Annals of Psychiatry and Treatment
Comparison of two questionnaires (PDQ-39 and SEIQoL) for assessment of the quality of life in idiopathic Parkinson’s disease
Lena Waldvogel1, Ketevan Toloraia1, Peter Fuhr1 and Ute Gschwandtner2*
2Neurology (Clinical Neurophysiology), University of Basel Petersgraben 4, 4031 Basel, Switzerland
Cite this as
Waldvogel L, Toloraia K, Fuhr P, Gschwandtner U (2023) Comparison of two questionnaires (PDQ-39 and SEIQoL) for assessment of the quality of life in idiopathic Parkinson’s disease. Ann Psychiatry Treatm 7(1): 018-026. DOI: 10.17352/apt.000049Copyright
© 2023 Waldvogel L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Parkinson’s disease is a neurodegenerative disease of the central nervous system that begins insidiously and progresses over time with a loss of nerve cells in certain brain regions.
People with chronic diseases often experience a change in their quality of life. For patients, relatives, and the whole community, a reduced quality of life can pose a significant burden. Therefore, it is imperative to reduce socioeconomic costs to preserve high health quality in patients with neurodegenerative disorders. Parkinson’s disease can cause people to have difficulty performing daily activities such as working or shopping. It is not uncommon for social interaction to be impaired, as patients sometimes struggle to participate in social life due to their symptomatology. The quality of life of Parkinson’s disease patients can be measured in different ways. A distinction can be made between Health-related Quality of Life and Individualised Quality of Life. Several questionnaires and screening tools are investigating the Quality of Life in patients with Parkinson’s disease. However, their validity and practicability are often not extensively analyzed.
In this paper, we will investigate whether the two questionnaires, “The Parkinson’s Disease Questionnaire (PDQ-39)” and “Schedule for the Evaluation of Individual Quality of Life (SEIQoL)”, measure the same quality of life in PD patients. The two questionnaires do not reach the same results, although they both measure the construct “Quality of Life” and should be used complementary to gain deeper insight into patients’ real-life problems.
Abbreviations
PDQ-39: Parkinson’s-Disease-Questionnaire 39; SEIQoL: Schedule for the Evaluation of Individual Quality of Life; PD: Parkinson`s Disease; H&Y: Hoehn and Yahr Scale; UPDRS: Unified Parkinson Disease Rating Scale; QoL: Quality of life
Introduction/Background
As the population continues to age, diseases such as Parkinson’s disease are becoming a focus of scientific interest. On average, about 2 out of 1,000 people have Parkinson’s disease, a neurodegenerative disorder that causes increasing disability. More than 15’000 people in Switzerland are affected by the as-yet incurable disease.
In addition to physical symptoms, Parkinson’s patients often experience psychological complaints. For example, in about 20 to 40% of patients, depression or depressive mood develops over the years. Parkinson’s disease is, therefore, a complex disorder that severely affects the patient’s quality of life.
Parkinson disease
Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s disease, and the disease’s key pathological feature is the loss of dopaminergic neurons in the brain [1]. Dopamine is a neurotransmitter responsible for the coordination and control of body movements and is produced in the neurons of the substantia nigra (black matter). This nerve structure is located in the basal ganglia. If these neurons are damaged or die, there is a decrease in dopamine levels [1]. When approximately 50%to 60% of the dopamine-forming cells have died, the main symptoms of the disease occur, which are tremors, bradykinesia, and rigor, which will be discussed later. It is not yet known which circumstance is responsible for the degradation of the nerve cells. In the past, the risk of developing PD was primarily attributed to adverse environmental factors [1]. However, most researchers point to a combination of genetic and environmental factors. There is no cure for Parkinson’s disease to date. However, in addition to the administration of dopamine and dopamine analogs, numerous non-drug therapies can help alleviate symptoms and slow the progression of the disease [2-4].
Incidence
The disease affects approximately 1% to 2% of adults over 65 years of age and 4% of adults over 80 years of age [2]. Approximately 10 million people worldwide are affected by Parkinson’s disease [5,6]. Most often, the disease is diagnosed between the ages of 50 and 60. Since the disease is much more common in old age, it is assumed that more people will be affected in the future due to increasing life expectancy. In addition, men are more frequently affected by the disease; the ratio of men to women is about 3:2 [2]. Prevalence appears to be higher in Europe, North America, and South America than in Africa, Asia, and Arab countries [1].
Motor symptoms
Parkinson’s disease can present with a variety of symptoms. In the early stages of the disease, the most obvious [7-9] symptoms are movement-related. Probably the most noticeable and often one of the first symptoms is a tremor, an involuntary, rhythmic, shaking movement of a body part. The tremor usually begins in the hand or fingers. Many Parkinson’s patients are affected by a resting tremor, so their muscles tremble when they are at rest, and the tremor decreases or may disappear when the person moves the muscles involved. In addition, the tremor may become stronger when the affected person is tired or stressed [10-12].
