Annals of Systems Biology
Salinity induced redox metabolic shift influence hormonal profile and germination performance of two contrasting indica rice cultivars
Nabanita Banik, Nivedita Dey and Soumen Bhattacharjee*
Cite this as
Banik N, Dey N, Bhattacharjee S (2022) Salinity induced redox metabolic shift influence hormonal profile and germination performance of two contrasting indica rice cultivars. Ann Syst Biol 5(1): 001-007. DOI: 10.17352/asb.000016Copyright License
© 2022 Banik N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The role of redox deviations under salinity on metabolic dysfunction associated with progression of seed germination is well documented. However, the correlative evaluation of the salinity induced changes in the redox system and hormonal profile in regulating germination are least studied and hence is the subject of present investigation. Imposition of post imbibitional salinity stress (PISS) to two contrasting rice genotypes differing in sensitivity towards salinity (Oryza sativa L., Cultivars Patnai and IR29) caused differential and significant redox-metabolic shift and germination performances. Biomarkers of oxidative stress like, accumulation of total ROS, in situ localization of hydrogen peroxide, radical scavenging property, and lipid peroxidation are assessed for the determination of salinity induced differential changes in redox status of both the experimental cultivars. Salt resistant cultivar Patnai exhibiting better redox regulatory property under PISS in terms of controlled generation of ROS (DCFDA oxidation, H2O2 content) with greater elicitation of total antioxidant capacity (DPPH radical scavenging property), contends lipid peroxidation (accumulation of TBARS) as compared to the salt-sensitive cultivar IR 29. RP-HPLC based estimation of PISS-induced alteration in hormonal pools showed strong correlation between altered redox status (assessed in terms of redox biomarkers) and hormonal profile (endogenous titer of gibberellic acid (GA3), abscisic acid (ABA) and jasmonic acid (JA)) and germination and other physiological phenotypes (t50 value, allocation index, relative water content, and Na+ / K+ ratio) of the experimental rice germplasms, suggesting the influence of differential shift in redox status on germination hormones and early growth performances. Taken as a whole, the work proposes close connection between salinity induced changes in oxidative windows and hormonal profile of germinating seeds, necessary for better management of salinity stress in agriculture.
Introduction
Salinity caused by unscientific irrigation practice, overuse of fertilizer, excessive plowing and intrusion of salt from coastal region negatively impact on crop productivity and harvest quality throughout the world [1-3]. Plants, apart from suffering ionic imbalance, dehydration or osmotic stress also suffer secondary oxidative stress under salinity [1,4,5]. Redox regulation seems to play a pivotal role under salinity that largely depends on the ROS-antioxidant interaction at metabolic interface, redox status, chemical reactivity of ROS as well as their spatio-temporal changes. The critical evaluation of redox regulation involves assessment of ROS-antioxidant interaction, genesis of internal redox cue, changes in hormonal titer, determination of oxidative stress. Overall, assessment of redox regulation under salt stress is extremely important and significant from the point of view of physiology of plants. Germplasm of rice exhibits variations in their redox regulatory mechanisms and hence their sensitivity towards salinity stress [6].
Plants exposed to salt stress in general trigger diverse signaling pathways involving phytohormones [7,8]. Altered hormone accumulation under abiotic stresses might trigger signaling through synergistic action or cross-talking with internal redox cues. There exist evidences of both feed forward and feed backward interactions between hormones and redox cue to regulate the developmental responses [7-9]. Further, some works specifically indicated the association of this phytohormone in controlling germination via crosstalk between ROS, ABA, GA, JA, etc. [10-13]. But despite the existing development made in this area of plant system biology, clear understanding of the impact of salinity induced redox shift in germinating tissue on hormonal changes regulating germination phenotypes are seldom studied and is the objective of the present investigation.
