Archive of Urological Research
Intranuclear Inclusions in Conventional Clear Cell Renal Cell Carcinoma (ccRCC): Diagnosis and Differential Diagnosis
Aryn McClain1, Lynne Sakowski2, Michele Conti2, Hui Zhang2, Qing Kay Li2*
2The Department of Pathology, Johns Hopkins Medical Institutions, Baltimore, MD 21224, USA
Cite this as
McClain A, Sakowski L, Conti M, Zhang H, Li QK (2018) Intranuclear Inclusions in Conventional Clear Cell Renal Cell Carcinoma (ccRCC): Diagnosis and Differential Diagnosis. Arch Urol Res 2(1): 005-001. DOI: 10.17352/aur.000003Intranuclear inclusions are important diagnostic features in many benign and malignant neoplasms. It has also been identified in major epithelial subtypes of renal cell carcinomas (RCCs), particularly in the chromophobe RCC. However, the finding in ccRCC has not been well studied. The finding of intranuclear inclusions may cause diagnostic difficulty, particularly in metastatic lesions. Herein, we reported a case of ccRCC with prominent intranuclear inclusions. The tumor also metastasized to local lymph nodes. Furthermore, in contrast to previous publications, we also found that intranuclear inclusions were immunoreactive with anti-PAX8 (paired box8) antibody. The potential diagnostic and clinical implications of intranuclear inclusions in ccRCC need to be addressed.
Introduction
Intranuclear inclusions are important diagnostic features in many benign and malignant neoplasms, such as adrenal cortical adenoma, thyroid papillary carcinoma, melanoma, meningioma, hepatocellular carcinoma, pancreatoblastoma, pulmonary blastoma and fetal-type adenocarcinoma, and lobular breast carcinoma [1-5] as well as recently reported ovarian serous carcinoma [2]. In renal epithelial cell tumors, the present of intranuclear inclusions has been reported in all three major subtypes, including chromophobe, papillary and ccRCC [3-5]. Among these tumors, the findings are more commonly seen in chromophobe RCCs [3]. Several previous publications demonstrated that intranuclear inclusions could be identified in ccRCCs with different frequencies [5-7], and it was more commonly seen in advanced stage of the ccRCC [5-7]. Clinically, 45% to 50% of patients with ccRCC present with locally advanced and/or metastatic disease at the time of diagnosis [6]. The common metastatic sites include lymph nodes (44%), abdominal and thoracic viscera (32%), lung (17%), thyroid and other anatomic sites [6]. Since intranuclear inclusions are common morphological findings in many tumors, an accurate diagnosis of ccRCC plays a critical role in the optimal management of the patient, particularly in patients with biopsy specimens.
The morphological features of ccRCC, such as clear cytoplasm, round to oval shaped nuclei, fine chromatin pattern, and present or absent prominent nucleoli depending on the ISUP nucleoli grade, are well documented [5-8]. Taken together, the finding of intranuclear inclusions in a subset of ccRCCs needs to be studied. Here, we report ccRCCs with prominent intranuclear inclusions and discuss the current publications and potential clinical impact of the finding.
Case presentation
Clinical presentation: We found five cases of ccRCC. The patient’s age ranged from 64 years to 73 years. All patient presented with history of micro- and macro-hematuria, mild abdominal pain, passing of multiple renal calculi, and sporadically halted urination. Past medical history included laser surgical treatment for renal calculi in one patient. No malignant history was found in all cases. Physical examination was unremarkable, and no palpable masses were noted. The patients’ creatinine level was ranged from 0.78 to 1.55 mg/dL (normal 0.6-1.2 mg/dL). A computed tomography (CT) scan of kidneys revealed masse, ranging from 4.0 to 7.5 cm. Tumors revealed a relatively well defined borders, and one tumor also found staghorn calculus. One case was found to have a suspicious left para-aortic lymph node and a 2 mm nodule in the right middle lobe of the lung. Clinically, ccRCCs were suspected. Radical nephrectomies were performed.
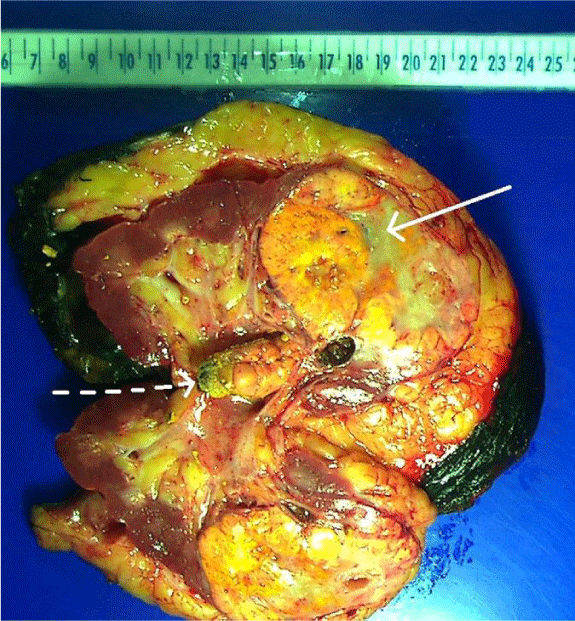
Pathological examination: A representative gross examination of bisected kidney revealed a solitary yellow-gray-colored 7.5 cm mass in the renal parenchyma (Figure 1). The tumor was well demarcated and demonstrated “pushing borders”. The cut surface of the tumor revealed focal hemorrhage and necrosis. The tumor did not invade the renal pelvis and other collecting duct system.