Another common symptom in the early stages of Parkinson’s disease is slowed movement, called bradykinesia. This symptom can cause patients to have difficulty performing everyday actions, such as buttoning a shirt, cutting food, or brushing their teeth. This symptom often leads to patients becoming frustrated and a decrease in their quality of life [13].
Muscle tension, also known as rigidity, is characterized by the inability of muscles to relax normally. This symptom can cause severe pain in the patients.
Non-motor symptoms
Meanwhile, the symptomatology of Parkinson’s disease is considered heterogeneous, with clinically significant non-motor [14]. As in all chronic diseases and especially in Parkinson’s disease patients, the psychological aspect is of great importance for the quality of life [14]. Parkinson’s disease is associated with many non-motor symptoms, such as fatigue, depression, anxiety disorders, and apathy [15]. These symptoms may appear many years or even decades before diagnosis. According to one study, the non-motor symptoms are often perceived as more burdensome than the motoric ones by affected individuals. Thus, alleviating these complaints can improve the quality of life of Parkinson’s disease patients [15-17]
Hoehn and yahr scale [18]
The Hoehn and Yahr Scale (H&Y) is a widely used clinical rating scale that ranks patients according to their current level of functioning [10,19-23]. The assessment instrument distinguishes between five levels. Its advantage is that it is simple and easy to use. Progression through the HY stages has been shown to correlate with motor decline and deterioration in the quality of life [24]. Because the scale does not address all aspects of the disease, it is often used in combination with other assessment tools to provide a more comprehensive picture of the condition.
Unified Parkinson disease rating scale [19]
The Unified Parkinson’s Disease Rating Scale (UPDRS) is a rating scale used to assess the severity and progression of PD symptoms. It consists of four sections that assess both motor and non-motor symptoms, as well as daily activities and treatment complications. Each section consists of different questions or statements that are scored on a scale of 0 to 4. The higher the total score, the more severe the symptoms of Parkinson’s disease. The UPDRS assesses disease progression and treatment effectiveness [25].
Quality of life in Parkinson’s disease
People are concerned not only about life expectancy but also about the quality of their lives. They want to live longer, but they also want to live better [26-28].
The assessment of a patient’s quality of life is becoming increasingly important in medicine, nursing, and the behavioral sciences. Quality of life is considered an essential indicator in studies of patients with chronic diseases [29]. It has a great influence on the experience and treatment of diseases. The focus should not only be on improving physical activity but, above all, on helping patients to cope with everyday situations [30-35].
According to the World Health Organization, quality of life is composed of six essential domains: physical, psychological, level of independence, social relationships, environment, and spirituality, and is defined as follows:
“Quality of life is a person’s subjective perception of their position in life about their culture and value systems and their goals, expectations, standards, and concerns. It is a broad concept that is influenced in many ways by a person’s physical health, psychological well-being, degree of independence, social relationships, and relationship to features of his or her environment.”
Thus, quality of life refers to the patient’s assessment of the impact of the illness. There are numerous reasons for the impairment of the quality of life of Parkinson’s patients, for example, emotional disturbances, falls, isolation, sleep disturbances, and many more. Many aspects of these disorders go unnoticed in clinical evaluation, and only assessing the quality of life makes it possible to evaluate them [28].
Therefore, quality of life assessment is subjective, individual, multidimensional, self-determined, and variable over time [28].
Measurement of abstract concepts such as “quality of life” requires prior operationalization, and quality of life is a multidimensional construct that can be assessed with various approaches. In clinical research, standardized and disease-specific measures, usually questionnaires, are used to inquire about the quality of life.
This paper discusses two measurement instruments for assessing the quality of life.
Materials and methods
The Parkinson’s Disease Questionnaire (PDQ-39)
People with Parkinson’s disease are often restricted in everyday situations due to their symptoms, which can reduce their quality of life.
Some questionnaires have been developed exclusively for Parkinson’s disease patients [36]. The PDQ-39 is the questionnaire most commonly used to assess the quality of life of people with PD in the last month. Peto and her colleagues developed the questionnaire and published it in 1995.
The items to be included in the questionnaire were developed through extensive interviews with individuals diagnosed with the disease [37]. Individuals were asked to describe the areas of their lives affected by their PD, which resulted in a large number of possible questionnaire items that could be included in the final questionnaire. These items were assessed for ambiguity and repetition. Initially, the questionnaire contained 65 questions, but it was later reduced to the most important 39 questions, which in turn are divided into eight domains (subscales) [38]. The subscales are Mobility, Activities of Daily Living, Emotional Well-Being, Stigma, Social Support, Cognition, Communication, and Physical Discomfort. Not all subscales contain the same number of questions (items). For example, the Mobility subscale is the most comprehensive, with ten questions, and the Social Support, Communication, and Physical Discomfort domains include only three questions each.
The resulting multidimensional measure was found to have good internal and test-retest reliability and construct validity [37].
The PDQ-39 is often used both before and after treatment to determine possible changes due to treatment.