Materials and methods
Imposition of post imbibitional salinity stress and assessment of germination phenotypes
Seeds of two indica rice cultivars (Oryza sativa L. Cultivars IR29 and Pokkali), selected as experimental materials were collected form. Central Rice Research Institute, Cuttack, Orissa, India and subsequently maintained at Crop Research and Seed Multiplication Farm ( The University of Burdwan, West Bengal ) between 2015 to 2021 for getting seeds necessary for experimentation. Seeds of the experimental rice cultivars (Oryza sativa L., Cultivars Patnai & IR29) were washed with distilled water and surface sterilized with 0.2% HgCl2 solution for five minutes. Surface sterilized seeds were washed and imbibed at sterile distilled water in darkness at 25ºC±2ºC, for 24hours and were plated to impose different magnitude of post-imbibitional salinity stress [150mM and 250mM NaCl for 24 hours at 25ºC with 14-hour photoperiod (270 µm m-1S-1) and 65±2% relative humidity]. Post-imbibitional salinity stressed (PISS), germinating seeds were subsequently allowed to grow for next 72 hours in an environmental chamber maintained at temperature 25ºC ± 2ºC, relative humidity 65±2% and 14-hour photoperiod with 270 µm m-1 S-1 illumination. For studying ROS-antioxidant interaction dynamics, redox biomarkers, and quantitative estimation of GA, ABA and JA,72-hour old tissues (after completion of PISS) were used.
Spectroflrometric estimation of total ROS generation
in vivo assay of total ROS was performed spectroflurometrically by placing seedling tissue (50 mg) in 8 mL 40 mM TRIS-HCl buffer (pH 7) in presence of 100 µM 2/, 7/-dichloroflorescin diacetate (DCFDA, Sigma) at 300C. The supernatant was removed after 60 minutes and fluorescence was monitored in a spectroflurometer (Hitachi, Model F-4500 FL Spectrophotometer) with excitation at 488 nm and emission at 521 nm [14].
DAB assay for in situ localization of hydrogen peroxide
For in situ localization of ROS DAB assay was performed following the procedure of Daudi, et al. (2012) [15]. For this, the leafy part of seedlings (approx. 1cm) were dipped into 2 mL of DAB stain (3, 3′-diaminobenzidin, 1 mg/mL, pH 3.8) and incubated for 8 h at room temperature under light. After incubation, the leaf parts were kept in absolute ethanol and then kept in a water bath (100 °C) till chlorophyll was completely removed from the leaf. Then the sample was cooled and kept immersed in 20% glycerol. The image was captured using a stereomicroscope (Leica Wild M3B stereo zoom microscope, Germany), and a dotted brown color in the tissue indicated the presence of H2O2.
DPPH (2,2/-diphenyl-1-pycryl hydrazyl) free radical scavenging activity
For the determination of DPPH+ free radical scavenging activity, the process of Mensor, et al. (2001) [16] was followed. One gram of dry sample was extracted with 30ml 80% methanol at 28oC for 24h in shaking incubator and centrifuged at 3500rpm for 20 min at 4oC, collected and filtered the supernatant. The filtrate was used for DPPH free radical scavenging activity. The reaction mixture containing 1mL filtrate with 3mL DPPH (0.04mg mL-1 ethanol) were incubated for 30min in darkness and then absorbance was measured at 517nm.
Estimation of membrane lipid peroxidation
To estimate malondialdehyde (MDA) membrane lipid peroxidation, the TBA (thiobarbituric acid) test was performed using the procedure of Heath and Packer (1968) [17]. Sample 200mg was homogenized in 5ml of 0.1% TCA, and then centrifuged at 10000 rpm for 5 min and finally supernatant was taken. To 1ml of supernatant, 3ml of 5% TCA (Trichloroacetic acid) containing 1% TBA was added and the mixture was heated in a hot water bath for 30min and cooled quickly in the cold bath and finally centrifuged at 10000 rpm for 10min. Thereafter, the absorbance of supernatant was measured at 530 nm. The concentration of MDA was calculated from its extinction coefficient of 155µM cm-1.
