Annals of Systems Biology
Blood and aqueous humor tumstatin concentrations associated with diabetic retinopathy
Oruc Y1 and Aydin S2,3*
2Cardiovascular Surgery Department, Elazig Fethi Sekin City Hospital, Health Science University, 23119 Elazig, Turkey
3Department of Anatomy, Elazig Fethi Sekin City Hospital, Health Science University, 23119 Elazig, Turkey
Cite this as
Oruc Y, Aydin S (2020) Blood and aqueous humor tumstatin concentrations associated with diabetic retinopathy. Ann Syst Biol 3(1): 025-028. DOI: 10.17352/asb.000008Aim: Diabetic Retinopathy (DR) is the most common microvascular complication of Diabetes Mellitus (DM). This study was carried out to determine blood and aqueous humor tumstatin level in patients (DM + cataract, in patients (DR+cataract), and patients only having cataract.
Methods: Blood and aqueous humor were collected from patients. Tumstatin measurement is performed by Enzyme-Linked Immunosorbent Assays in Biological Samples (ELISA).
Results: Blood and aqueous humor tumstatin were significantly raised in patients (diabetes mellitus + cataract) and in patients (diabetic retinopathy+cataract) as compared to patients only having cataract (their p values were less than 0.05). Aqueous humor tumstatin were higher than blood tumstatin having p values, 0.001.
Conclusion: Taken together, these results demonstrate that diabetic retinopathy is associated with high levels of tumstatin and this peptide might be a novel potential therapeutic agent in diabetic retinopathy in the future.
Introduction
Diabetic Retinopathy (DR) is blood vessel damage in the retina that occurs due to long-standing Diabetes Mellitus (DM) [1]. It is estimated that 642 million people are to live with diabetes worldwide by 2040 [2]. Therefore, the global prevalence of diabetic retinopathy is also expected to increase significantly over the past 20 years, due to the rise in the number of people diagnosed with diabetes [3]. However, incidence rate of DR might be decreased by aggressive control of hyperglycemia and hypertension [4,5]. As known in diabetes, increased blood sugar concentrations damage the blood vessels of the retina [6]. Retina is composed of blood vessels, nerve cells (neurons), and photoreceptor cells [7]. These damaged blood vessels leak fluid, bleed, and do not provide adequate oxygen to the retina, leading to retinal ischemia (microaneurysms, hemorrhages, intraretinal microvascular abnormalities, cotton-woolspots, venous caliber abnormalities, and neovascularization [8,9]. As a result, vision loss can begin to due to vitreous hemorrhage and/or retinal detachment, macular edema, and retinal capillary nonperfusion and the retina is unable to function properly [10]. If it is find out other underline mechanism of DR fully might delay at least the onset of diabetic retinopathy besides controlling blood sugar. As mentioned above, damage to the blood vessels deprives the retina of oxygen. Angiogenesis is new blood vessel formation from the preexisting vasculature, but uncontrolled neovascularization is associated with a number of pathological disorders including rheumatoid arthritis and diabetic retinopathy [11,12]. Angiogenesis might help damaged blood vessels in order provide the oxygen and other essential nutrients in the retinal ischemia [12]. Recent studies have suggested a connection between tumstatin involved in newly formed vessel network in a corneal neovascularization [13]. The role of Tumstatin in DR is unknown and remains an essential research interest until now. There might be a link between Tumstatin, cataract, DM+ cataract and DR. Therefore, the involvement of Tumstatin in the progression of DR has yet to be elucidated.
Tumstatin (an angiogenesis inhibitor) is a protein with a molecular weight of 28 kDa [14]. This protein belongs to the type IV collagen C4 domain (or collagen IV NC1 domain) of the α3 chain of type IV collagen (α3 [IV], the 1.9-a crystal structure of the noncollagenous (NC1) in smooth muscle basement membranes. Tumstatin deficient mice [type IV collagen α3 chain knockout (COL4A3−/−)] affects angiogenesis or regulate the secretion of various growth factors [15]. Blood Tumstatin concentration might play a crucial role in inhibiting in vivo neovascularization induced by Vascular Endothelial Growth Factor (VEGF) and progression of vessel formation [14].
