Annals of Psychiatry and Treatment
Theta-Burst Stimulation over the pre-Supplementary Motor Area in Schizophrenia and comorbid substance use disorder: Preliminary clinical data
Stefano Pallanti1,2*, Michele Di Ponzio1, Nikos Makris3-6# and Marek Kubicki3-5#
2Department of Psychiatry and Behavioural Science, Albert Einstein College of Medicine, Bronx, NY, USA
3Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
4Center for Morphometric Analysis, Departments of Psychiatry, Neurology and A. A. Martinos Center for Biomedical Imaging, Harvard Medical School, Boston, Massachusetts, USA
5Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
6Department of Anatomy and Neurobiology, Boston University Medical School, Boston, USA
#Authors contributed equally
Cite this as
Pallanti S, Di Ponzio M, Makris N, Kubicki M (2022) Theta-Burst Stimulation over the pre-Supplementary Motor Area in Schizophrenia and comorbid substance use disorder: Preliminary clinical data. Ann Psychiatry Treatm 6(1): 028-032. DOI: 10.17352/apt.000042Copyright
© 2022 Pallanti S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Schizophrenia (SZ) is a debilitating disorder, which tremendously impacts the psychological, social, and financial aspects of a patient’s life. Frequently, SZ patients present with poor insight, which can even worsen the symptomatology. Antipsychotic medications frequently result in suboptimal outcomes, especially the ones concerning negative and cognitive symptoms. Accordingly, new therapeutic options are warranted. Transcranial Magnetic Stimulation (TMS) has been adopted in SZ with promising results. Continuous Theta burst stimulation (cTBS) is a particular brief and effective form of TMS. It has been successfully applied in patients with poor cognitive control (e.g., gambling disorder patients) targeting the pre-Supplementary Motor Area (pre-SMA). Given that poor cognitive control has been regarded as a core deficit in SZ, 11 patients with SZ were included in this study and treated with cTBS for a total of 10 sessions during a two-week period. Patients were divided into two groups: patients with a diagnosis of SZ in comorbidity with Substance Use Disorder (SZ + SUD) vs SZ. Patients were evaluated before and after treatment, assessing executive functions, awareness, and nicotine craving. Within-group comparisons showed a significant reduction in the Scale to assess Unawareness in Mental Disorders (SUMD) scores (p < 0.05) and in the test of Fagerstrom (to assess nicotine dependence) scores (p < 0.001) before and after treatment in the SZ + SUD group. These results showed the efficacy of cTBS for craving reduction as well as in improving awareness of the illness and of treatment. This can be considered a remarkable result since better insight has been previously associated with an improved quality of life in SZ.
Introduction
Schizophrenia (SZ) is a disorder characterized by positive, negative, and cognitive symptoms [1]. Among cognitive symptoms, poor cognitive control has been regarded as a core deficit in SZ and is associated with clinical presentation and poor daily functioning of people with this illness [2]. Moreover, SZ frequently presents in comorbidity with Substance Use Disorder (SUD) [3] and this may exacerbate cognitive and executive function alterations [4].
The concept of schizophrenia has been partially rectified but is an object of critique which is leading to new hypotheses like the one by Guloksuz and Os [5] who prospected the introduction of a psychosis spectrum disorder. In this spectrum, a translational research approach based on empirical data [6] is needed with the aim to redefine the concept of schizophrenia and highlight the main features of this spectrum. In this sense, insight could represent a key dimension of the psychosis spectrum.
The majority of schizophrenic patients possess poor insight, with around 70% of them with poor awareness of the mental disorder and of the achieved effects of medication, and 80% of them with poor awareness of social consequences [7]. Poor insight in SZ has been related to worse symptomatology, including depression comorbidity and neurocognitive deficits [8], specifically with low executive functions [7]. Moreover, lack of insight has been regarded as one of the main causes of treatment nonadherence in SZ [8], strengthening the idea of the importance of insight in the SZ clinical picture.
