Archives of Pulmonology and Respiratory Care
The spin of dioxygen as the main factor in pulmonology and respiratory care
Boris Minaev1,2*
2Department of Physics and Astronomy, Uppsala University, Uppsala, Sweden
Cite this as
Minaev B (2022) The spin of dioxygen as the main factor in pulmonology and respiratory care. Arch Pulmonol Respir Care 8(1): 028-033. DOI: 10.17352/aprc.000081Copyright License
© 2022 Minaev B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Not many doctors are aware of the most important obstacle in pulmonology and respiration care which is determined by the electronic structure of molecular oxygen. In great contrast to a huge majority of chemically stable substances, the O2 molecule possesses two non-paired electrons with parallel spins. That means dioxygen (O2) is a paramagnetic gas, while many other substances are diamagnetics including almost all organic molecules, which are stable at ambient conditions. Organic components of an alive cell consist of molecules (proteins, DNA, RNA, lipids, sugars, etc.), which possess an even number of electrons all being paired with anti-parallel spins. An electron is a tiny magnet; its magnetic arrow (spin S=½) has only two possible orientations determined by the quantum nature of this elementary particle. Many atoms have non-paired electrons, but entering a molecule they produce spin pairing which accompanies the formation of chemical bonds. The O=O molecule has a double chemical bond, but because of the special symmetry of the O2 valence shell, its ground state (the most stable one with the lowest internal energy) keeps two non-paired electrons with parallel spins (↑↑). Such a state has total electronic spin S = ½+½ =1 with three possible projections on molecular axis Ms= 0±1 (in units of Planck constant h/2π); thus, the state is called a triplet. The common molecules with antiparallel spins (↑↓) have total electronic spin S =0 (no intrinsic magnetic moment), which corresponds to a singlet diamagnetic state. Fe(II) ion in hemoglobin is also paramagnetic and its coupling with O2 is a complicated process depending on the exchange and spin-orbit coupling. This review describes spin-dependent essential features of dioxygen involvement in aerobic life.
Introduction
It has been long known, that dioxygen from the air could be either chemically inert or extremely active depending on the presence of radicals in the close environment [1-23]. In various processes from combustion to respiration, this simple transparent gas could show furious activity, for all that being billion years passive in the Earth’s atmosphere without visible changes in the great gaseous ocean [1,2]. Photosynthesis started to fulfill our atmosphere with O2 about 2.4 billion years ago [2]. First dioxygen was spent for metal oxidation and then utilized during long aerobic life evolution until the high 21% level was achieved. Nowadays, dioxygen protects the life on the Earth from solar far Ultraviolet (UV) radiation through its absorption in the Schumann-Runge band (175 nm - 205 nm), creates a stratospheric ozone layer protecting us from the softer UV light, and provides respiration of aerobic organic cells. Thus, the majority of organic substances (M) are diamagnetics because their covalent bond saturation requires spin pairing and quenching of the total spin to a singlet ground state [6]. Therefore, their reactions with triplet dioxygen from the air are spin-forbidden processes since the products (P) of such oxidation are known to be also the singlet-state molecules (H2O, N2, and CO2) [7]:
M (↑↓) + O2 (↑↑) ≠ P (↑↓) (↑↓) (1)
The spin-flip is necessary to occur to complete such a reaction, Eq. (1). The flip of the spin magnetic moment could be induced only by interaction with the magnetic field in Eq. (1) [6]. It is well-known, however, that organic oxidation reactions can proceed without external magnetic fields [7]. All chemical processes are determined mainly by pure electric forces (interactions between charged micro-particles, electrons, and nuclei) [1]. Pure magnetic interactions are extremely weak in organic molecules from light atoms; thus, they are reasonably neglected in traditional quantum chemistry [7]. This is one of the reasons why the reaction in Eq. (1) is forbidden by the Wigner-Witmer selection rule for spin conservation [4]. How combustion and respiration can be supported by dioxygen? The real mechanism of the combustion process, denoted by Eq. (2), is known to include numerous intermediate chain reactions [2] initiated by short-lived species - radicals (R), which possess non-paired electrons:
M (↑↓) + O2 (↑↑) + R (↓) = P (↑↓) (↑↓) + R’ (↑) (2)
A newborn radical R’ is able to react with dioxygen again without spin prohibition since the total spin (S = 1/2) together with its projection (Ms = +1/2) would be the same as in the right and left parts of Eq. (2). The trick is that the particular spin orientation in Eq. (2) is possible upon O2 (↑↑) and R (↓) collision [9]. A new organic molecule M can react with dioxygen providing the same diamagnetic P(↑↓) product through the involvement of the intermediate radical. Thus, combustion proceeds as the radical chain reaction [2,7]; one of the chain links is presented by Eq. (2).