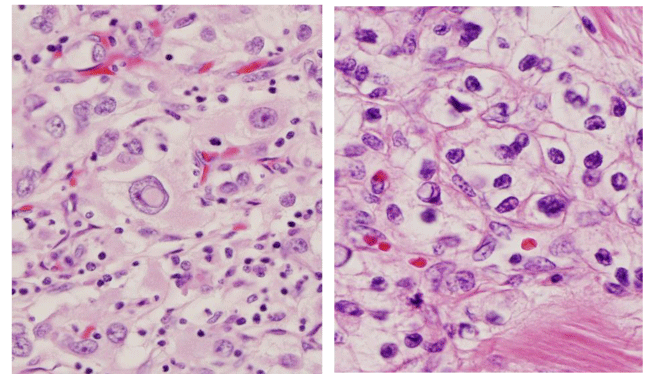
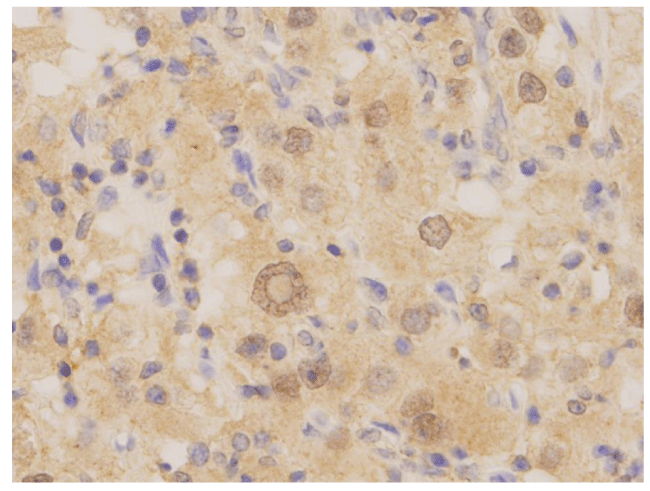
On microscopic examination, tumor cells were arranged in solid and acinar patterns, with prominent small thin-walled blood vessels, focal hemorrhage and necrosis. Individual tumor cells were intermediate to large in size with moderately pleomorphic nuclei and coarse granular chromatin patterns. The cytoplasm of the tumor cells was abundant, with a wispy and/or a lacy appearance. Many of the cells also had abundant clear, granular or vacuolated cytoplasm. The cell borders were indistinct. The nucleoli were readily identifiable using 10X objective (X100 magnification) and consistent with the ISUP (International Society of Urological Pathology) nucleoli grade 3. In addition, prominent intranuclear inclusions were also identified in tumor cells (Figure 2). In one of our patients, the tumor had metastasized to two regional lymph nodes. The pathological stage of the tumor was pT2a N1. Immunohistochemical stains were performed, including PAX8, PAX5, PAX2, P53, PTEN, TFE3, and cadherin. The intranuclear inclusions were only highlighted by PAX8 immunostains (Figure 3).
Discussion
In renal epithelial cell tumors, the presence of intranuclear inclusions has been reported in all three major subtypes [3-5,8]. For example, Granter SR, et al studied morphological features of chromaphobe RCC and found that intranuclear inclusions were present in four of six cases and were numerous in two cases [3]. They considered the presence of intranuclear inclusions as one of characteristic findings of chromophobe RCC. However, they did not find any prognostic impact of intranuclear inclusions. Furthermore, Granter SR, et al. also studied 40 cases of papillary RCCs and found 12.5% had intranuclear inclusions [4]. Recently, Lee JH, et al. studied 110 cases of RCCs, including 54 cases of clear cell, 25 cases of papillary and 31 cases of chromophobe RCCs [5]. In the study, intranuclear inclusions were identified in 39% of ccRCCs. They also compared the number of intranuclear inclusions among different types of RCCs and correlated them with patients’ survivals. They found that ccRCC of high inclusion scores were correlated with ISUP nucleoli grade 3 and 4, and a poor survival rate in comparison to tumors of low inclusion scores [5]. Taken together, these studies demonstrated that the feature of intranuclear inclusions is not an uncommon finding and could potentially relate to poor prognosis.