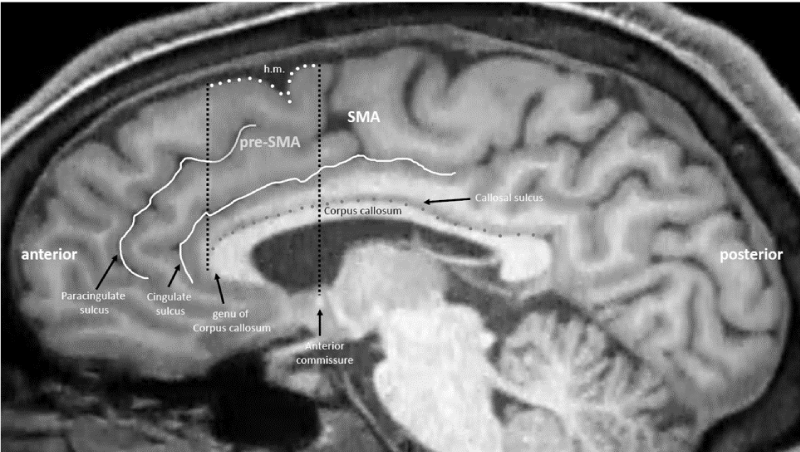
Pre-Supplementary Motor Area (pre-SMA) is a key node of the cognitive and executive control network, and its neural activity has been also related to the awareness of intentions to act [9], and to the sense of agency [10]. In SZ, pre-SMA volumetric abnormalities have been reported to be associated with impaired motor sequences [11]. Moreover, abnormalities in major mesocortical, dopaminergic connections of the pre-SMA, namely the Medial Forebrain Bundle (MFB), have been associated with delusions and grandiosity [12].
Antipsychotic medications, the mainstay of treatment for schizophrenia, frequently result in suboptimal outcomes [13] and, while helping in correcting positive symptoms like hallucinations and delusions, little impact on the more disabling negative and cognitive symptoms has been observed. It is therefore imperative to examine novel therapeutic avenues that not only target the resistant, positive symptoms but also improve negative, cognitive symptoms of SZ and increase insight.
Transcranial magnetic stimulation (TMS) has been adopted in SZ with promising results, mainly targeting the DorsoLateral Prefrontal Cortex (DLPFC) or the left temporoparietal cortex [14]. One study also reported the effect of TMS over DLPFC in increasing insight [15]. Theta-Burst Stimulation (TBS) is a particular brief and effective form of TMS that can be either inhibitory (continuous or cTBS) or excitatory (intermittent or iTBS). TBS appears to be particularly suitable for SZ patients due to its brevity. Pre-SMA has been successfully targeted with traditional TMS and TBS for impulse-control disorders [16]. This approach has never been, however, tested in SZ. Therefore, considering the preSMA-related cognitive control dysfunctions in SZ [2], we hypothesized that this region could be the target of cTBS for treating patients with SZ.
In this preliminary, naturalistic study, cTBS over pre-SMA has been prescribed in SZ patients and SZ in comorbidity with SUD in order to assess the cognitive profile of patients before and after treatment, regarding executive functions and awareness levels.
Methods
Participants
Eleven patients (5 females, mean age: 37) (Table 1) were included in this study and divided into two groups based on the diagnosis: SZ vs SZ + SUD. The diagnosis was based on the criteria of the DSM-5 TR [17] assessed through clinical interviews with a licensed clinician. Patients were all screened to exclude the presence of dementia and personality disorders. Patients with encephalitis, urinalysis, the prevalence of syphilis, HIV, hepatitis, hormonal disarrange, the presence of a risk of seizure or epilepsy, and implanted devices were excluded. All patients were treated stably for two months with second-generation antipsychotic treatments at an olanzapine equivalent dosage from 5.144 mg to 15.432 mg, determined on the basis of the work by Leucht, et al. [18]. Patients’ data were obtained from a database of patients of the Institute of Neuroscience (Florence, Italy). Informed consent was obtained for each participant. Anonymity was guaranteed to all participants and the ethical requirements of the Declaration of Helsinki have been met.