It is known that combustion and respiration are identical in the exothermic reaction effects and in the oxidation products (all organic fuels are completely oxidized to H2O, N2, and CO2) [2]. When the bio activator provides “a match” to initiate radical appearance and respiration by dioxygen in the cell? It is obvious that respiration cannot proceed through the radical-chain reaction mechanism; the radicals would burn the cell. This is the Krebs cycle of tricarboxylic acid - the complicated multistep process where dioxygen is activated by enzymes in the presence of paramagnetic metal ions [2,9]. The ion provides non-paired spin which interacts with the triplet dioxygen by exchange coupling leading to an efficient spin-allowed process, similar in a way to Eq. (3); however, the spin of the metal ion is not a radical being tightly bound with enzyme ligands [7]. The Fe(II) ion in hemoglobin is also paramagnetic and its coupling with O2 is a complicated process depending on the exchange and spin-orbit coupling [10].
Two basic processes of aerobic life, photosynthesis, and respiration represent spin-forbidden reactions, which are activated by the presence of paramagnetic Mg (II) and Fe(II) ions [7,8]. O2 (↑↑) from the air penetrates through the lung alveolus and being bound by Fe(II) ions of hemoglobin is transported to a cell [10]. Besides respiration dioxygen provides many other useful oxidation reactions being activated by various oxidases, mono- and di-oxygenases [12]. Many of them also contain cofactors with paramagnetic metal ions, but many others include flavins and pterins as cofactors without any paramagnetic particles [12-18]. Their mechanism of dioxygen activation to overcome the spin prohibition is not known in the modern biomedical community [12]. Moreover, several new enzymes are known now that have no cofactor at all but can activate dioxygen for substrate oxidation [12-15]. Meanwhile, the physically grounded concept of strong internal magnetic spin-orbit coupling (SOC) perturbation inside the intermediate O2- anion radical [6] can explain all peculiarities of metal-free [7] and even cofactor-free oxidases and oxygenases [9]. Though some practical biochemists [15,23], have already accepted the idea of strong magnetic torque in superoxide anion-radical [6,10] the common biomedical community is not still informed [12] about the SOC theory of the O2-• Species as the main factor of aerobic metabolism and respiration.
How oxidases and oxygenases can overcome spin prohibition for O2 activation
Flavin- and pterin-dependent enzymes are ubiquitous in aerobic life [12]. Their isoalloxazine and pterin cycles can undergo multistep redox chemistry [7,9]. Various redox states of flavins and pterin play essential roles in electron transfer processes being crucial for important biological functions, such as apoptosis, protein folding, DNA repair, cytoskeleton dynamics, detoxification, methylation of RNA, neural development, biosynthesis, energy production, oxidation, and biodegradation [21-26]. Various forms of transient radical pair (RP) intermediates can be generated during reactions catalyzed by flavin- and pterin-dependent enzymes, including FADH+(↑) …O2-•(↓) radical pair [21-28].