Also, administration of tumstatin has been demonstrated to reduce glomerular hypertrophy, hyperfiltration, and albuminuria in streptozotocin-induced diabetic mice [16]. Monocyte/macrophage accumulation was also inhibited by tumstatin peptide [16,17]. Based on above given information it is possible that tumstatin may have a decisive role in a number of ocular pathologies including retinopathy of prematurity and diabetic retinopathy. Therefore, purpose of this was to determine levels of tumstatin in the blood and Aqueous humor (Aq) of patients with diabetes mellitus and cataract with and without diabetic retinopathy.
Materials and methods
This retrospective study was approved by Institutional Review Board of the Firat University. 20 patients aged 37-68 years with type 2 diabetes having DR+cataract, 20 patients aged 35-59 years with type 2 diabetes having cataract and 20 patients aged 36-71 years with cataract were included in the study. Patients with type 1 diabetes, or previous intra-ocular surgery or any other systemic disease including hypertension, laser photocoagulation and intra-vitreal corticosteroids were excluded from the study. Diabetic retinopathy was diagnosed with a comprehensive dilated eye exam [18] and according to the ADA guidelines [19] and cataract diagnosis was done by ophthalmologists [20]. Biological fluids (blood and aqueous humor) were obtained while Phaco surgery was performed between January 2016 and December 2019 as indicated in detail in our previously published article [10]. Blood and aqueous humor are transferred to the archives and stored -400C for further analysis as explained earlier [21]. Tumstatin concentrations were analyzed from our biological fluid archives while other parameters were obtained from patient’s files. Fasting blood glucose and triglycerides levels were measured with standard methods by biochemistry analyzer. The Body Mass Index (BMI) was calculated by dividing the body weight in kilograms to the square of the height in meters [body weight/height2].
Quantitation of blood and aqueous humor tumstatin
Tumstatin measurement is carried out by enzyme-linked immunosorbent assays (Bioassay Technology Laboratory catalog no: E4234Hu Shanghai, CHINA) in blood and aqueous humor (ELISA). To determine whether the Tumstatin ELISA kit accurately measured the parameters in our samples, the detectability and reliability of this assay was assessed in our laboratory according to previously published procedure [21]. The measurement range of the human Tumstatin kit was 0.2-70 ng/mL, the intraassay Coefficient of Variation (CV) value, <8%, and the interassay CV value , <10%. Aq samples were diluted with phosphate-buffered saline (pH 7.4 at a 1:2 ratio); Plates were washed using the automatic washer Bio-Tek ELX50 (BioTek Instruments, Winooski). Optical density values were measured at a wavelength of 450 nm using the spectrophotometric microplate reader ChroMate P4300 (Awareness Technology Instruments, FL).
Statistical analyses
The mean comparisons between groups were performed using Statistical Package for the Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA). The significance level was set at p<0.05. Kruskal-Wallis test was used for comparison of means between groups for all the parameters.
Results
The demographic characteristics and some biochemical parameters of the participants are seen in Table 1.The average age of the cataract, DM and DR patients in this study was 56.10±3.38, 57.40±3.10 and 59.38±2.83 years, respectively. It was also found that for blood samples, the intraassay CV value and interassay CV values were 8%, and 10%, respectively. For aqueous humor samples, the intraassay CV value and interassay CV values were 10%, and 12%, respectively. Recovery results of the kits ranged from 81% to 129%. Linearity in the measurements was observed at dilutions of 1:2, 1:4, 1:8, and 1:16. Based on our tests, the tumstatin ELISA kit for aqueous humor samples were as sensitive as blood samples. DR is also more common in women than men.
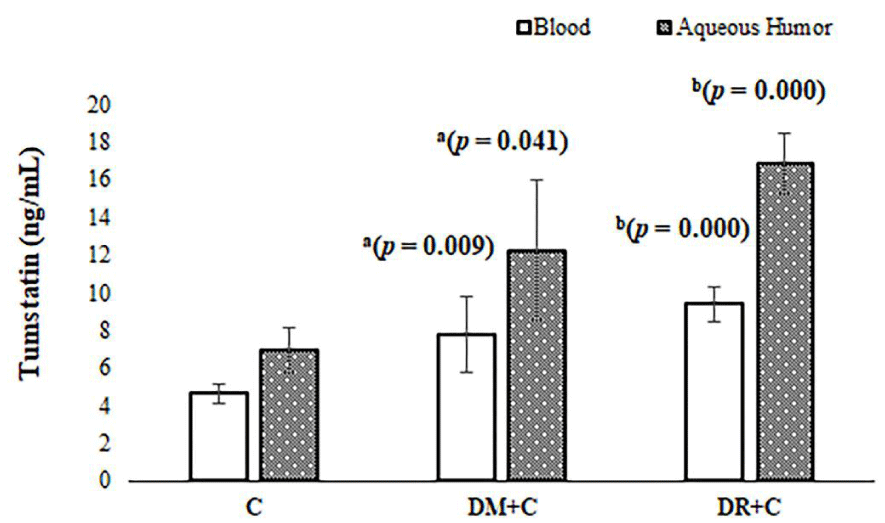
Blood tumstatin levels were significantly elevated in DR patients with cataract as compared with diabetic subjects with cataract (without DR) and patients with only cataract (control) having p values; 0.009, 0.000, respectively as indicated in Figure 1. Aqueous humor tumstatin levels were also significantly elevated in DR patients with cataract as compared with diabetic subjects with cataract (without DR) and patients with only cataract (control) having p values; 0.041, 0.000, respectively as indicated in Figure 1. Aqueous humor tumstatin levels were higher than that of blood tumstatin levels as seen Figure 1.