RP-HPLC based quantification of phytohormones
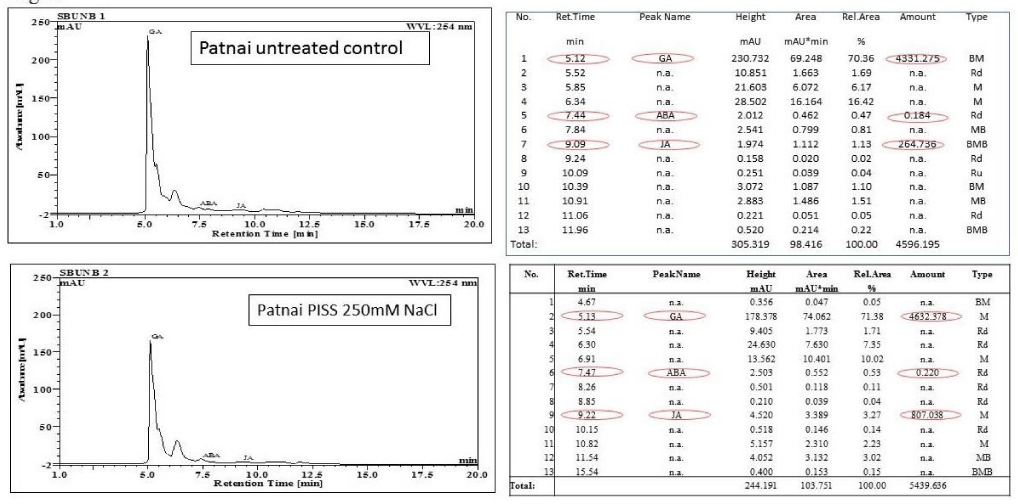
Treated and control seedlings were collected and put it in liquid nitrogen to make them powder. Powdered sample were homogenized with cold acetonitrile. Homogenates were kept at 4oC for 12h and centrifuged at 2000 rpm for 10 min at 4oC. Supernatants were taken in centrifuge tubes and mixed with 1.5 mL 0.1M phosphate buffer (pH-7.1). These mixtures were kept at -80oC for 30 minutes and then thawed adequately at 4oC. The mixture was extracted with 2.5 ml of ethylacetate thrice after addition of 1 mL HCl. After centrifugation ethylacetate phase was collected and with the help of rotary vacuum evaporator the sample was dried. The dry sample was dissolved in 1ml of mobile phase containing methanol and water (1:1). Finally, extract was filtered through 0.22mm membrane filter for HPLC in C18 column and UV detection. GA3, ABA and JA peaks were detected by using absorbance at 254 nm
Determination of germination phenotypes and other physiological traits
For studying the impact of PISS on germination of two experimental rice cultivars (Patnai and IR29), T50 value of germination was performed according to the procedure of Rubio-Casal, et al. (2003) [18] and Bhattacharjee (2008) [19].
For the estimation of allocation index, Na+ / k+ ratio and relative water content the processes of USDA handbook (1954) [20] and Barrs and Weatherly (1962) [21] were followed.
Statistical analysis
Experiments for all metabolic parameters were performed thrice with three replicates each and results were calculated as mean of three replicates ± standard error. For statistical analysis of the data for significance, “t-test, paired two samples for means” was used in Microsoft Excel 2010.