Clinically, 45% to 50% of patients with ccRCC present with locally advanced and/or metastatic disease at the time of diagnosis [6]. Intranuclear inclusions may cause diagnostic difficulty when a ccRCC metastasizes, as intranuclear inclusions are common morphological findings in many other tumors, particularly in liver and thyroid neoplasms [9]. For example, Gritsman AY, et al. reported a case of RCC metastasis to the thyroid, and the tumor demonstrated intranuclear inclusions [7]. The presence of intranuclear inclusions in the metastatic RCC may cause a diagnostic confusion with the primary papillary thyroid carcinoma (PTC), since the intranuclear inclusion is the diagnostic feature in PTC [9]. Thus, it is important to recognize the presence of intranuclear inclusions in primary and metastatic RCCs, particularly in tumors with high nucleoli-grade.
With regard to morphogenesis, intranuclear inclusions in RCCs may be formed by cytoplasmic invagination, similar to thyroid papillary carcinoma [5,7,9]. The condensed chromatin is found to be located at the periphery of the intranuclear inclusions [5, 9]. One study using electron microscopy (EM) demonstrated that intranuclear inclusions had the same component found in the cytoplasm—the lipid droplets that give RCCs their clear color properties—suggesting that intranuclear inclusions are formed by cytoplasmic invagination [5]. In our study, we performed several immunohistochemical stains, including PAX8, PAX5, PAX2, P53, PTEN, TFE3, and cadherin. The intranuclear inclusions were only highlighted by PAX8 staining. PAX8 is a transcription factor and belongs to the PAX family of proteins [10,11]. It plays an important role in the regulation of renal lineage differentiation and is also involved in the proliferation of tumor cells via Wnt/beta-catenin pathway [10,11]. Overexpression of PAX8 has been detected in almost all subtypes of RCCs, as well as in thyroid, ovarian, bladder, prostate and endometrial carcinomas [10]. The overexpression of PAX8 has been linked to anti-apoptosis in tumor cells. Several studies have also demonstrated that downregulation of PAX8 expression inhibits cell growth and induces apoptosis [10,11]. Further study is needed to clarify the mechanism of the formation of intranuclear inclusions and the role of PAX8 in ccRCC.
In summary, the finding of intranuclear inclusions is a useful feature for the morphological diagnosis of ccRCC. In addition to the standard TNM staging protocol and the ISUP nucleoli grade, the presence of intranuclear inclusions in ccRCC should be carefully evaluated, since it may cause a diagnostic confusion in metastatic tumors and potentially relate to the prognosis of the disease.
This work is partially supported by Drs. Ji and Li Family Cancer Research Foundation, and NIH U01CA152813 (Hui Zhang and Qing Kay Li).
- Ip YT, Dias Filho MA, Chan JK (2010) Nuclear inclusions and pseudoinclusions: friends or foes of the surgical pathologist? Int J Surg Pathol 18: 465-481. Link: https://goo.gl/sXFi8m
- Naka M, Ohishi Y, Kaku T, Watanabe S, Tamiya S, et al. (2015) Identification of intranuclear inclusions is useful for the cytological diagnosis of ovarian clear cell carcinoma. Diagn Cytopathol 43: 879-884. Link: https://goo.gl/5BD8PZ
- Granter SR, Renshaw AA (1997) Fine-needle aspiration of chromophobe renal cell carcinoma: analysis of six cases. Cancer 81: 122-128. Link: https://goo.gl/ETWLqb
- Granter SR, Perez-Atayde AR, Renshaw AA (1998) Cytologic analysis of papillary renal cell carcinoma. Cancer 84: 303-308. Link: https://goo.gl/xP17gh
- Lee JH, Han EM, Lin ZH, Wu ZS, Lee ES, et al. (2008) Clinicopathologic significance of nuclear grooves and inclusions in renal cell carcinoma: image database construction and quantitative scoring. Arch Pathol Lab Med 132: 940-946. Link: https://goo.gl/TttoxZ
- Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, et al. (2013) The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37: 1490-1504. Link: https://goo.gl/yVxwBy
- Gritsman AY, Popok SM, Ro JY, Dekmezian RH, Weber RS (1988) Renal-cell carcinoma with intranuclear inclusions metastatic to thyroid: a diagnostic problem in aspiration cytology. Diagn Cytopathol 4: 125-129. Link: https://goo.gl/nH2rT9
- Linsk JA, Franzén S (1984) Aspiration cytology of metastatic hypernephroma. Acta Cytol 28: 251-260. Link: https://goo.gl/bjBtsZ
- Gray A, Doniach I (1969) Morphology of the nuclei of papillary carcinoma of the thyroid. Br J Cancer 23: 49-51. Link: https://goo.gl/kCHC37
- Sharma R, Sanchez-Ferras O, Bouchard M (2015) Pax genes in renal development, disease and regeneration. Semin Cell Dev Biol 44: 97-106. Link: https://goo.gl/fv3xVL
- Tan PH, Cheng L, Rioux-Leclercq N, Merino MJ, Netto G, et al. (2013) Renal tumors: diagnostic and prognostic biomarkers. Am J Surg Pathol. 37: 1518–1531. Link: https://goo.gl/zZtwRj
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
