Annals of Systems Biology
Spectrum of Leishmania donovani infection in the Southwest of Sudan: A rapid epidemiological mapping
Sharief AH1*, Khalil EAG1, Elmagzoub RM2 and Omer SA2
2Department of Immunology, Tropical Medicine Research Institute, National Centre for Research, Khartoum, Sudan
Cite this as
Sharief AH, Khalil EAG, Elmagzoub RM, Omer SA (2019) Spectrum of L. donovani infection in the Southwest of Sudan: a rapid epidemiological mapping. Ann Syst Biol 2(1): 008-011. DOI: 10.17352/asb.000003This epidemiological survey was based on simple immunological techniques (leishmanin skin reactivity and the direct agglutination tests), combined with clinical history to obtain data about the spectrum of L. donovani infection in communities at risk of developing visceral Leishmaniasis. The data was depicted in map format to give an enhanced visual impact.
In this study clinical history and immunological tests, together with the past history of the infection, were combined and conducted in 1781 randomly selected volunteers in Southwestern Sudan.
The overall leishmanin skin reactivity of ≥5mm was 24.5% and children (<15 years) had higher leishmanin reactivity (≥5mm) of 13.2% compared to 11.3% in adult (>years). The DAT results showed that 13% (238/1781) had titres above the diagnostic cut-off level of 1:3200, while 87% had titre under the cut-off point.
The study concluded that the use of clinical interview combined with simple immunological tests can give valuable information about the pattern of L. donovani infection and predict future prevalence of VL in a short time. Leishmanin non-reactive individuals are a useful piece of data to plan for future vaccine efficacy studies. The interactive dynamic map that was produced in the GIS can act as a nidus for development of a leishmania network in Sudan and the surrounding countries that are endemic for VL.
Introduction
From the early 1900s, visceral leishmaniasis (VL) has been among the most important health problems in Sudan. The disease is historically endemic in a wide belt extending from the western bank of the White Nile and the Sudan–Ethiopian border. This area includes the southern areas of Central and Eastern states and the north-eastern part of Upper Nile State. Since 1989, VL has spread in the western part of Upper Nile State, the disease has never been reported before. Cases have also been reported from Darfur State in western Sudan, the Nuba mountains area (Kordofan State) and the Kapoeta area in Eastern Equatoria [1].
Between 1984 and 1994, an estimated 100,000 were died in the Upper Nile Province (southern Sudan) due to visceral leishmaniasis (VL); because of the isolation of the area caused by the war, the epidemics went unnoticed until 1988. A number of causes were proposed for this outbreak in previously non-endemic areas; regeneration of A. seyal and B. aegyptica forests after they were destroyed in the 1960’s, introduction of the parasite from VL-endemic areas along the Ethiopian.
Borders by movement of military personnel, discontinuation of residual insecticide spraying for malaria because of the civil war, and malnutrition of the people.
The advent of GIS has greatly facilitated the mapping process and, in particular, enables maps to be updated rapidly and presented in different ways. CL and VL could possibly be mapped using GIS and database of the incidence and/or prevalence rates in a selected area using census data and official CL/VL records from the available health centres or peripheral hospitals [2-4].
In this study we aimed to identify Leishmania spectrum of infection in an endemic area in Western Sudan by using rapid assessment method that included: Clinical history, leishmanin skin test, Direct Agglutination Test (DAT), as a rapid epidemiological assessment scheme which is very much needed to prevent unnecessary loss of lives.
Methods
Study area
Outskirts of Muglad and Babnousa cities (southwest Kordofan State), western Sudan were selected for this study. The area witnessed a VL epidemic that particularly hit Bentue area in late eighties and took lives of hundreds of thousands from the Dinka of the Nuer tribe. The area (about 457 miles western Khartoum) located between Latitudes 11.03˚C and Longitudes 27.73˚C. The approximate population of the area according to national census of 2007 was about 10.000 individuals. The soil is sandy and the rainy season in the area usually starts in June and extends to October; the average rainfall is about 651mm, and the temperature ranges from 25˚C to 40˚C. The nature and green vegetation of the area is characterized by rich savannah where the majority of the land covered by acacia and balanities forests. The elevation of the study area is 434 meter (1472 feet). The area is mainly inhibited by the Mesairya (a nomadic tribe) who are principally cow owners. They are continuously moving from one place to another looking after their cattle (pastoralism). During their movement, the Mesairya takes a trip from southern states (Bentue) towards the north (Abugabra) and returning back in the rainy season to settle in Bahr El-Arab area during the summer (non rainy times).