Because patients complete the questionnaire themselves, it is a self-assessment instrument. If the patient can no longer complete the questionnaire independently, care must be taken to ensure that the assistant does not ask any suggestive questions, as this may lead to bias.
Calculation of the subscales
There are five answer options, which are coded as follows: 0 = never, 1 = rarely, 2 = sometimes, 3 = often, 4 = always, or I can’t at all. The respondent must select one of the five responses.
The PDQ scores of each domain are added together to calculate the domain scores. To be able to compare the individual scale values with each other, the following transformation must be carried out:
(raw scale value x 100) / (maximum scale value)
(Example for a patient who has a raw score = 4 in the domain “Communication” ? 4 x 100 / (3 x 4) = 33.3).
In this example, a score of 33.3 for the domain “Communication” means that the patient has achieved a score that is 33.3% worse than the best possible score. Higher scores reflect a lower quality of life, so 0 would be the best score and 100 the worst.
PDQ-39 sum score
In addition to calculating the individual domains, the total sum score can also be calculated. It represents the mean value of the eight subscales, weighted by the number of items. For its calculation, the PDQ values of each scale are added up for each patient, and the sum is divided by 8 (=number of scales).
[Mobility + Activities of Daily Living + Emotional Well-Being + .... + Physical Discomfort] / 8 = PDQ-39SI
The Schedule for the Evaluation of Individual Quality of Life (SEIQoL)
In contrast to the PDQ-39, the SEIQoL is not only a questionnaire for patients with Parkinson’s disease but also an instrument for assessing the quality of life of patients with any disease. In the SEIQoL, quality of life is also assessed from the subjective point of view of the person.
There are two versions: the full version and a shortened version of the questionnaire, the SEIQoL-DW (SEIQoL-Direct Weighting). The questionnaire was developed in the early 1990s by researchers at the Royal College of Surgeons in Ireland.
Standardized instruments such as the PDQ-39 have been criticized for potentially ignoring areas that are important to the individual patient while including other areas that may be less important. In addition, they assume that physical limitations must, by default, result in decreased quality of life [39].
Whereas the PDQ-39 presents the patient with a predetermined list of questions that may be relevant to the individual patient, the SEIQoL questionnaire is based on the premise that quality of life is subjective: the domains that contribute to the quality of life vary across individuals, as does the importance the individual places on each domain. Thus, the limitation of predetermined measures is overcome.
The SEIQoL uses a semi-structured interview to collect data and allows individuals to name domains freely. Thus, patients first indicate the areas of their lives that are important to them. Next, individuals indicate how well they can perform these domains, and last, they rate the subjective importance of each domain. If a person finds it difficult to name five domains, a standard list of examples is used [39].
In contrast to the PDQ-39, the SEIQoL takes an idiographic approach to measure the quality of life, in which the individual determines the reference variables entirely. This approach focuses on examining the individual to understand and interpret the uniqueness of the individual in a historical and social context [39].
However, the use of the SEIQoL is limited in clinical practice due to the time and human resources involved.
Calculation
The index score is calculated by multiplying the score of each domain by the weight of the same domain and then adding the products [39]. This results in the SEIQoL index, where 0 represents the lowest quality of life, and 100 represents the best possible quality of life. Therefore, SEIQoL scores are unique to each person and cannot be summed into an average score.
Based on the literature mentioned above, the following question was derived: Can the two questionnaires, PDQ-39 and SEQoL, be used equivalently to measure the quality of life of PD patients?
The following two hypotheses were derived:
- The PDQ-39 and the SEIQoL have different quality of life scores.
- The SEIQoL is superior to the PDQ-39 because the questionnaire overcomes the limitations of standardized measures, according to the study by Wettergren, et al. [39].
Results
Literature review
This paper is based on a literature search and includes only previously published research. The literature search was conducted in Google Scholar and PubMed from October 2022 to February 2023. The keywords “PDQ-39 AND SEIQoL” were used.
Selection criteria
The search query was adjusted to display only studies published between 2005 and 2023 to narrow the search and find recent literature. The period was chosen because the study by Lee, et al. [40] was published in 2006 and was highly relevant to this work.