Procedure
cTBS was administered with the MagVenture MagPro R30 stimulator with add-on Theta Burst option (MagVenture INC.) using a Cool D-B80 figure-of-eight coil. TBS consists of bursts of 3 pulses separated by 20 ms (i.e., 50 Hz). Stimulus intensities were set at 80% of the Resting Motor Threshold (RMT). The bilateral pre-SMA was targeted using a neuronavigation system. RMT was defined as the minimum magnetic flux needed to elicit a threshold EMG response (50 mV in peak-to-peak amplitude) in a resting target muscle (abductor pollicis brevis or APB) in 5/10 trials using single-pulse TMS administered to the contralateral primary motor area.
Patients received cTBS over the pre-SMA (Figure 1) for 2 weeks (10 sessions).
Assessment
Patients were assessed before and at the end of the treatment with the Scale for the Assessment of Positive Symptoms (SAPS), the Barkley Deficits in Executive Functioning Scale – Short Form (BDEFS-SF), the Scale to assess Unawareness of Mental Disorders (SUMD) and with the Test of Fagerstrom (to assess nicotine dependence).
Statistical analysis
Depending on the distribution, assessed with the Shapiro-Wilk test, t-test or Wilcoxon test was also used for within-group comparison in time (pre- vs post-treatment). A two-way ANalysis Of Variance (ANOVA) was run to assess the interaction between groups and time. Analyses were performed with R software. Statistical significance was set at p < .05, two-sided. Bonferroni correction for multiple comparisons was applied.
Results
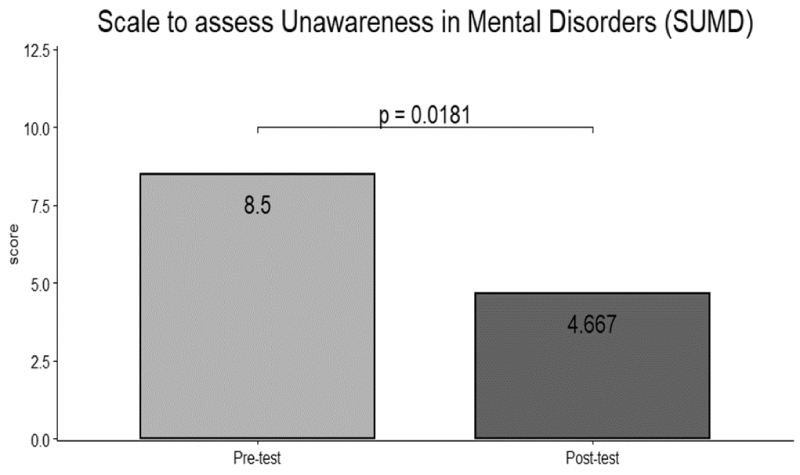
At baseline, between groups comparisons showed no significant differences. Within-group comparisons showed a significant difference in SUMD scores (d = 1.41; p < 0.05) (Figure 2) and FAGE scores (d = 1.65; p < 0.001) before and after treatment in the SZ + SUD group. No significant difference was found between scores before and after treatment for any measure in the SZ group, although the presence a reduction in scores (Table 2).
ANOVA analysis showed no differences in changes during treatment between the two groups for any measure.
Discussion
This is the first study to report data concerning cTBS stimulation over the pre-SMA in patients with SZ. The main results of this preliminary study are represented by a significant reduction in craving in the SZ + SUD group and an improvement in awareness.
All patients in the SZ + SUD group were nicotine addicted. It is noteworthy to highlight that, during treatment, the patients did not participate in specific nicotine craving reduction programs. Therefore, it was a spontaneous reduction, obtained after treatment with cTBS. It is important to highlight that reduction in nicotine assumption per day was not related to a ponderal increase. This result appears particularly important, considering that other studies using TMS at high frequency (20 Hz) over the DLPFC failed to reduce smoking cravings in SZ [23]. Therefore, it can be proposed that nicotine consumption reduction in SZ following TMS is potentially specific for protocols of cTBS over pre-SMA.