Results and discussion
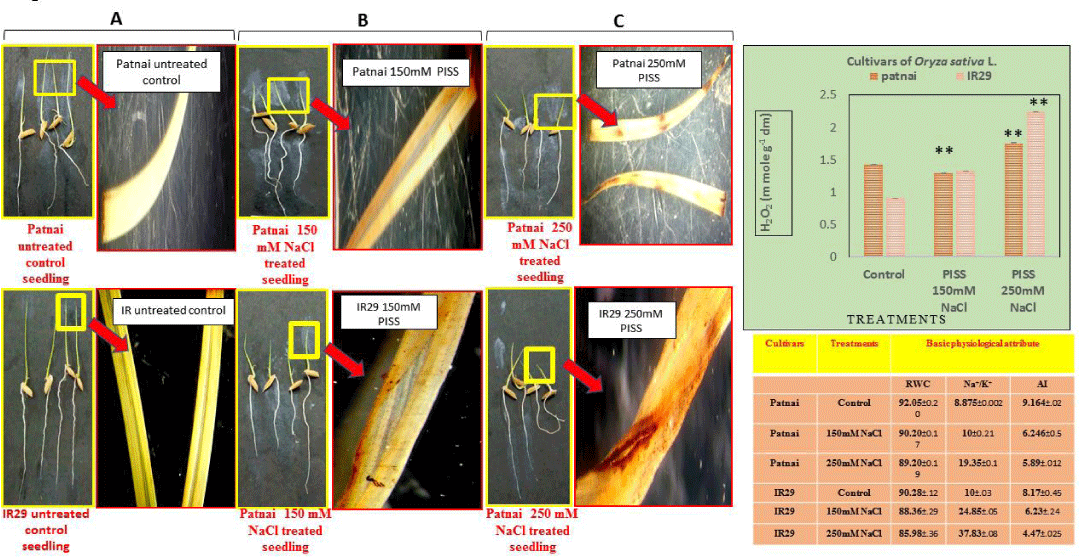
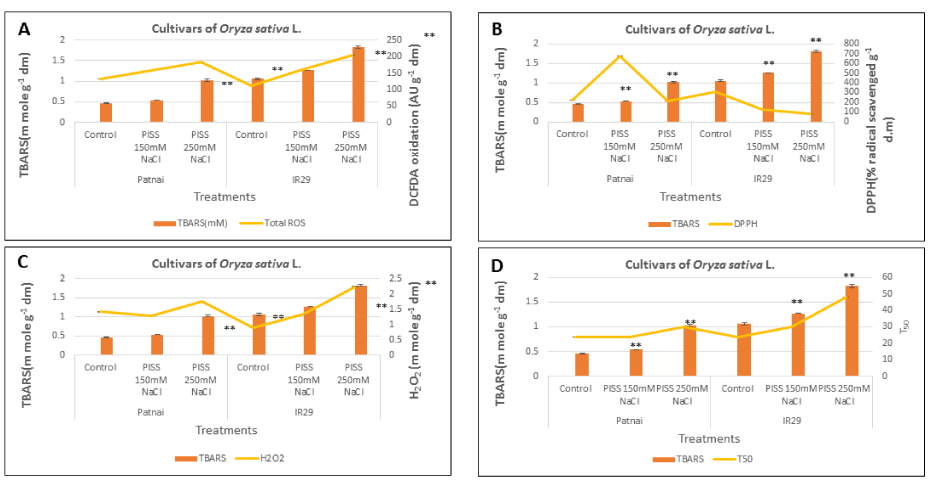
The redox metabolic shift suffered by the experimental rice cultivars imposed to PISS was assessed in terms of prooxidant-antioxidant status and changes in standard redox biomarkers of the germinating seedlings. In Figure 1, the brown spots indicate the presence of H2O2 in shoot tissues of the germinating seedlings as detected by DAB staining in both the rice cultivars raised under different magnitudes of salinity stress [150 mM NaCl (Figure 1B), 250 mM(1C)] vis-à-vis their untreated control [Figure 1(A)] seedlings. The cultivar Patnai exhibited a significantly lower accumulation of H2O2 at cellular level under same magnitude of PISS as compared to IR29, hinting at the controlled generation of ROS in salt-resistant experimental rice cultivar. Further, altered redox status of PISS raised seedlings was estimated in terms of accumulation of total ROS and H2O2, total antioxidant capacity and lipid peroxidation (thiobarbituric acid reactive substances) (Figure 2). The result substantiates the data of in-situ localization of ROS, with significantly greater accumulation of both total and individual ROS and associated lipid peroxidation (TBARS level) in salt-sensitive cultivar IR 29 as compared to salt-resistant cultivar Patnai, grown under the same magnitude of PISS [Figure 2(A), (C)]. A comparison of total antioxidant competence assessed in terms of DPPH radical scavenging assay, further revealed significant up-regulation for the cultivar Patnai as compared to IR29, confirming the reduction of level of TBARS associated with redox-regulatory attributes of salt-resistant cultivar Patnai [Figure2 (B), Figure 2D].