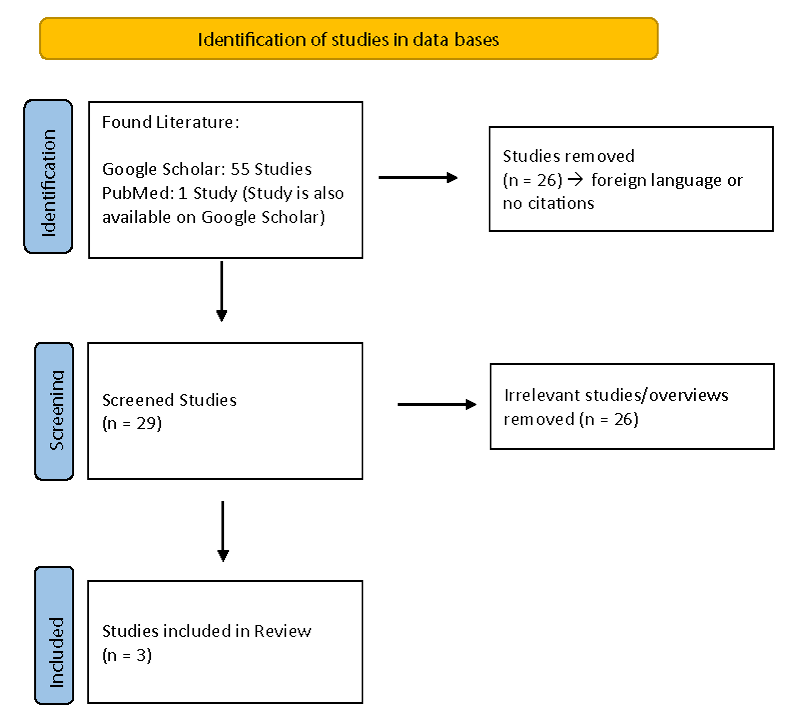
The search resulted in a total of 55 articles, of which four could already be excluded due to the language. Another 22 articles could be excluded because they have never been cited. The remaining 29 documents were evaluated as potentially relevant articles for this work. The studies had to focus on PD and compare the two questionnaires. Since only three studies proved useful for the present work, the search criteria were again adjusted so that a study published in 1998 could also be included. Thus, a total of three studies were used for hypothesis testing (Figure 1).
Empirical studies on the topic
The results of the three selected studies for testing the hypotheses are discussed below.
Quality of life in people with Parkinson’s disease: The relevance of social relationships and communication [41]
Takahashi, et al. [41] investigated whether there was a correlation between the PDQ-39 total score and the SEIQoL index. The sample of this study consisted of 15 subjects (mean age = 80 years, SD = 10.3 years), which included ten women and five men. The subjects were recruited with flyers in the hospital. Subjects were eligible for participation in the study if they were (1) diagnosed with idiopathic PD, (2) currently in Hoehn and Yahr stages 1-4, and (3) able to reflect and report on their current and past lives cognitively. The quality of life of the subjects was assessed using two different measurement instruments: the SEIQoL and the PDQ-39. The study collected the following demographic data over five months: Gender, age, number of years since diagnosis, marital status, current living situation, caregiver, and severity of illness. During a 60-minute interview, subjects completed the SEIQoL and the PDQ-39 and were subsequently tested with the UPDRS-III. The SEIQoL items reported by the 15 subjects were categorized into several domains to define the quality of life constructs of interest: physical, psychological, level of independence, social relationships, environment, and spirituality/religion/personal beliefs. Spearman correlation coefficients were calculated, with the significance level set at 5%, to test the relationships between the SEIQoL index and the PDQ-39 total and subscale scores.
The results showed no significant correlations between the PDQ-39 total score and the SEIQoL index. However, a significant correlation was found between the PDQ-39 dimension “communication” and the SEIQoL index. The items related to this dimension were “difficulty speaking,” “unable to communicate properly with others,” and “feeling ignored by others, which suggests that communication is essential for maintaining social relationships.
The most frequently mentioned item in the SEIQoL questionnaire was “family” (13 patients, 87%), followed by “friends” (10 patients, 67%) and “hobbies” (10 patients, 67%).
Individualised assessment of quality of life in idiopathic Parkinson’s disease [40]
This study aimed to assess patients’ quality of life with Parkinson’s disease. Originally, 161 subjects were recruited for the study, but only 123 completed it. Ethical approval for a descriptive cross-sectional survey was obtained from the ethics committees of the Newcastle and North Tyneside health authorities.
To be included in the study, subjects had to (1) have Parkinson’s disease and (2) be under medical care. Patients who were unable to sign an informed consent form or were not under medical treatment were excluded from the study. The mean age of the subjects was 75.4 years, and 52% of the sample was female.
Subjects were interviewed with the PDQ-39 and the SEIQoL, and in addition, the Mini MentalState Examination (Folstein, et al. 1975), the Beck Depression Inventory (Beck, 1961), a qualitative pain assessment, and the Palliative Assessment Tool (for symptoms) were administered.
The SEIQoL questionnaire was well understood by most subjects and took an average of 20 minutes to complete. There were 87 different domains mentioned. The domain “family” was mentioned by 87.8 % of the subjects and is thus of great importance for the majority of the subjects. Only 65 subjects named “health” as an important domain.
The SEIQoL index score was predicted by depression but not by stage of illness. The PDQ-39, on the other hand, was predicted mainly by disease stage, number of symptoms, and depression.
Because much of the data collected were nonparametric, Spearman correlation coefficients were used. Spearman’s correlation coefficients were used to assess the association of the variables. The Mann-Whitney tests were used for comparing groups, but should the distributions have been normal, the test for two independent samples (t-test) would have been applied.