Ethical consideration
This study was ethically approved by the Ethical Boards of Institute of Endemic Diseases, University of Khartoum and Federal Ethical Committee, Ministry of Health, Khartoum, Sudan.
Sample size for seroepidemiological screening
We randomly recruited 1781 individuals; demographic and clinical data was collected in a specially designed questionnaire by direct interviewing and entered into the computer. Data from laboratory tests was also entered.
Volunteer’s assessment
All selected volunteers were primarily subjected to clinical interview for their previous exposure to VL infections. They were then subjected to leishmanin skin testing (LST) as described by Zijlstra et al. The test was read after 48 or 72 hours using the ballpoint pen method [5]. The presence of induration +/- erythema of ≥5 was considered a positive reaction [6]. Direct agglutination test (DAT) was also performed as described by [7]. The test is a direct reaction between antigen (L.donovani whole parasite antigen) and antibodies (patients serum). Briefly, patients’ sera were diluted (1:100–1:102400) in gelatine diluents. 50µL of the antigen was added in each well of V-shaped microtiter plates. The test was read in the next day and a titer of ≥1:3200 was considered as a cut-off for a positive test.
Parasite isolation and cultivation
A lymph node aspiration was performed for all suspected VL patient who had lymphadenopathy. Those whose diagnosis were not confirmed by direct microscopic examination but were still highly suspected were contacted in the next day and preceded for bone marrow aspiration. Aspirations from enlarged lymph node were done using a sterile needle, the epitrochlear lymph node was punctured and then the materials were injected into a vacutainers containing NNN culture medium. Prior to bone marrow aspiration, patients were firstly being locally anaesthetized, and then using a bone marrow aspiration needle, a few drops of the bone marrow were taken from the posterior superior spinae. The materials were also dispensed into containers of culture medium. Samples were kept at 26˚C before being transferred to the laboratory where it was properly kept at 27˚C for 2 weeks until promastigotes reached their stationary phase.
Map drawing
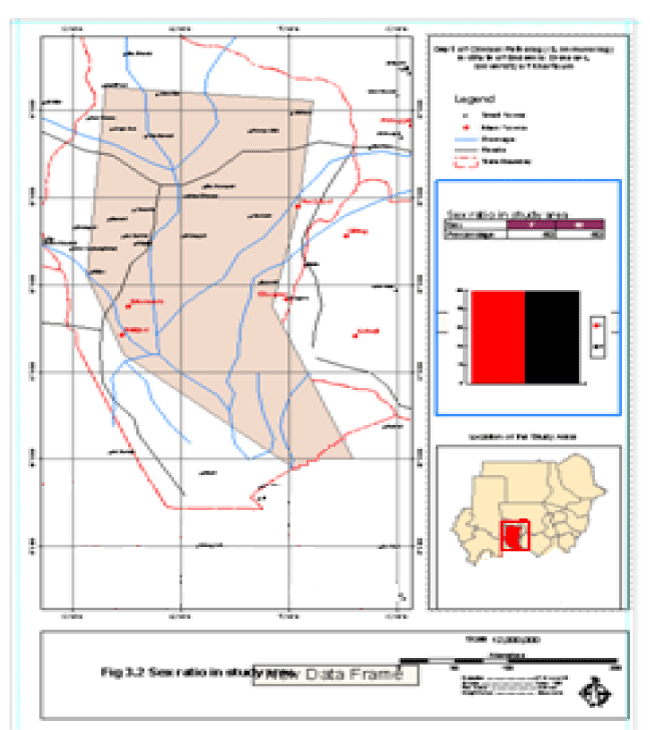
The study area coordinates were obtained using GPS 12 XS, Garmin International, Kansas, USA. ArcGIS Version 9.0, ESRI, USA was used to construct a project called VL/GIS project (VL/GIS.apr) and files contained layers coverage’s e.g. political boundaries; populated areas; drainage network, drainage points and roads network were downloaded. The interactive dynamic map was drawn and all coverage layers were placed in one view. Tables of leishmanin reactivity, DAT titres, age and sex distribution for the selected villages were added to the table and appropriate charts were also made in the project. Different layouts were prepared to show map themes and then the study area was constructed. The weather phenomena including total temperature, rainfall average, humidity, and wind directions were collected from National Metrological Centre, Khartoum, Sudan.