Insight is considered a continuous and multidimensional construct that includes awareness of having a mental illness, awareness of the need for treatment, awareness of the social consequences of mental disorders, awareness of symptoms, and attribution of symptoms to a mental disorder [24-26]. Awareness represents an important component of the SZ clinical picture [27]. Associations exist between lack of insight and psychopathology in schizophrenia. Indeed, a higher level of insight at baseline is significantly associated with a lower level of schizophrenia symptoms [28]. Also, neuroimaging studies using fMRI in patients with schizophrenia in comparison with healthy controls have shown that impaired insight is related to brain activation during self-reflection in the left insula, left inferior frontal gyrus, and left inferior parietal lobule, and the bilateral ventromedial prefrontal cortex [29]. Yu, et al. [30], found that anodal Transcranial Direct Current Stimulation over pre-SMA effectively increased BOLD response in the pre-SMA and ventromedial PreFrontal Cortex (vmPFC). These results suggest that pre-SMA brain stimulation modulates the medial-frontal activity and preSMA-vmPFC functional connectivity, a finding that can also explain our results.
Concerning the SZ group, no statistically significant improvements in rating scale measures have been reported, although a reduction in percentage after treatment. In this sense, it is necessary to consider the limited sample size. Therefore, further studies are needed with larger sample sizes.
Two hypotheses could possibly explain the results here reported. First, awareness improvement in SZ + SUD group is an effect of the reduction of craving and therefore these results emerged only in the group with comorbid nicotine addiction. Second, SZ in comorbidity with SUD should be regarded as a unitary disorder, with peculiar features. In this sense, it has been hypothesized that SZ and SUD share a genetic determinant that increases the risk of SZ and makes them more vulnerable to substance abuse [31]. This genetic risk may lead to a dysfunctional mesocorticolimbic brain reward circuit. Indeed, alteration in reward-related activation was observed in the right ventral striatum in schizophrenia, an area characterized by functional connectivity primarily with the lateral prefrontal cortex and the pre-SMA [32].
Limitations
As previously reported, one of the main limitations of this study concerns the low sample size. Our results need to be expanded, with the definition of a randomized prospective study, as well as with the inclusion of a control group. Moreover, we lacked information regarding the levels of nicotine in the patients’ blood. Future studies could also shed light on the two hypotheses here formulated.
Conclusions
In case the improvement of awareness in SZ following cTBS will be confirmed by further evidence, they will have great implications for SZ treatment. Indeed, SZ has been indicated as a disorder of awareness and insight [26,33,34]. Insight is likely to have dynamic relationships with all these dimensions and with responses to personal events and contextual factors. Impaired insight directly affects patients’ quality of life [35], as well as treatment outcomes [36]. Therefore, the possibility of increasing awareness, with its multifaceted nature, could have a great impact in clinical settings. Moreover, we used an efficient TMS protocol (cTBS) with the potential to induce longer-lasting clinical effects with very short treatment sessions, hence increasing the potential for both cost-effectiveness and public health impact.
We acknowledge support from the National Institutes of Health (RO01MH112748, R01MH125860, R01NS125307, R01MH125860, and K24MH116366).
Author contributions
Conceptualization: S.P., N.M., M.K.; Formal analysis: M.D.P.; Investigation: S.P., M.D.P., N.K., M.K.; Data curation: S.P., M.D.P.; writing—original draft preparation: S.P., M.D.P., N.M., M.K.; Writing—Revision and editing: S.P., M.D.P.; Supervision: S.P., N.K., M.K. All authors have read and agreed to the published version of the manuscript.
Ethics: Anonymity was guaranteed to all participants and the ethical requirements of the Declaration of Helsinki have been met.
Informed consent statement: Informed consent was obtained from all subjects involved in the study.
Data availability statement: The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders .
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011 Jan;36(1):316-38. doi: 10.1038/npp.2010.156. Epub 2010 Sep 15. PMID: 20844478; PMCID: PMC3052853.
- Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990-2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018 Oct 1;191:234-258. doi: 10.1016/j.drugalcdep.2018.07.011. Epub 2018 Aug 22. PMID: 30153606.
- Thoma P, Daum I. Comorbid substance use disorder in schizophrenia: a selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin Neurosci. 2013 Sep;67(6):367-83. doi: 10.1111/pcn.12072. Epub 2013 Jul 25. PMID: 23890122.
- Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018 Jan;48(2):229-244. doi: 10.1017/S0033291717001775. Epub 2017 Jul 10. PMID: 28689498.
