compounds also have a decisive influence on the development of DNA (Hutvágner Getal 2001, Zhou et al. 2014 Koch, et al. 2013; Kucukali, et al. 2014). Some proteins perform a specific function on histones to create landmarks to connect to other proteins that make protein domains like Chromodomain, (methylate branches) and the Bromodomain (that facilitate the acetylation): The repressing of DNA has been performed by DNA methylation and by this function, the gene couldn’t be expressed [14]. However, this process of domain reveals some intercourses, and the mentioned complex through the creation of the histone octamers, or remodeling of the other octameric histones, performing the transfer of DNA molecule to other DNA. Histon Argenine as an amino acid in its structure is Methylated in the H3 histone (R17-R23) and Lysine is methylated in H3 (K20-K4) and this methylation could be used in the translation process [3] and in the other forms of methylation for other amino acids would cause a miss translation and make unsuitable manufacturing of new protein. The Lysine acetylation will take place in H2A (K5) and H2 B (K12) this one causes the translation process to perform a rule and result in any modifications in the mentioned process, which would take place and lead to mutation and produce malignancies like breast cancer. Histone modifications and DNA methylations are courses for epigenetic changes that are modified by nutrients and thereby express genes that would experience some changes from embryonic extension and would cause carcinogenesis [3]. Two essential pieces of information that cooperate in the development of an organism, belonged to the information of Genome assembly of a living organism and referred to their Genetic and Epigenetic [18]. The manufacture of proteins related to the sequences of the pivotal genetic information as they create a living organism.However, the resent discovered precious information about Genetic structure, provides details about the quality of printed genetic alphabets and makes functional instructions on how this information should be used, in fact they are describing the details of information in what manner they would be used and when is their appropriate time to perform the persuaded functions.
Conclusion
The re-methylation process that has been performed by methyl group donor for methylation reactions applicable for RNA, DNA, Neurotransmitters, and proteins such as histones and methylation of lipids also would be included too for this cellular ongoing reaction, the impact of micronutrients on the epigenetic programming is the methylation of DNA that would be performed through the CpG islands by binding to proteins known as a methyl-CpG-binding domain. This DNA methylation mechanism is done by DNA methyltransferase and binds methyl groups to the position of 5 ‘nucleotiticity [15]. Methyltransferase, catalysis the formation of 5-methylcytosine [13] and in this way the DNA methyltransferase (DNMTs), is methylated cytosine residues in CpG dinucleotide. According to the new findings , the dual functions of some nutrients caused a new apprehension on the safety of dietary advices about the consumption of some micronutrients which might be the probable reasons for common health problems like breast cancer. Donating of a methyl group, although doesn’t produce any particular protein and doesn’t interact the inheritance of new characteristics, but this miniature change on the nucleotide initiates the inactivation of various genes (Moss and Wallrath, 2007, Geraghty AA, et al. 2016). The post-translational stage is affected by biomedical factors that affect the bioavailability of selected nutrients like folic acid. Disorders in the methylation process or the different Histone variants may cause dysregulation in epigenetic assembly [19-31], that finally could lead to breast cancer. Researches should focus more on Future interventional trials need to be done to investigate the role of nutrients with dual function on epigenetic changes in other destinations such as other types of cancer and other health issues.
- Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, et al. (2014) Interspecies Systems Biology Uncovers Metabolites Affecting C.elegans Gene Expression and Life History Traits. Cell 156: 759-770. Link: https://bit.ly/3fgyRDE
- Ambros V (2001) microRNAs: Tiny Regulators with Great Potential. Cell 107: 823-826. Link: https://bit.ly/33bvxHv
- Choi SW, Friso S (2010) Epigenetics: A New Bridge between Nutrition and Health. Adv Nutr 1: 8-16. Link: https://bit.ly/3fgwocv
- Wise IA, Charchar FJ (2016) Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Epigenetic Modifications in Essential Hypertension Int J Mol Sci 17: 451.