Results
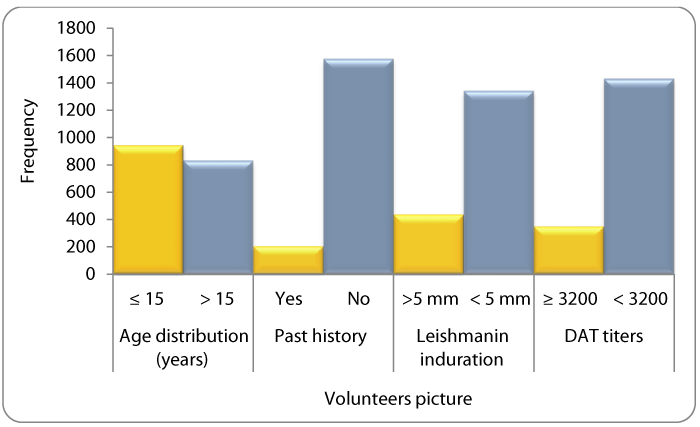
The mean age of the volunteers was 20.7 years (range 1-90). Children (<15 years) who are the most susceptible group to VL formed 53% of the study volunteers. Past history of VL (treated at nearby hospitals) was reported in 11%. History of skin rash following VL treatment was not reported. The majority of the volunteers (75.5%) did not react to the leishmanin antigen, but those above 15 years of age were more likely to be leishmanin reactive (induration >5mm) compared to those below that age. DAT titres <3200 have been noticed in 88% of the volunteers, whereas 11.7 had a titers ≥3200. A comparative summary of all of these results is shown in Figure 1.
Moreover, 63 individuals had a history of parasitology confirmed VL within the previous 12 months. Some of them presented and were diagnosed during the survey period. The mean age of these patients was 9.7+/- 6.0 years, with an equal male: female ratio. All were non-reactive to leashmanin skin testing and their DAT titers were equal and/or above 3,200.
Mapping the results
The constructed project was named VL/GIS. The project contained views that were formed from political boundaries, drainage network and road network themes. Tables of age groups, leishmanin reactivity and DAT titres were added to the cases to the project. Charts were constructed from the tables. Layouts for printing were formed and printed Figures 2,3.
Discussion
Visceral leishmaniasis (VL) is a major health problem in eastern and southern parts of the Sudan and it mainly affects children below 15 years Zijlstra et al., [8-10].
The western parts of the country are not known to be endemic with cutaneous leishmaniasis, but sporadic VL cases were detected. These areas witnessed a huge outbreak that has killed an estimate of 100.000 lives in late eighties. Such outbreak revealed the inadequacy of health services in dealing with such disasters that are more likely to occur from time to time [11].
The demographic data collected showed that the communities under study are mainly of the young age groups with approximate equal numbers of males and females.
The past history of VL infection was lower than the rate of leishmanin conversion; this may simply point to the possibility of subclinical infection [7,12]. Past history was considered as knowledge about VL in that area is fair enough as well as people’s diagnosis about the disease is very good. Leishmanin skin test (LST) was chosen as a simple and cheap test that correlates well with development of cell-mediated immunity and exposure to leishmania as was reported previously from eastern Sudan [8-10,13]. In this study, the LST positivity increased in direct proportion with age indicating that as individuals get older they will be increasingly exposed; some will develop overt disease while others remain asymptomatic (subclinical infection). This was extensively reviewed Bucheton et al., [12].