Flavoenzymes oxidation was studied for many decades [12]. The reduced flavin (Flred) can be oxidized to semiquinone radical (Flsq•) interacting with O2 and following by subsequent flavoperoxide formation [5]. In 1994 Vincent Massey postulated electron transfer (ET) from reduced flavin to dioxygen and the intermediate RP formation between superoxide ion O2-• and semiquinone Flsq• in the following form, where the spin-flip occurs at the triplet RP stage [5]:
Flred(↓↑) +O 2(↑)(↑) → [Flsq• ↑↑ O2-•]→[Flsq• ↑↓ O2-•]→FlOO-→FlOOH (3)
For example, the reduced deprotonated flavin in the form of the FADH- anion at the start of oxidative half-reactions of glucose oxidase (GO) can give up an electron to the triplet O2 molecule and form the triplet radical pair between semiquinone Flsq• (↑) and superoxide O2-• (↑), Eq. (3). Vincent Massey did not comment the origin of magnetic force responsible for the spin-flip in Eq. (3) [5]. But we can suspect that he took in mind the radical pair theory (RPT) [20], which was quite popular in the eighties for the explanation of magnetic field effects in chemistry. The RPT considers the triplet-singlet (T-S) transition in radical reactions as being induced by hyperfine (HF) interaction between nuclear and electron spins in the separated radicals inside a non-bound RP [20]. The T and S states possess the same energy (degenerate) in RPT; thus, a weak HF interaction can induce the T-S transition [20]. The radical pair theory could be applied to free flavin in solvents but does not apply to real enzymes, where FADH and O2-• are tightly bound in the GO enzyme active site [7,21]. To uncover the driving force origin of the spin-flip in Eq. (3) is the main problem of O2 activation by numerous enzymes; knowledge of the such mechanism is crucial for many practical biochemical and medical applications [12-30]. (like respiration care and lung diseases treatment with a magnet). Extremely weak HF interaction cannot induce a fast spin-flip between triplet and singlet states of RP in the enzyme active center, Eq. (3) [9]. The rate of T-S transition has to compete with the RP triplet state dissociation, Eq. (3), which will lead to the dangerous superoxide release into the cytoplasm. Thus, the origin of driving force for the T-S transition in Eq. (3) is so important to understand.
Explanation of the T-S transition in Eq. (3) has been proposed in Ref. [6] with an account of two possible electronic configurations in the degenerate (πg)3 open shell of superoxide ion (↑)(↓↑) and (↓↑)(↓), where brackets refer to πg,x and πg,y molecular orbitals (MO) of dioxygen [7]; it means, the T-S transition in the radical pair, Eq. (3), can be presented as
3[Flsq•(↑)…(↑)(↓↑)O2-•] → SOC → 1[Flsq•(↑)…(↓↑)(↓)O2-•] (4)
The triplet and singlet states in Eq. (4) differ by electronic configurations inside the superoxide ions. The T-S transition includes orbital rotation πg,x → πg,y for the red-denoted electron with the simultaneous spin flip. The orbital rotation creates magnetic torque which is responsible for spin inversion [13]. The T and S states in Eq. (4) are connected by the strong spin-orbit coupling (SOC) which is equal to ½ Aso, where Aso is a SOC constant of the ground X2Π state of the diatomic O2-• molecule [7,31]. In a simple approximation [7] the Aso (X2Π, O2-•) constant is equal to the SOC constant of the ground O(3P) state of oxygen atom Aso = ζO, which is close to 160 cm-1 [1]. This simple analysis is fully supported by fine structure experimental measurement of the O2-• ion [31]. This energy is millions of times larger than the hyperfine interaction between nuclear and electron spins in the RP theory [20,25]. The above simple theory of O2 activation should be included in all textbooks about respiration care. It is relevant now in connection with pulmonology to consider shortly the external magnetic field effects in biology and medicine [22-30].
External magnetic fields effects in biology and medicine. The role of radical pair theory and spin-orbit coupling in pulmonology
Weak magnetic field effects (MFEs) are abundant in modern biophysics and biomedicine [22-29]. Sensitivity to MFE was detected in the circadian clock, bird’s magnetoreception, in brain activity, memory, anxiety, analgesia, genetics, and many other physiological functions [22-27]. The great achievements of MFE mechanisms understanding are connected with the radical pair theory (RPT) [25] which was applied to electron transfer in cryptochrome, neuronal activities, stem cells, DNA, action potentials in brain, and Reactive Oxygen Species (ROS) [25-29].
The RPT mechanism was applied in significant detail for the bird’s magnetoreception and their navigation in the Earth’s weak magnetic field during the bird’s intercontinental flights [25-27]. RPT considers two radicals, typically each having one non-paired electron spin; thus, the RP can possess either singlet (↑↓) or triplet (↑↑) spin state. Two radicals can recombine in the S state but only scatter in the T state spin orientation since it does not lead to covalent bonding. If triplet RP collides in a solvent, the radicals go apart and experience T-S transition during their diffusion in the solvent cage; (the rate of such spin flip depends on external magnetic field) [20]. Thus, these radicals can recombine in the second collision [20]. Spin interaction with the magnetic field of a few millitesla (mT) is very weak and smaller than thermal energy; thus, only RPT can explain such weak MFEs.