- An J, Kim K, Rhee SM, Chae H, Nephew KP, et al. (2013) Genome-wide analysis and modeling of DNA methylation susceptibility in 30 breast cancer cell lines by using CpG flanking sequences. J Bioinform Comput Biol 11: 1341003. Link: https://bit.ly/2DppioQ
- Ciappio ED, Mason JB, Crott JW (2011) Maternal one-carbon nutrient intake and cancer risk in offspring: Micro RNA Formation1. Nutr Rev 69: 561-571. Link: https://bit.ly/311yhEQ
- Dominguez-Salas P, Cox Sharon E, Prentice Andrew M, Hennig Branwen J, Moore Sophie E (2012) Maternal nutritional status, C1 metabolism and offspring DNA methylation: a review of current evidence in human subjects. Proc Nutr Soc 71: 154-165. Link: https://bit.ly/39G3EIX
- Jazayeri SB, Saadat S, Ramezani R, Kaviani A (2015) Incidence of primary breast cancer in Iran: Ten-year national cancer registry data report. Cancer Epidemiol 39: 519-527. Link: https://bit.ly/3hUjW3H
- Kotsopoulos J, Kim YI, Narod SA (2012) Folate and breast cancer: what about high-risk women?. Cancer Causes Control 23: 1405-1420. Link: https://bit.ly/2PaYuuT
- Dong Y, Zhao H, Li H, Li X, Yang S (2014) DNA methylation as an early diagnostic marker of cancer (Review). Biomedical Rep 2: 326-330. Link: https://bit.ly/2PdxRFS
- Zhang Y, Li E (2014) DNA methylation in mammals. Cold Spring Harb Perspect Biol 6: a019133. Link: https://bit.ly/2BOMKLI
- McKay J, Mathers J (2011) Diet induced epigenetic changes and their implications for health. Acta Physiol 202: 103-118. Link: https://bit.ly/2BOkk4s
- Liu J, Ward RL (2010) Folate and one-carbon metabolism and its impact on aberrant DNA methylation in cancer. Adv Genet 71:79-121. Link: https://bit.ly/39U6uKB
- Moore Lisa D, Le Thuc and Fan Guoping (2014) DNA Methylation and Its Basic Function, Neuropsychopharmacology 38: 23-38. Link: https://bit.ly/3jVFvmg
- Thomson JP, Skene PJ, Selfridge J, Clouaire T, Guy J, et al. (2010) CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 464: 1082-1086. Link: https://go.nature.com/313jyZT
- Wurtman RJ (2011) Non-nutritional uses of nutrients. Eur J Pharmacol 668: S10- S15. Link: https://bit.ly/315zjzC
- Xu X, Gammon MD, Jefferson E, Zhang Y, Cho YH, et al. (2011) The influence of one-carbon metabolism on gene promoter methylation in a population-based breast cancer study. Epigenetics 6: 1276-1283. Link: https://bit.ly/311HJrC
- Jin B, Robertson KD (2013) DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol 754: 3-29. Link: https://bit.ly/30fujJx
- Binda O, Sevilla A, LeRoy G, Lemischka IR, Garcia BA, et al. (2013) SETD6 mono-methylates H2AZ on lysine 7 and is required for the maintenance of embryonic stem cell self-renewal. Epigenetics 8: 177-183. Link: https://bit.ly/2Djxgj6
- Arefhosseini SR, Ebrahimi-Mamaeghani M, Mohammadi S (2014) MicroRNAs Regulation by Nutrients, the New Ray of Hope in Obesity RelatedGlucose and Lipid Metabolic Disorders. J Metabolic Synd 3: 2-12. Link: https://bit.ly/3gfp4iB
- McKay JA, Mathers JC (2016) Maternal folate deficiency and metabolic dysfunction in offspring. Proc Nutr Soc 75: 90-95. Link: https://bit.ly/3fgu70X
- McStay CL, Prescott SL, Bower C, Palmer DJ (2017) Maternal Folic Acid Supplementation during Pregnancy and Childhood Allergic Disease Outcomes: A Question of Timing? Nutrients 9: E123. Link: https://bit.ly/3hMI4VQ
- Nazki FH, Sameer AS, Ganaie BA (2014) Folate: metabolism, genes, polymorphisms and the associated diseases. Gene 533: 11-20. Link: https://bit.ly/3gbxzLo
- Oosting A, van Vlies N, Kegler D, Schipper L, Abrahamse-BerkeveldM, et al. (2014) Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. BrJ Nutr 111: 215-226. Link: https://bit.ly/315ywyE
- Paniagua JA, Escandell-Morales JM, Gil-Contreras D, Berral de la Rosa FJ, Romero-Jimenez M, et al. (2014) Central obesity and altered peripheral adipose tissue gene expressioncharacterize the NAFLD patient with insulin resistance: Role of nutrition and insulin challenge. Nutrition 30: 177-185. Link: https://bit.ly/3gjdliT
- Sevilla A, Binda O (2014) Post-translational modifications of the histone variant H2AZ. Stem Cell Res 12: 289-295. Link: https://bit.ly/3fbpWDj
- Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, et al. (2004)Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 286: E272–E279. Link: https://bit.ly/2PaprPB
- Golchin N, Khodadi E, Yaghooti SH, Jaseb K, Shahjahani M, et al. (2017) Immunophenotype, microRNA expression and cytogenetic characterization of acute leukemias of ambiguous lineage. Comp Clin Pathol 26: 261-267. Link: https://bit.ly/2EwvBHr
- Vanhees K, Vonhögen IG, van Schooten FJ, Godschalk RW (2014) You are what you eat, and so are your children: the impact of micronutrients on the epigenetic programming of offspring. Cell Mol Life Sci 71: 271-285. Link: https://bit.ly/39U5g1X
- Jürgen V, Manel E (2010) Breast Cancer Epigenetics: From DNA Methylation to microRNAs. J Mammary Gland Biol Neoplasia 15: 5-17. Link: https://bit.ly/2ECmEMY
- Zhang CX, Ho SC, Chen YM, Lin FY, Fu JH, et al. (2011) Dietary folate, vitamin B6, vitamin B12 and methionine intake and the risk of breast cancer by oestrogen and progesterone receptor status. Br J Nutr 106: 936-943. Link: https://bit.ly/2BOL1Ge
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