Volunteers who had DAT titers equal and above 6,400 match closely those who gave past history of VL. As reported by [14,15], that positive DAT test results may be obtained from patients with active VL, subclinical infection, and those who were infected more than 7 years previously (DAT titre will remain high for many years following successful treatment). The findings of this study are in agreement with that reported earlier by Hashim et al., [11] and recently by Mohammed et al., [16], who described an outbreak of VL among the Mesairya tribe in Kordofan area, this tribe moves annually towards Bentue areas looking after their cattle in search of water and suitable pasture, and they may acquired the infection during their stay in that areas. There is no transmission of VL in the Misairiya homelands around Muglad and Babnusa, but some of them may contract the infection during their daily movement and keeping close to the animals i.e. cow odours are very attractant to sand flies, which could easily be hidden in the animal hairs and then the flies activates and could easily transmit the parasites Baleela et al., [17].
Several authors use GIS technology for mapping leishmaniasis using spatial data and environmental factors [4,18]. Golpayegani et al., [3], investigated the relationship between environmental factors (vegetation and elevation) affecting the prevalence of cutaneous leishmaniasis and visceral leishmaniasis. After mapping the prevalence data from 144 studies, considering non-parametric ANOVA, the study indicated tendency of zoonotic visceral leishmaniasis to presence in high elevation and high vegetation was more than anthroponotic and zoonotic cutaneous leishmaniasis. Ostad et al., [2], produced distributional maps of CL over five years and evaluate the role of GIS in control of CL in Khuzestan province where an endemic area of CL in Iran. The study explored the trends in spatio-temporal dynamics of VL endemicity at a meso-scale level in Vaishali District, based on geographical information systems (GIS) tools and spatial statistical analysis. Moreover, High-case incidence rate (CIR) plays a major role in the spatial dispersion of the VL cases between non-endemic and endemic villages [4].
In this study the geographic information system (GIS) have been used as an early alarm system to determine those naïve individuals who are at risk of contracting the diseases and to avoid any sudden future outbreaks may occur. The GIS constructed interactive map where epidemiological information was linked to locations allows easy access to information and helps in graphical display. The information can easily be updated according to the current situation of the disease. The map can easily be shared through the internet, which allows faster transfer of the latest assessment of the disease in Sudan and/or other neighbouring countries. The interactive map that was produced in the GIS can be used as another point (with that produced map for the eastern of the Sudan) for a more comprehensive map for Sudan and other surrounding countries that are endemic for visceral leishmaniasis.
- WHO (2005) Communicable Disease Profile. Sudan. 42-45.
- Ostad M, Shirian S, Pishro F, Abbasi T, Armin A, et al. (2014) Control of Cutaneous Leishmaniasis Using Geographic Information Systems from 2010 to 2014 in Khuzestan Province, Iran. PLoS One. Link: http://bit.ly/2kMEm6S
- Golpayegani AA, Moslem AR, Akhavan AA, Zeydabadi A, Mahvi AH, et al. (2018) Modeling of Environmental Factors Affecting the Prevalence of Zoonotic and Anthroponotic Cutaneous, and Zoonotic Visceral Leishmaniasis in Foci of Iran: A Remote Sensing and GIS Based Study. J Arthropod Borne Dis 12: 41-66. Link: http://bit.ly/2knm102
- Mandal R, Kesari S, Kumar V, Das P (2018) Trends in spatio-temporal dynamics of visceral leishmaniasis cases in a highly-endemic focus of Bihar, India: an investigation based on GIS tools. Parasit Vectors 11: 220. Link: http://bit.ly/2knm7Vs
- Sokal JE (1975) Editorial: Measurement of the delayed skin-test responses. N Engl J Med 293: 501-502. Link: http://bit.ly/2mhO95h
- Khalil EAG, El-Hassan AM, Zijlistra EE, Hashim FA, Ibrahim ME, et al. (1998) Treatment of visceral leishmaniasis in Sudan: management of those who do not respond. Ann Trop Med Parasitol 92: 151-158. Link: http://bit.ly/2kNLLTg
- Harith A, Kolk AHJ, Leeuwenburg J, Muigai R, Huigen E, et al. (1988) Improvement of a direct agglutination test for field studies of visceral leishamaniasis. J Clin Microbiol 26: 1321-1325. Link: http://bit.ly/2ko5hWw
- Zijlstra EE, el-Hassan AM (2001) Leishmaniasis in Sudan. Visceral leishmaniasis. Trans R Soc Trop Med Hyg 95: S27-S58. Link: http://bit.ly/2kBMGq8
- Sharief A, Khalil E, Theander T, Kharazmi A, Omer S, et al. (2006) Rapid epidemiological assessment of L. donovani infection in eastern Sudan: immune surveillance and application of GIS. J Thromb Haemost5:1733-1735.