Multiple regression analysis of the SEIQoL index with the other study parameters showed that the only predictor of quality of life was the depression score. The negative beta coefficient showed that as the depression score increased, the SEIQoL score decreased. Age, stage of illness, duration of illness, pain, or the number of symptoms did not contribute to the model. A single index score from the PDQ-39 was created for each person in the study. Multiple regression analysis of this score with the other study parameters showed that the PDQ-39 single index score predictors were the number of symptoms, stage of illness, and depression. Thus, the SEIQoL index score was predicted by depression but not by disease stage. However, PDQ-39 was predicted by disease stage, number of symptoms, and depression.
The Spearman coefficient for the correlation between the two scores is 0.404, and thus it is a moderate but significant negative correlation between the two scores. Thus, an inverse relationship was observed between all beta coefficients and the SEIQoL index. Thus, when the PDQ-39 single index increases, the SEIQol index score decreases.
A direct comparison of the instruments confirmed that the SEIQoL index score was predicted by the following three domains: social support, cognitive impairment, and emotions. None of the other domains (e.g., mobility, activities of daily living, or physical pain) contributed significantly to the model. Spearman correlations of the SEIQoL index with the individual questions of the PDQ-39 showed that the strongest associations with the SEIQoL index were for the questions “Did I feel isolated and lonely?” and “Did you have difficulty getting dressed?” and “Did you feel unable to communicate properly with other people?” All other associations were less than 0.32.
A direct comparison between the PDQ-39 and the SEIQoL showed that social support, cognitive impairment, and emotion were the only PDQ-39 domains that predicted the SEIQoL index. Neither the domains of mobility nor activities of daily living contributed. Thus, the quality of life predictors differed between the PDQ-39 and the SEIQoL.
In addition, the patient’s quality of life could not be predicted by the stage of Parkinson’s disease. This finding has been shown in the past in other diseases and is consistent with previously published studies, but the study by Lee, et al. was one of the first to show this finding in PD. Therefore, maintaining the quality of life despite increasing age could be due to adaptation to the disease process.
In this study, the authors also mention that only negative questions are asked in PDQ-39. However, what effect this might have on the patients was not pursued further in the study.
Even though Lee, et al. criticized the points mentioned above in the PDQ-39 questionnaire, they concluded that an instrument is nevertheless a useful tool. However, when using the questionnaire, one should be aware of its limitations.
However, even the SEIQoL questionnaire is not considered error-free by Lee, et al. Since patients have much freedom with the SEIQoL, they may be deliberately exploiting this (e.g., in the form of manipulation).
In summary, Lee, et al. believe that a single test is not sufficient to assess the quality of life but that it is best to use several tests, which is very time-consuming.
An introduction to the concept of “quality of life in Parkinson’s disease” [28]
Unlike the other two studies, Martinez-Martin et al. compared more than two scales. The Movement Disorder Society task force was commissioned to evaluate the psychometric quality of the available health-related quality of life scales in Parkinson’s disease. The scales were considered from three perspectives:
- Is the questionnaire used in practice in Parkinson’s disease patients?
- Has the questionnaire been used by several research groups?
- What are the clinical characteristics of the questionnaire?
Finally, a final classification was made as “recommended”, “suggested”, or “listed”.
The task force members examined 17 different questionnaires to investigate the quality of life of Parkinson’s patients and identified the scales’ problems and limitations. Both the PDQ-39 and the SEIQoL were included in the study.
The authors concluded that the PDQ-39 is the most thoroughly tested and applied questionnaire. Since all three PDQ-39 questions mentioned above can be answered with “yes”, the questionnaire is classified as “recommended”. The authors confirm that the questionnaire has adequate psychometric properties and adequately covers the physiological, mental, and social domains. They note that the measurement instrument has already been used in various epidemiological studies and clinical trials.
However, they also mention that the questionnaire does not include the domains of “night sleep” and “sexuality.”
In contrast to the PDQ-39, the SEIQoL is only rated as “recommended.” The SEIQoL has not yet been used as often with Parkinson’s patients as the PDQ-39, possibly due to the high time and personnel expenditure. In contrast to the PDQ-39, the SEIQoL can also be used for other diseases, and acceptable psychometric properties were found for non-Parkinson patients. Content validity is considered satisfactory, but acceptability and stability in known groups have not been tested in PD patients.
The authors affirm that quality of life measurement is an indispensable resource in clinical research. It provides information about the impact of treatment from the unique perspective of the person affected in a more reliable way than the information interview. In clinical practice, quality-of-life assessment helps prioritize interventions and understand essential aspects of the disease and treatment that cannot be captured by any other method.
In addition, the research group noted that various health-related quality-of-life measurement instruments already exist and therefore discourage the development of a new scale (Table 1).
Discussion
This paper aimed to compare the PDQ-39 and the SEIQoL and to investigate them based on two hypotheses. For this purpose, three exemplary studies were selected, and their results were explained. The hypothesis that the two questionnaires would yield different results was confirmed by the study of Takahashi and colleagues [41] and the study of Lee and colleagues [41]. Takahashi and colleagues [41] could not find a significant correlation between the two measurement instruments based on their study, while Lee and colleagues could find a significant correlation between three PDQ-39 domains and the SEIQoL index.