Discussion
In the present study, we first time demonstrate that tumstatin significantly increased in the blood and Aq of patients with DM + cataract, with DR+ cataract when compared with patients bearing only cataract. It was also observed that Tumstatin significantly increased in the blood and Aq of patients with DR + cataract than that of patients with DM + cataract. The rise in tumstatin in the blood and aqueous humor might lead to compensatory mechanisms aimed at ameliorating alterations in the early stage of diabetes. Supporting this notion it has been showed that the therapeutic effect of tumstatin in ameliorating alterations in the early stage of diabetic nephropathy induced by Streptozotocin (STZ) in mice [16].
These effects of Tumstatin were supposed to be mediated through downregulation of pro-angiogenic factors and Endothelial Growth Factors (VEGF). Increased expression of VEGF has been reported in diabetic retinopathy [22]. Tumstatin also inhibits in vivo neovascularization induced by VEGF, suggesting its potential therapeutic efficacy in diabetic nephropathy [16], since upregulation of VEGF has been reported in diabetic nephropathy [22].
Furthermore, administration of anti-VEGF neutralizing antibodies has been indicated to fall hyperfiltration, albuminuria, and glomerular hypertrophy in diabetic rats or mice [23,24]. Also, it has been reported that treatment with tumstatin remarkably suppressed glomerular hypertrophy, hyper filtration, and urinary albumin excretion as well as the accumulation of mesangial matrix [16]. That study was suggestive of the involvement of angiogenesis in the development of glomerular alterations in diabetic nephropathy similar to diabetic retinopathy [25].
The level of tumstatin was significantly high in aqueous humor as compared with the level of tumstatin in the blood. The marked increase of tumstatin in aqueous humor suggests that aqueous humor might be another source of tumstatin and considering simultaneous upregulation with blood and that might pass to aqueous humor via blood tumstatin saturation and possibly associated with diabetery disease response. These results suggest the biological function of tumstatin peptide as an antiangiogenic peptide in a DR similar to its efficacy reported on diabetic nephropathy model [26].
Diabetic Retinopathy (DR) patients have high levels of tumstatin. So that blood and aqueous humor tumstatin can probably be considered as one of the best biological markers reflecting diabetic retinopathy compared with control. The increased amount of tumstatin in patient with diabetic retinopathy must be taken into account and the increased amount of tumstatin might be associated with pathophysiology of diabetic retinopathy. If neovascularization contributes significantly to the pathogenesis of diabetic retinopathy, then tumstatin (antiangiogenic) therapy might be a novel approach to retard the progression of diabetic retinopathy in the future.
- Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H (2018) Early detection of diabetic retinopathy. Surv Ophthalmol 63: 601-608. Link: https://bit.ly/2S3wdYZ
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, et al. (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128: 40-50. Link: https://bit.ly/3eOXN64
- Delcourt C, Massin P, Rosilio M (2009) Epidemiology of diabetic retinopathy: expected vs reported prevalence of cases in the French population. Diabetes Metab 35: 431-438. Link: https://bit.ly/2S7kWXU
- Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, et al. (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977-986. Link: https://bit.ly/2S454Wa
- Schrier RW, Estacio RO, Esler A, Mehler P (2002) Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 61: 1086-1097. Link: https://bit.ly/3cHedf1
- Lutty GA (2013) Effects of diabetes on the eye. Invest Ophthalmol Vis Sci 54: ORSF81- ORSF87. Link: https://bit.ly/2VSWFpA
- Country MW (2017) Retinal metabolism: A comparative look at energetics in the retina. Brain Res 1672: 50-57. Link: https://bit.ly/2VxSfoT
- Lasta M, Palkovits S, Boltz A, Schmidl D, Kaya S, et al. (2012) Reproducibility of retinal vessel oxygen saturation measurements in healthy young subjects. Acta Ophthalmol 90: e616- e6120. Link: https://bit.ly/2Kw3asZ
- Kawasaki R, Xie J, Cheung N, Lamoureux E, Klein R, et al. (2012) Retinal microvascular signs and risk of stroke: the Multi-Ethnic Study of Atherosclerosis (MESA). Stroke 43: 3245-3251. Link: https://bit.ly/2VwnVem
- Oruc Y, Celik F, Ozgur G, Beyazyildiz E, Ugur K, et al. (2020) Altered Blood And Aqueous Humor Levels Of Asprosin, 4-Hydroxynonenal, And 8-Hydroxy-Deoxyguanosine In Patients With Diabetes Mellitus And Cataract With and Without Diabetic Retinopathy. Retina. Link: https://bit.ly/2VxAvtS
- Sisto M, Lisi S, Ingravallo G, Lofrumento DD, D'Amore M, et al. (2014) Neovascularization is prominent in the chronic inflammatory lesions of Sjögren's syndrome. Int J Exp Pathol 95: 131-137. Link: https://bit.ly/2Y37Kai
- Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298-307. Link: https://bit.ly/3azgBD1
- Gajjar DU, Vasavada AR, Patel P, Praveen MR, Shah SR (2019) Evaluation of collagen derived antiangiogenic factors and matrix metalloproteinases in anterior lens epithelial cells of pediatric eyes with persistent fetal vasculature. Indian J Ophthalmol 67: 1618-1622. Link: https://bit.ly/2VSXaQu
- Boosani CS, Sudhakar YA (2011) Proteolytically Derived Endogenous Angioinhibitors Originating from the Extracellular Matrix. Pharmaceuticals (Basel) 4: 1551-1577. Link: https://bit.ly/3ayqgdg
- Hamano Y, Kalluri R (2005) Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun 333: 292-298. Link: https://bit.ly/2xNrKmC
- Yamamoto Y, Maeshima Y, Kitayama H, Kitamura S, Takazawa Y, et al. (2004) Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes 53: 1831-1840. Link: https://bit.ly/2KxajcE
- Van der Velden J, Harkness LM, Barker DM, Barcham GJ, Ugalde CL, et al. (2016) The Effects of Tumstatin on Vascularity, Airway Inflammation and Lung Function in an Experimental Sheep Model of Chronic Asthma. Sci Rep 6: 26309. Link: https://bit.ly/3aAp2hw
- Beaser RS, Turell WA, Howson A (2018) Strategies to Improve Prevention and Management in Diabetic Retinopathy: Qualitative Insights from a Mixed-Methods Study. Diabetes Spectr 31: 65-74. Link: https://bit.ly/3aFWd3r
- American Diabetes Association (2018) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 41: S13-S27. Link: https://bit.ly/2KxRk1I
- Gogate P, Wood M (2008) Recognising “high-risk” eyes before cataract surgery. Community Eye Health 21: 12-14. Link: https://bit.ly/2S3oYAe
- Aydin S (2015) A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 72: 4-15. Link: https://bit.ly/2yHDkiY
- Gupta N, Mansoor S, Sharma A, Sapkal A, Sheth J, et al. (2013) Diabetic retinopathy and VEGF. Open Ophthalmol J 7: 4-10. Link: https://bit.ly/3eNjdR5
- Tufro A, Veron D (2012) VEGF and podocytes in diabetic nephropathy. Semin Nephrol 32: 385-393. Link: https://bit.ly/34Zx6r1
- Ferrara N (2001) Role of vascular endothelial growth factor in the regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280: C1358- C1366. Link: https://bit.ly/3eQbMsg
- Lee WJ, Sobrin L, Lee MJ, Kang MH, Seong M, et al. (2014) The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3). Invest Ophthalmol Vis Sci 55: 6547-6553. Link: https://bit.ly/3ePOwdU
- Ichinose K, Maeshima Y, Yamamoto Y, Kitayama H, Takazawa Y, et al. (2005) Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 54: 2891-2903. Link: https://bit.ly/3569fWK
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