- Cuthbert BN, Morris SE. Evolving Concepts of the Schizophrenia Spectrum: A Research Domain Criteria Perspective. Front Psychiatry. 2021 Feb 25;12:641319. doi: 10.3389/fpsyt.2021.641319. PMID: 33716834; PMCID: PMC7947312.
- Choudhury S, Khess CR, Bhattacharyya R, Sanyal D. Insight in schizophrenia and its association with executive functions. Indian J Psychol Med. 2009 Jul;31(2):71-6. doi: 10.4103/0253-7176.63576. PMID: 21938098; PMCID: PMC3168088.
- Buckley PF, Wirshing DA, Bhushan P, Pierre JM, Resnick SA, Wirshing WC. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-41. doi: 10.2165/00023210-200721020-00004. PMID: 17284095.
- Fried I, Mukamel R, Kreiman G. Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron. 2011 Feb 10;69(3):548-62. doi: 10.1016/j.neuron.2010.11.045. PMID: 21315264; PMCID: PMC3052770.
- Moore JW, Ruge D, Wenke D, Rothwell J, Haggard P. Disrupting the experience of control in the human brain: pre-supplementary motor area contributes to the sense of agency. Proc Biol Sci. 2010 Aug 22;277(1693):2503-9. doi: 10.1098/rspb.2010.0404. Epub 2010 Apr 7. PMID: 20375048; PMCID: PMC2894930.
- Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr Res. 2006 Jun;84(2-3):386-96. doi: 10.1016/j.schres.2006.03.013. Epub 2006 Apr 19. PMID: 16624528.
- Bracht T, Viher PV, Stegmayer K, Strik W, Federspiel A, Wiest R, Walther S. Increased structural connectivity of the medial forebrain bundle in schizophrenia spectrum disorders is associated with delusions of paranoid threat and grandiosity. Neuroimage Clin. 2019;24:102044. doi: 10.1016/j.nicl.2019.102044. Epub 2019 Oct 18. PMID: 31678911; PMCID: PMC6978276.
- Kane JM, Correll CU. Pharmacologic treatment of schizophrenia. Dialogues Clin Neurosci. 2010;12(3):345-57. doi: 10.31887/DCNS.2010.12.3/jkane. PMID: 20954430; PMCID: PMC3085113.
- Cole JC, Green Bernacki C, Helmer A, Pinninti N, O'reardon JP. Efficacy of Transcranial Magnetic Stimulation (TMS) in the Treatment of Schizophrenia: A Review of the Literature to Date. Innov Clin Neurosci. 2015 Jul-Aug;12(7-8):12-9. PMID: 26351619; PMCID: PMC4558786.
- Dlabac-de Lange JJ, Bais L, van Es FD, Visser BG, Reinink E, Bakker B, van den Heuvel ER, Aleman A, Knegtering H. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med. 2015 Apr;45(6):1263-75. doi: 10.1017/S0033291714002360. Epub 2014 Oct 30. PMID: 25354751.
- Salerno L, Grassi E, Makris N, Pallanti S. "A Theta Burst Stimulation on Pre-SMA: Proof-of-Concept of Transcranial Magnetic Stimulation in Gambling Disorder". J Gambl Stud. 2022 May 21:1–9. doi: 10.1007/s10899-022-10129-3. Epub ahead of print. PMID: 35596900; PMCID: PMC9123619.
- American Psychiatric Association (2022). Diagnostic and statistical manual of mental disorders – Text Revised.
- Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014 Mar;40(2):314-26. doi: 10.1093/schbul/sbu001. Epub 2014 Feb 3. PMID: 24493852; PMCID: PMC3932104.
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008 Nov;9(11):856-69. doi: 10.1038/nrn2478. Epub 2008 Oct 9. PMID: 18843271.
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005 Jan 26;15(2):122-8. doi: 10.1016/j.cub.2005.01.006. PMID: 15668167; PMCID: PMC2648721.
- Nachev P, Wydell H, O'neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36 Suppl 2(3-3):T155-63. doi: 10.1016/j.neuroimage.2007.03.034. Epub 2007 Mar 31. PMID: 17499162; PMCID: PMC2648723.