Zheng, et al. have shown that a static magnetic field of 4 mT can regulate the proliferation, migration, and differentiation of human dental pulp stem cells [30]. It was shown that exposure of human monocytic U937 cells to the external magnetic field of 6 mT decreases macrophagic differentiation [24]. Cortical astrocytes and renal cell cultures were influenced by weak (0.6 mT) MFE [26]. The external magnetic field can induce dopamine-dependent change in cortical excitability for patients with Parkinson’s disease [26]. All these MFEs are supposed to be explained with the radical pair theory, though the biochemical mechanisms are far from detailed analysis. Turin, et al. [29] have shown that oxygen gas was necessary for observing spin changes during xenon-induced anesthesia in Drosophila. Accounting for these observations, the authors [28] proposed that the single electron transfer related to xenon’s anesthetic action that was observed by Turin, et al. [29] would play a role in the recombination dynamics of the radical par between tryptophan cation and superoxide anion. We can comment that such RP recombination will be driven by the SOC-induced mechanism of the Eq. (3) type (not by radical pair theory).
One should mention that many studied MFEs are poorly reproduced and the influence of static magnetic field on oxygen functionality [10-13,23] is also rather suspicious. The only solid conclusion about the internal magnetic interaction influence on O2 reactivity in aerobic creatures is connected with the role of spin-orbit coupling in oxygenation enzymes, Eq. (4) [16].
O2 transport and supply to the cell
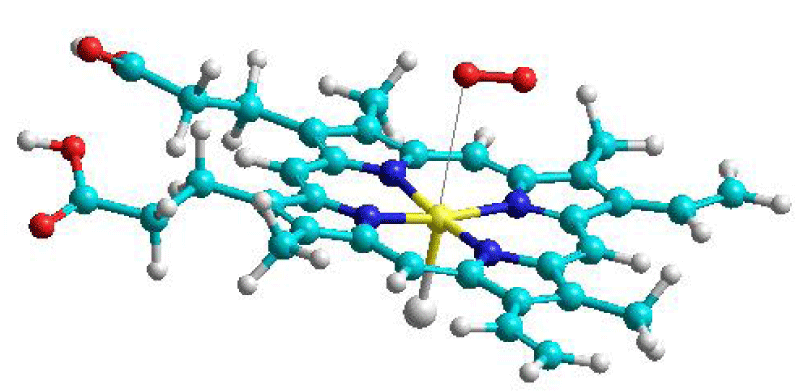
Myoglobin (Mb) and hemoglobin (Hb) are abundant globular proteins that reversibly bind the dioxygen molecule. The mechanism of this binding is crucial for respiratory care. Myoglobin is distributed in muscle сells, where it stores O2 supplying it to working muscles and providing them with oxidation energy [10]. Hemoglobin is the dioxygen carrier in the red blood cells; Hb is a tetramer of myoglobin molecules. Both proteins contain ferrous Fe(II) iron in a heme group; the late is often simulated by Fe(II) porphyrin, with iron ion being tetra-coordinated to the nitrogen atoms of the tetrapyrrole cycles [10]. One proximal histidine residue of the side chain protein of Mb is usually tightly bound to the ferrous ion leaving a vacant empty position in the Fe(II) octahedral coordination sphere. (In Figure 1 the histidine residue is simulated by Cl- ion). Both paramagnetic proteins, Hb and Mb, can bind several gaseous molecules besides dioxygen, e.g. carbon and nitrogen oxides, CO, and NO [10,11,19]. Quantum chemical calculations with SOC account afford to get new ideas on all diatomic gases coupling with heme models [10,11,19]. The ferrous ion provides four non-paired spins and the quintet ground state (S=2) for the Mb ground state with the close-lying triplet excited state [10]. The late one becomes the lowest during the O2 approach to heme. After such a spin flip, the dioxygen binding reaction includes the interaction of two triplet species which can produce the quintet (S=2), triplet (S=1), and singlet (S=0) final states. Oxidation of the iron ion (Fe2+ → Fe3+) is known to be responsible for the color of blood and muscle. Such electron transfer occurs during Fe3+binding and superoxide involvement in the heme structure.
Quantum chemical calculations show that the ground state of the O2 + heme recombination product (the oxy-heme) is a singlet state with an open electronic shell in agreement with the Messbauer spectra and EPR experiments [10]. Therefore, the reaction of dioxygen binding to heme is also the spin forbidden complicated process. It was shown that the O2 binding by heme includes the stage of Fe3+…O2- interaction which includes large SOC energy for the T-S transition and spin-flip produced by two possible superoxide electronic configurations, like in Eq. (4). For the heme reaction this SOC energy is accompanied by additional SOC contribution from the Fe3+ ion.