Further, the total antioxidant capacity competence which exhibited significant enhancement for the cultivar Patnai, showed significantly reduced accumulation of TBARS as compared to the cultivar IR29 under the same magnitude of PISS. In fact, the magnitude of lipid peroxidation, which exhibited close relationship with the redox status of the germinating seedlings grown under PISS (assessed in terms of accumulation of total and individual ROS and radical scavenging properties), showed a negative correlation with germination (Figure 2).
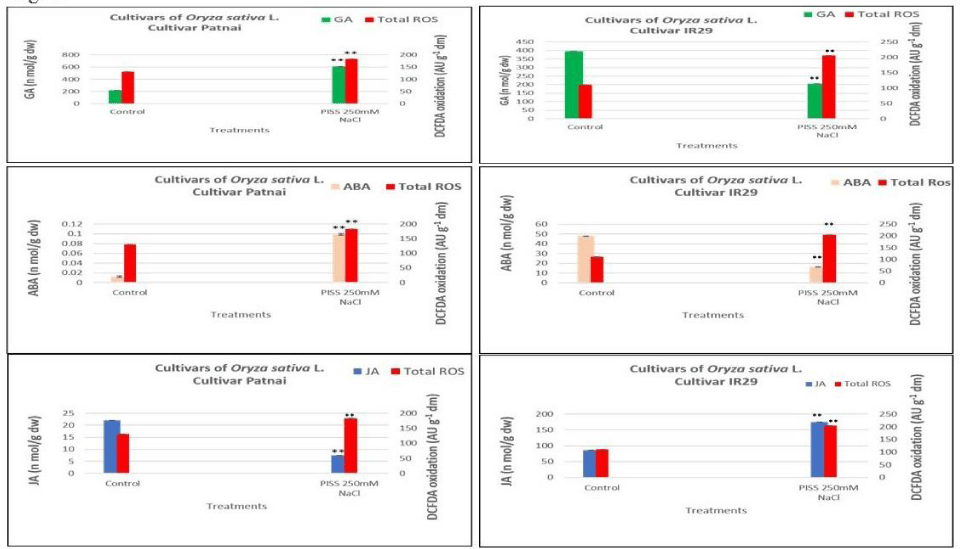
For the assessment of corelative evaluation of the impact of Post Imbibitional Salinity Stress (PISS) on redox and hormonal changes, and RP-HPLC based quantification of three important hormones GA, ABA and JA have been done in the germinating seedlings of both the experimental rice cultivars. There was in general PISS induced alteration in hormonal pools, which showed strong correlation with the alter redox status assessed in terms of the accumulation of total ROS (DCFDA oxidation) (Figures 3,4). Extraction and quantification of GA from PISS-raised seedlings of both the experimental rice cultivars revealed a differential response (Figures 3,4). PISS to the magnitude of 250 mM NaCl imposed to the salt-resistant cultivar Patnai not only evades reduction but also exhibited an up-regulation in the accumulation of GA over untreated control (Figures 3,4). When compared with the salt susceptible cultivar IR29, a contrasting response has been noticed (Figures 3,4), where PISS found to down-regulate GA accumulation significantly. A correlative evaluation of PISS-raised changes in redox states and GA accumulation for both the experimental rice cultivars showed that the cultivar showing restricted accumulation of ROS through redox regulation is able to up-regulate GA accumulation vis-à-vis with the cultivar showing greater ROS accumulation exhibiting a down regulatory response in GA accumulation (Figure 3-5). So, the ability of redox regulation in terms of ROS accumulation seems to have a direct impact on the accumulation of germination hormone GA under PISS. The same trend of result has been noticed when ABA was extracted and quantified by RP-HPLC from PISS raised seedlings of experimental rice cultivars. A significant positive correlation between the accumulation of ABA and accumulation of total ROS has been noticed for PISS-raised germinating seedling of the cultivar Patnai (Figures 3,4). The salt susceptible cultivar IR29 on the other hand exhibited a down-regulation in ABA accumulation showing an inverse relationship with decontrol ROS generation under PISS (Figures 3-5). The hormone JA on the other hand, showed the significant down-regulation in accumulation in PISS-raised seedlings of salt resistant cultivar Patnai indicating an inverse relationship with regulated ROS accumulation (Figures 3,4).