The second hypothesis that the SEIQoL is superior to the PDQ-39 because the questionnaire overcomes the limitations of standardized measures could not be confirmed or rejected. However, the authors of all three studies agree that the SEIQoL solves the problem of pre-formulated questions.
The study by Lee, et al. [40] questions the role of the PDQ-39 as an instrument to measure the quality of life because the PDQ-39 emphasizes the physical aspects that may be impaired due to PD. Thus, external value systems are imposed on the patients instead of letting them determine for themselves what is important to them. Thus, the construct “quality of life” is assumed to mean the same thing to everyone. On the other hand, Martinez-Martin and colleagues [28] rank the PDQ-39 as more appropriate than the SEIQoL. The SEIQoL questionnaire showed that quality of life is broad and highly individualized and determined more by psychosocial than physical aspects. Thus, the SEIQoL includes more psychological aspects than the PDQ-39, as these are considered relevant by patients concerning their quality of life. Lee and colleagues [40] found that the SEIQoL index was predicted by the following three domains: social support, cognitive impairment, and emotions. In contrast, age, disease stage, disease duration, pain, and number of symptoms had no significant effect on QoL. In contrast, PDQ-39 was predicted by disease stage, number of symptoms, and depression.
In both the study by Lee and colleagues [40] and the study by Takahashi and colleagues [41], the domain “family” was cited as the most crucial factor in patients’ quality of life. In the study by Takahashi and colleagues [41], the item was mentioned by 87% of patients, and in the study by Lee and colleagues [40], it was mentioned by 87.8%.
This result shows that the item “social relationships” is most important in relation to the quality of life of people with PD.
Takahashi and colleagues [41] also observed a significant correlation between the SEIQoL index and the “communication” dimension of the PDQ-39. This result suggests that feeling “able to communicate” is vital for maintaining social relationships with family and relatives. Thus, social bonding is an important factor in the quality of life of people with PD. Furthermore, in the study by Takahashi and colleagues [41], the PDQ-39 total score correlated strongly with the “physical domain” and “degree of independence.”
However, the study by Lee and colleagues [40] and Takahashi and colleagues [41] also differ in certain respects. Takahashi and colleagues’ [41] study seems to cover other domains, especially concerning “social relationships.” Takahashi and colleagues [41] cited cultural values as a possible explanation for this difference. While the study by Lee and colleagues [40] reflects Western cultures, where independence is particularly important, patients in Japan and other Eastern countries are more likely to assign a high value to social relationships. According to all three studies, therapists and other health professionals should also place a high value on social relationships and communication, as these areas are associated with increased quality of life and thus have positive outcomes for patients.
Limitations
When interpreting the present results, the following limitations should be noted.
First, the selected studies were all conducted with a relatively small sample (N = max. 123), which limits external validity and thus makes it difficult to generalize the results.
Difficulties have arisen, especially in the literature search, due to the lack of studies on this topic.
The amount of available material was further limited, as only studies that directly compared the two questionnaires were included.
Conclusion
To maintain the quality of life of Parkinson’s disease patients, it is necessary to know which factors influence the quality of life. For this, valid measurement instruments are needed. In this paper, the most important differences between health-related Quality of Life and individualized Quality of Life were detected in the domain of communication and social relationships. Especially the SeiQoL index showed that the only predictor of Quality of Life in Patients with Parkinson’s disease was the depression score. The quality of life of PD patients is best measured by a combination of a standardized (PDQ-39) and an adapted measurement instrument (SEIQoL), whereby the cultural background must also be considered. Therefore, all future working and research groups should use both questionnaires to receive a deeper insight into the patient’s daily life problems.
- Kalia LV, Lang AE. Parkinson’s disease. The Lancet. 2015; 386(9996):896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
- Capriotti T, Terzakis K. Parkinson's disease (PD) is a progressive neurodegenerative disease that affects one million people in the United States. This article reviews the etiology and pathophysiology of PD, risk factors, clinical manifestations, diagnostic criteria, and treatment of this common disease. Implications for home care clinicians are included. 2016.
- Damiano AM, Snyder C, Strausser B, Willian MK. A review of health-related quality-of-life concepts and measures for Parkinson's disease. Qual Life Res. 1999 May;8(3):235-43. doi: 10.1023/a:1008823222574. PMID: 10472154.
- Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000 Nov;15(6):1112-8. doi: 10.1002/1531-8257(200011)15:6<1112::aid-mds1008>3.0.co;2-a. PMID: 11104193.
- Ball N, Teo WP, Chandra S, Chapman J. Parkinson's Disease and the Environment. Front Neurol. 2019 Mar 19;10:218. doi: 10.3389/fneur.2019.00218. PMID: 30941085; PMCID: PMC6433887.
- Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson's disease: the relative importance of the symptoms. Mov Disord. 2008 Jul 30;23(10):1428-34. doi: 10.1002/mds.21667. PMID: 18543333.
- Appleman ER, Stavitsky K, Cronin-Golomb A. Relation of subjective quality of life to motor symptom profile in Parkinson's disease. Parkinsons Dis. 2011 Mar 7;2011:472830. doi: 10.4061/2011/472830. PMID: 21437183; PMCID: PMC3062095.
- Den Oudsten BL, Van Heck GL, De Vries J. Quality of life and related concepts in Parkinson's disease: a systematic review. Mov Disord. 2007 Aug 15;22(11):1528-37. doi: 10.1002/mds.21567. PMID: 17523198.
- Nowak K, Meyer A, Chaturvedi M, Hadinia A, Gschwandtner U, Fuhr P. Quality of life measured by PDQ39 and SEIQoL during anti-stress training for Parkinson’s disease (PD) patients. Parkinsonism & Related Disorders. 2016; 22:e13. https://doi.org/10.1016/j.parkreldis.2015.10.527
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A. on behalf of the PRIAMO study group. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Movement Disorders. 2009; 24(11):1641–1649. https://doi.org/10.1002/mds.22643
- Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord. 2005 Feb;20(2):224-30. doi: 10.1002/mds.20279. PMID: 15384126.
- Kuhlman GD, Flanigan JL, Sperling SA, Barrett MJ. Predictors of health-related quality of life in Parkinson's disease. Parkinsonism Relat Disord. 2019 Aug;65:86-90. doi: 10.1016/j.parkreldis.2019.05.009. Epub 2019 May 7. PMID: 31118162.
- Ehlers C, Timpka J, Odin P, Honig H. Levodopa infusion in Parkinson's disease: Individual quality of life. Acta Neurol Scand. 2020 Sep;142(3):248-254. doi: 10.1111/ane.13260. Epub 2020 May 22. PMID: 32383152.
- Crispino P, Gino M, Barbagelata E, Ciarambino T, Politi C, Ambrosino I, Ragusa R, Marranzano M, Biondi A, Vacante M. Gender Differences and Quality of Life in Parkinson's Disease. Int J Environ Res Public Health. 2020 Dec 29;18(1):198. doi: 10.3390/ijerph18010198. PMID: 33383855; PMCID: PMC7795924.
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017 Mar 23;3:17013. doi: 10.1038/nrdp.2017.13. PMID: 28332488.
- Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderowf A, Welsh M, Poewe W, Rascol O, Sampaio C, Stebbins GT, Goetz CG, Schrag A. Health-related quality-of-life scales in Parkinson's disease: critique and recommendations. Mov Disord. 2011 Nov;26(13):2371-80. doi: 10.1002/mds.23834. Epub 2011 Jul 6. PMID: 21735480.
- Martinez-Martin P. What is quality of life and how do we measure it? Relevance to Parkinson's disease and movement disorders. Mov Disord. 2017 Mar;32(3):382-392. doi: 10.1002/mds.26885. Epub 2016 Dec 2. PMID: 27911002.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998 Feb;50(2):318 and 16 pages following. doi: 10.1212/wnl.50.2.318. PMID: 9484345.
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L; Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004 Sep;19(9):1020-8. doi: 10.1002/mds.20213. PMID: 15372591.
- Jenny AL, Meyer A, Handabaka I, Calabrese P, Fuhr P, Gschwandtner U. Nonmotor-Related Quality of Life in Parkinson's Patients with Subjective Memory Complaints: Comparison with PDQ-39. Parkinsons Dis. 2020 Apr 20;2020:7953032. doi: 10.1155/2020/7953032. PMID: 32377331; PMCID: PMC7189320.
- Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, Collerton D, Taylor JP, Burn DJ. Severity of mild cognitive impairment in early Parkinson's disease contributes to poorer quality of life. Parkinsonism Relat Disord. 2014 Oct;20(10):1071-5. doi: 10.1016/j.parkreldis.2014.07.004. Epub 2014 Jul 18. PMID: 25074728; PMCID: PMC4194347.
- Velseboer DC, Broeders M, Post B, van Geloven N, Speelman JD, Schmand B, de Haan RJ, de Bie RM; CARPA Study Group. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013 Feb 12;80(7):627-33. doi: 10.1212/WNL.0b013e318281cc99. Epub 2013 Jan 23. PMID: 23345637.
- Zhao N, Yang Y, Zhang L, Zhang Q, Balbuena L, Ungvari GS, Zang YF, Xiang YT. Quality of life in Parkinson's disease: A systematic review and meta-analysis of comparative studies. CNS Neurosci Ther. 2021 Mar;27(3):270-279. doi: 10.1111/cns.13549. Epub 2020 Dec 28. PMID: 33372386; PMCID: PMC7871788.