Figure 1 presents the model for heme– O2 interaction in myoglobin used in our calculations of the multiconfiguration self-consistent field approach with spin-orbit coupling account [10]. The recent and more realistic models of heme– O2 interaction [32] support the main details of our analysis of spin effects in dioxygen binding by myoglobin. New time-resolved spectroscopy studies of the light-induced photo-dissociation of oxy-hemoglobin are necessary to provide experimental proof for spin transitions during heme– O2 interaction.
Spin effects in reactive oxygen species (ROS) and in the oxidative stress
The singlet excited oxygen O2(a1Δg) and superoxide O2-• (X2Πg) radicals are the most important reactive oxygen species (ROS) [17,33].
Excessive generation of ROS and oxidative stress play a crucial role in the pathogenesis of many diseases [34,35]. Respiration produces adenosine triphosphate (ATP) molecules through glycolysis and oxidative phosphorylation (OxP), along with dioxygen and glucose consumption. The OxP occurs in mitochondria producing most of the ATP for the cell’s needs. Thus, cell respiration affects most physiological functions and behaviors [34]. Dysfunction of mitochondria causes numerous predictable defects in most tissues [33-38].
Ion-radical O2-• can leave enzymes and destroy mitochondria when the spin-flip in Eq. (3) is not so fast to compete with the RP dissociation in the enzyme active site; the spin-flip rate can deviate from the normal regime because it depends on the O2-• radical rotation and vibration in the protein scaffold environment [9,13]. Vibrational movement of the nearest amino-acid residues also influences the spin-orbit coupling in the radical pair and the spin-flip rate, Eq. (4) [37-39].
The small O2 molecule can freely penetrate through the cell membrane; thus, the decreased dissolved O2 concentration around the cells can be used to indicate oxygen consumption by the cell [35]. Microfluidic chips which use electrochemical micro-electrodes can provide accurate detection of dissolved oxygen. Optical sensors using luminescence are also useful to monitor O2 concentration [35,36]. The ROS O2(a1Δg) can be detected in tissue by its weak near-infra-red luminescence at 1.27 μm in the spin-forbidden singlet-triplet transition O2(a1Δg) → O2(X3Σg) [7]. Quantum-chemical analysis of dioxygen wave functions in the electronic O2(πg)2 open shell and its spectrum helps to shed new light on the role of internal magnetic interactions, which makes it possible to overcome spin prohibitions in both fundamental phenomena - in biological oxidation and in light emission by molecular oxygen [37].
Concerning the ROS importance, one can remind the whole history of aerobic evolution connected with the role of spin prohibition for the O2(X3Σg) chemical reactivity and spectroscopy [37,38]. The triplet nature of dioxygen and spin restriction explains why our world had not been consumed by fire during the Great Oxygenation Event (GOE) [39] when the green-blue algae and photosynthetic bacteria started to fulfill our Earth’s atmosphere by O2(X3Σg) molecules billion years ago. That time is also known as the Oxygen Catastrophe when primordial anaerobic forms of life and archaea had started to perish and been substituted by eukaryotes [39]. Thus, a new more efficient type of life evolution had started [40]. The primordial atmosphere before GOE does not contain dioxygen; with its occurring the new players - mitochondria - were created and evolved. Mitochondria provide OxP and electrochemical proton gradient generated across inner membranes by the electron transport chain [37]. However, the role of mitochondria extends far beyond the oxidation of glucose via oxidative phosphorylation in living organisms. A new role of mitochondria discovered recently [17]; this is their involvement in ion homeostasis and apoptosis through the signaling functions of ROS. Scientists believe that ROS appeared on the Earth simultaneously with the first photosynthetic O2 [17]. High level of ferrous ions Fe(II) in primordial ocean led to dioxygen reduction. Then, superoxide can dismutate to form hydrogen peroxide H2O2, which interacts with the soluble Fe(II) ions in water by the Fenton reaction to produce a highly reactive OH radical [13,41]. All these molecules, together with the singlet O2(a1Δg) produced by solar radiation, constitute reactive oxygen species that occurred in a natural way at the GOE beginning. Latter on all ROS play important role in life evolution. Superoxide dismutase (SOD) is the oldest enzyme on Earth developed to scavenge ROS very effectively. SOD was found in all kingdoms of life and evolved even before archaea-eukaryotes differentiation [13,40]. The Oxygen Catastrophe left numerous fingerprints on the mountain rocks and these records show very clearly the SOD history in the early Earth’s evolution. The role of spin factors and spin-orbit coupling in the ROS and SOD activity is described in a number of references [7-13,37,38].