The results also exhibited strong correlation between PISS-induced modulation of redox metabolic shift in germinating tissue with germination and other basic physiological traits (allocation index, relative water content and Na+ / k+ ratio). Salt resistant cultivar Patnai showed better physiological performances in terms of regulation of Na+ / k+ ratio, RWC, allocation of solutes and germination phenotype under PISS as compared to its counterpart IR29 grown under same condition, advocating the role of redox regulation associated with salt tolerant rice germplasm.
Several workers noticed negative consequences of surplus ROS accumulation under salinity induced redox metabolic shift on lipid peroxidation, enzyme inhibition, protein oxidation, membrane and nucleic acid damage etc. affecting physiological performances of the crop [22-24]. The steady state redox status which depends on the relative rates of generation and decomposition, determine the subsequent role of the internal redox cue, toxic or signaling [5,8]. In fact, upregulation of antioxidant competence may be considered as proactive stress acclimation rejoinder which down regulates prooxidant status and exhibit tolerance to oxidative threat and salinity stress [1,5]. Several workers also showed close ROS – hormone interaction in regulating salinity tolerance. Hormones like GA3, ABA and JA found to have decisive roles in the salt stress response, regulating ROS homeostasis and homeostasis, orchestrating salt stress-responsive gene expression necessary for stress acclimation [3,25]. Salt-induced enhancement of ABA accumulation help to upregulate antioxidative defense and alleviate the harmful effects of oxidative stress [3,8].
Conclusion
Taken as a whole, the present work suggests the role of salinity induced redox metabolic shift in regulating differential redox cue necessary for stress acclimation in indica rice cultivars. The work further explored the close connection between salinity induced changes in oxidative windows and hormonal profile of germinating seeds in context of two contrasting experimental rice genotypes, for successful germination.
Authors acknowledge thanks to UGC (CAS) & DST-FIST for instrumentation facility to the Department of Botany, University of Burdwan for Research funding and facility to the Department [No.F.5-13/2012 (SAP-II) & SR/FST/LS-1/2018/188©]. ND acknowledges Government of West Bengal for State Funded Senior Research Fellowship [No. FC(Sc.) /RS/SF/BOT./2014-15/ 103/ (3)/1(3)]. Special thanks are extended to the Director, Rice Research Institute, Chinsurah, Hooghly, West Bengal, India for providing the seeds of the experimental germplasms. Gratitude is also extended to the Director, Crop Research and Seed Multiplication Farm, the University of Burdwan, West Bengal, India, for providing the experimental field.