- Caviness VS Jr, Meyer J, Makris N, Kennedy DN. MRI-Based Topographic Parcellation of Human Neocortex: An Anatomically Specified Method with Estimate of Reliability. J Cogn Neurosci. 1996 Nov;8(6):566-87. doi: 10.1162/jocn.1996.8.6.566. PMID: 23961985.
- Kozak K, Sharif-Razi M, Morozova M, Gaudette EV, Barr MS, Daskalakis ZJ, Blumberger DM, George TP. Effects of short-term, high-frequency repetitive transcranial magnetic stimulation to bilateral dorsolateral prefrontal cortex on smoking behavior and cognition in patients with schizophrenia and non-psychiatric controls. Schizophr Res. 2018 Jul;197:441-443. doi: 10.1016/j.schres.2018.02.015. Epub 2018 Feb 24. PMID: 29486960; PMCID: PMC6108952.
- David AS. Insight and psychosis. Br J Psychiatry. 1990 Jun;156:798-808. doi: 10.1192/bjp.156.6.798. PMID: 2207510.
- Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM. Assessment of insight in psychosis. Am J Psychiatry. 1993 Jun;150(6):873-9. doi: 10.1176/ajp.150.6.873. PMID: 8494061.
- Belvederi Murri M, Amore M. The Multiple Dimensions of Insight in Schizophrenia-Spectrum Disorders. Schizophr Bull. 2019 Mar 7;45(2):277-283. doi: 10.1093/schbul/sby092. PMID: 29939361; PMCID: PMC6403083.
- Touskova T, Bob P. Consciousness, awareness of insight and neural mechanisms of schizophrenia. Rev Neurosci. 2015;26(3):295-304. doi: 10.1515/revneuro-2014-0063. PMID: 25741942.
- Mohamed S, Rosenheck R, McEvoy J, Swartz M, Stroup S, Lieberman JA. Cross-sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes in chronic schizophrenia. Schizophr Bull. 2009 Mar;35(2):336-46. doi: 10.1093/schbul/sbn067. Epub 2008 Jun 26. PMID: 18586692; PMCID: PMC2659303.
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010 May;34(6):935-46. doi: 10.1016/j.neubiorev.2009.12.004. Epub 2009 Dec 14. PMID: 20015455.
- Yu J, Tseng P, Hung DL, Wu SW, Juan CH. Brain stimulation improves cognitive control by modulating medial-frontal activity and preSMA-vmPFC functional connectivity. Hum Brain Mapp. 2015 Oct;36(10):4004-15. doi: 10.1002/hbm.22893. Epub 2015 Aug 7. PMID: 26248582; PMCID: PMC6869595.
- Khokhar JY, Dwiel LL, Henricks AM, Doucette WT, & Green AI. (2018) The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophrenia research. 194: 78-85.
- Chase HW, Loriemi P, Wensing T, Eickhoff SB, & Nickl‐Jockschat T (2018) Meta‐analytic evidence for altered mesolimbic responses to reward in schizophrenia. Human brain mapping. 39: 2917-2928.
- Lysaker PH, Vohs J, Hillis JD, Kukla M, Popolo R, Salvatore G, Dimaggio G. Poor insight into schizophrenia: contributing factors, consequences and emerging treatment approaches. Expert Rev Neurother. 2013 Jul;13(7):785-93. doi: 10.1586/14737175.2013.811150. PMID: 23898850.
- Mishara AL, Lysaker PH, Schwartz MA. Self-disturbances in schizophrenia: history, phenomenology, and relevant findings from research on metacognition. Schizophr Bull. 2014 Jan;40(1):5-12. doi: 10.1093/schbul/sbt169. Epub 2013 Dec 6. PMID: 24319117; PMCID: PMC3885311.
- Boyer L, Aghababian V, Richieri R, Loundou A, Padovani R, Simeoni MC, Auquier P, Lançon C. Insight into illness, neurocognition and quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012 Mar 30;36(2):271-6. doi: 10.1016/j.pnpbp.2011.10.008. Epub 2011 Oct 14. PMID: 22019603.
- Schwartz RC. The relationship between insight, illness and treatment outcome in schizophrenia. Psychiatr Q. 1998 Spring;69(1):1-22. doi: 10.1023/a:1022141322657. PMID: 9536472.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