- Sharief AH, Khalil EAG (2017) Rapid Assessment of Patterns of L. donovani Infection in Eastern Sudan: Immune Surveillance and Application of Geographic Information System. Ann Clin Cytol Pathol 3: 1070-1076. Link: http://bit.ly/2mk7oLH
- Hashim FA, Ali MS, Satti M, el-Hassan AM, Ghalib HW, et al. (1994) An outbreak of acute Kala-azar in anomadic tribe in western Sudan: features of the diseases in a previously non-immune population. Trans R Soc Trop Med Hyg 88: 431-432. Link: http://bit.ly/2kL6M11
- Mueller YK, Nackers F, Ahmed KA, Boelaert M, Djoumessi JC, et al. (2012) Burden of Visceral Leishmaniasis in Villages of Eastern Gedaref State, Sudan: An Exhaustive Cross-Sectional Survey. PLoS Negl Trop Dis 6: e1872. Link: http://bit.ly/2kyZBZT
- Khalil EA, El Hassan AM, Zijlstra EE, Mukhtar MM, Ghalib HW, et al. (2000) Autoclaved leishmania major vaccine for prevention of visceral leishmaniasis: a randomized, double-blind, BCG-controlled trial in Sudan. Lancet 356: 1565-1570. Link: http://bit.ly/2lXNwxp
- Harith AE, Kolk AH, Kager PA, Leeuwenburg J, Muigai R, et al. (1986) A simple and economical direct agglutination test for sero-diagnosis and sero-epidemiological studies of visceral Leishmaniasis. Trans R Soc Trop Med Hyg 24: 364-367. Link: http://bit.ly/2lR83nq
- Jacquet D, Boelaert M, Seaman J, Rijal S, Sundar S, et al. (2006) Comparative evaluation of reeze‐dried and liquid antigens in the direct agglutination test for serodiagnosis of visceral leishmaniasis (ITMA‐DAT/VL). Trop Med Int Health 11: 1777-1784. Link: http://bit.ly/2lT1yR1
- Mohammed AM, Khalid NM, Aboud MA (2018) Kala-azar in Darfur: Evidence for indigenous transmission in Al-Malha Locality, North Darfur, western Sudan. Parasit Vectors 11: 14. Link: http://bit.ly/2lSd8vI
- Mukhtar MM, Sharief AH, el Saffi SH, Harith AE, Higazzi TB, et al. (2000) Detection of antibodies to L. donovani in animals in a kala-azar endemic region in eastern Sudan: a preliminary report. Trans R Soc Trop Med Hyg 94: 33-36. Link: http://bit.ly/2lVQmTC
- Iliopoulou P, Tsatsaris A, Katsios I, Panagiotopoulou A, Romaliades S, et al. (2018) Risk Mapping of Visceral Leishmaniasis: A Spatial Regression Model for Attica Region, Greece. Trop Med Infect Dis 3: 83. Link: http://bit.ly/2mdpZZv
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