- Liu H, Xin Z, Zhang Z (2011) Changes in activities of antioxidant related enzymes in leaves of resistant and susceptible wheat inoculated with Rhizoctonia cerealis. Agric Sci China 10: 526–533. Link: https://bit.ly/3niOvFi
- Horie T, Schroeder JI (2004) Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol 136: 2457–2462. Link: https://bit.ly/335Ns4X
- van Zelm E, Zhang Y, Testerink C (2020) Salt Tolerance Mechanisms of Plants. Annu Rev Plant Biol 71: 403-433. Link: https://bit.ly/3fe1Ydh
- Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6: 66-71. Link: https://bit.ly/3zSHjVn
- Hossain MS, Dietz KJ (2016) Tuning of Redox Regulatory Mechanisms, Reactive Oxygen Species and Redox Homeostasis under Salinity Stress. Front Plant Sci 7: 548. Link: https://bit.ly/3FiKmri
- Zagorchev L, Teofanova D, Odjakova M (2016) Ascorbate-glutathione cycle: Controlling the redox environment for drought tolerance. Hossain MA, Wani SH, Bhattacharjee S, Burritt DJ, Phan Tran L-S (Eds.) Drought stress tolerance in plants, Spinger Nature, Switzerland 1: 187-226. Link: https://bit.ly/3GpslZC
- Choudhary P, Pramitha L, Rana S, Verma S, Aggarwal PR, et al. (2021) Hormonal crosstalk in regulating salinity stress tolerance in graminaceous crops. Physiol Plant 173: 1587-1596. Link: https://bit.ly/33r5MVM
- Bhattacharjee S (2019) Reactive oxygen species in Plant Biology. Springer Nature, New Delhi 1–31. https://bit.ly/3qlU3B3
- Oracz K, Karpiński S (2016) Phytohormones Signaling Pathways and ROS Involvement in Seed Germination. Front Plant Sci 7: 864. https://bit.ly/3K7de9l
- Hoss Hussain S, Zhang ZJ, Zhong C, Zhu L, Cao XC, et al. (2017) Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. Journal of Integrative Agriculture 16: 2357–2374. Link: https://bit.ly/3zOO9eJ
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, et al. (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key actors of cellular signaling during germination. Plant Physiol 150: 494–505. Link: https://bit.ly/3HVIndX
- Lee S, Kim SG, Park CM (2010) Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol 188: 626–637. Link: https://bit.ly/3nj2Wt6
- Wituszyńska W, Slesak I, Vanderauwera S, Szechyńska-Hebda M, Kornas A, et al. (2013) Lesion Simulating Disease1, enhanced disease susceptibility1, and phytoalexin deficient4 conditionally regulate cellular signaling homeostasis, photosynthesis, water use efficiency, and seed yield in Arabidopsis. Plant Physiol 161: 1795–1805. Link: https://bit.ly/3HWUM1h
- Simontacchi M, Caro A, Fraga CG, Puntarulo S (1993) Oxidative stress affects-tocopherol content in soyabean embryonic axes upon imbibitions. Plant Physiol 103: 943–953. Link: https://bit.ly/3zO4Cjo
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, et al. (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287. Link: https://bit.ly/3fcUnvw
- Mensor LI, Menezes FS, Leitao GG, Reis AS, dos Santos T, et al. (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15: 127-130. Link: https://bit.ly/3niDOmn
- Heath RL, Packer L (1968) Photo-oxidation in isolated chloroplasts: kinetics and stoichiometry of fatty acid oxidation. Arch Biochem Biophys 125: 189–198. Link: https://bit.ly/336mTN0
- Rubio-Casal AE, Castillo JM, Luque CJ, Figueroa EM (2003) Influence of salinity on germination and seeds viability of two primary colonizers of Mediterranean salt pans. J Arid Environ 53: 145-154. Link: https://bit.ly/3Gni0NF
- Bhattacharjee S (2008) Calcium-dependent signaling pathway in the heat-induced oxidative injury in Amaranthus lividus. Biol Plantarum 52: 137. Link: https://bit.ly/3K27ldI
- Allison LE, Bernstein L, Bower CA, Brown JW, Fireman M, et al. (1954) Diagnosis and improvement of saline and alkali soils. Richards LA (Ed) Agriculture Handbook of United States Department of Agriculture Government printing office, Washington 60: 96. Link: https://bit.ly/3fj9Vhj
- Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15: 413-428. Link: https://bit.ly/31T4O4m
- Mishra A, Tanna B (2017) Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front Plant Sci 8: 829. Link: https://bit.ly/3zTBIyq
- Srivastava S, Dubey RS (2011) Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regulation 64: 1-16. Link: https://bit.ly/3nj0KSa
- Habib SH, Kausar H, Saud HM (2016) Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. Bio Med Res Int 6284547. Link: https://bit.ly/3r4qrqI
- Liu CW, Chang TS, Hsu YK, Wang AZ, Yen HC, et al. (2014) Comparative proteomic analysis of early salt stress-responsive proteins in roots and leaves of rice. Proteomics 14: 1759-1775. Link: https://bit.ly/3fhRjOA
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