- Bhidayasiri R, Tarsy D. Tremor-Dominant Parkinson’s Disease. In R. Bhidayasiri & D. Tarsy, Movement Disorders: A Video Atlas (S. 8–9). 2012. https://doi.org/10.1007/978-1-60327-426-5_4
- Galperin I, Hillel I, Del Din S, Bekkers EMJ, Nieuwboer A, Abbruzzese G, Avanzino L, Nieuwhof F, Bloem BR, Rochester L, Della Croce U, Cereatti A, Giladi N, Mirelman A, Hausdorff JM. Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson's disease. Parkinsonism Relat Disord. 2019 May;62:85-90. doi: 10.1016/j.parkreldis.2019.01.022. Epub 2019 Jan 29. PMID: 30718220.
- Chrischilles EA, Rubenstein LM, Voelker MD, Wallace RB, Rodnitzky RL. Linking clinical variables to health-related quality of life in Parkinson's disease. Parkinsonism Relat Disord. 2002 Jan;8(3):199-209. doi: 10.1016/s1353-8020(01)00044-x. PMID: 12039432.
- Karlsen KH, Tandberg E, Arsland D, Larsen JP. Health related quality of life in Parkinson's disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000 Nov;69(5):584-9. doi: 10.1136/jnnp.69.5.584. PMID: 11032608; PMCID: PMC1763406.
- Martínez-Martín P. An introduction to the concept of "quality of life in Parkinson's disease". J Neurol. 1998 May;245 Suppl 1:S2-6. doi: 10.1007/pl00007733. PMID: 9617714.
- Marinus J, Ramaker C, van Hilten JJ, Stiggelbout AM. Health related quality of life in Parkinson's disease: a systematic review of disease specific instruments. J Neurol Neurosurg Psychiatry. 2002 Feb;72(2):241-8. doi: 10.1136/jnnp.72.2.241. PMID: 11796776; PMCID: PMC1737742.
- Candel-Parra E, Córcoles-Jiménez MP, Delicado-Useros V, Ruiz-Grao MC, Hernández-Martínez A, Molina-Alarcón M. Predictive Model of Quality of Life in Patients with Parkinson's Disease. Int J Environ Res Public Health. 2022 Jan 7;19(2):672. doi: 10.3390/ijerph19020672. PMID: 35055498; PMCID: PMC8775752.
- Dowding CH, Shenton CL, Salek SS. A review of the health-related quality of life and economic impact of Parkinson's disease. Drugs Aging. 2006;23(9):693-721. doi: 10.2165/00002512-200623090-00001. PMID: 17020395.
- Duncan GW, Khoo TK, Yarnall AJ, O'Brien JT, Coleman SY, Brooks DJ, Barker RA, Burn DJ. Health-related quality of life in early Parkinson's disease: the impact of nonmotor symptoms. Mov Disord. 2014 Feb;29(2):195-202. doi: 10.1002/mds.25664. Epub 2013 Oct 7. PMID: 24123307.
- Karimi M, Brazier J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? Pharmacoeconomics. 2016 Jul;34(7):645-9. doi: 10.1007/s40273-016-0389-9. PMID: 26892973.
- McGee HM, O'Boyle CA, Hickey A, O'Malley K, Joyce CR. Assessing the quality of life of the individual: the SEIQoL with a healthy and a gastroenterology unit population. Psychol Med. 1991 Aug;21(3):749-59. doi: 10.1017/s0033291700022388. PMID: 1946863.
- Slezakova Z, Zavodna V, Vcelarikova A. (o. J.). Quality of life in patients with Parkinson´s disease.
- Hagell P, Nilsson MH. The 39-Item Parkinson's Disease Questionnaire (PDQ-39): Is it a Unidimensional Construct? Ther Adv Neurol Disord. 2009 Jul;2(4):205-14. doi: 10.1177/1756285609103726. PMID: 21179529; PMCID: PMC3002633.
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997 Sep;26(5):353-7. doi: 10.1093/ageing/26.5.353. PMID: 9351479.
- Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998 May;245 Suppl 1:S10-4. doi: 10.1007/pl00007730. PMID: 9617716.
- Wettergren L, Kettis-Lindblad A, Sprangers M, Ring L. The use, feasibility and psychometric properties of an individualised quality-of-life instrument: a systematic review of the SEIQoL-DW. Qual Life Res. 2009 Aug;18(6):737-46. doi: 10.1007/s11136-009-9490-2. Epub 2009 Jun 3. PMID: 19496021.
- Lee MA, Walker RW, Hildreth AJ, Prentice WM. Individualized assessment of quality of life in idiopathic Parkinson's disease. Mov Disord. 2006 Nov;21(11):1929-34. doi: 10.1002/mds.21099. PMID: 16986143.
- Takahashi K, Kamide N, Suzuki M, Fukuda M. Quality of life in people with Parkinson's disease: the relevance of social relationships and communication. J Phys Ther Sci. 2016 Jan;28(2):541-6. doi: 10.1589/jpts.28.541. Epub 2016 Feb 29. PMID: 27065542; PMCID: PMC4793007.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