Aerobic respiration and photosynthesis as multistep complicated processes are essentially spin-forbidden reactions being activated by exchange perturbations in the presence of paramagnetic metal ions. At the same time, numerous metabolic O2-initiated oxygenation reactions are known to be catalyzed by enzymes without metals. They proceed through the step of electron transfer from organic cofactor (C) to dioxygen and ion O2-• generation in the active site of the enzyme. Just at this step, the spin-flip occurs being induced by the superoxide-internal magnetic force determined by spin-orbit coupling inside the O2-• open shell. This SOC provides intersystem crossing in the C+.…O2-• radical pair [6]. Such a mechanism has nothing in common with the well-known RP theory [20,26]. It includes a huge SOC energy (160 cm-1) while in RP theory spin-orbit coupling is neglected [23,25]. Such neglection is well-grounded for most organic radical pairs in solvents, but not for radicals with degenerate open shells, like superoxide [7]. The new mechanism [7] is widely spread in O2 biochemistry, being the only way to activate the T-S spin flip in dioxygen without external paramagnetic assistance. It can explain O2 activation by glucose oxidase and by other enzymes containing flavin cofactor, as well as cofactor-free enzymes like Rubisco or oxoquinaldine 2,4-dioxygenase [13,30]. Magnetic torque in the open shell of superoxide ions is one of the main driving forces for O2 activation in many other oxygenases and oxidase enzymes without cofactor [37].
Conclusion
Interaction of paramagnetic dioxygen from the air with myoglobin and hemoglobin, as well as the whole Crebs cycles of citric acid, are very specific chemical processes forbidden by the formal chemical rules because of the spin conservation concept [4]. The fundamental background of respiration care and new therapeutic and diagnostic pulmonology methods will strongly depend on the theoretical physics and quantum chemistry progress in studies of spin interactions between paramagnetic dioxygen and biopolymers.
Thus, the chemical reactivity of dioxygen strongly depends on the spin properties of electrons. Despite marvelous discoveries in molecular electronics and spintronics, modern theoretical chemistry still keeps in silence many mysteries of dioxygen bioactivation [13].
Even nowadays the chemistry of dioxygen continues to challenge modern branches of research in physics, biology, and medicine, despite the attention drawn for ~200 years since Faraday’s and Lavoisier’s discoveries, which established paramagnetism of O2 and its ability to support respiration and combustion [1,2]. Quantum chemistry explained dioxygen paramagnetism by spin properties, but the spin-forbidden biochemical puzzles are still not fully understood.
The triplet O2 dioxygen exhibits sluggish chemical reactivity when there are no organic radicals or paramagnetic metal ions in the nearest environment. In their absence, the two parallel spins of the O2 molecule hinder dioxygen reactivity significantly despite its apparent inclination to burn and oxidize everything around. Any paramagnetic relief of spin prohibition could be used by dioxygen to insert into organic matter. The external magnetic field effects (not fully understood nowadays) will promise new therapy advantages in future pulmonology. Many enzymes are developed by Nature to provide various magnetic reliefs using particular magnetic properties of the O2 molecule and reactive oxygen species.
This work was supported by the Ministry of Science and Education of Ukraine (project 0122U000760) and by the Swedish Wenner-Gren Foundations (project GFU 2022-0036). The authors express gratitude to Dr. Hans Agren and Dr. V.A. Minaeva for useful discussions.
- Minaev BF, Murugan NA, Ågren H. Dioxygen spectra and bioactivation. International Journal of Quantum Chemistry. 2013; 113 (14): 1847-1867.
- Lane N. Oxygen. The Molecule that Made the World. Oxford: University Press. 2002; 365.
- Mulliken RS. Oxygen triplet spin state. Nature. 1928; 122: 505-507.
- Wigner E, Witmer EE. Zeits. F. Physik. 1928; 51: 859-864.
- Massey V. Activation of molecular oxygen by flavins and flavoproteins. Journal of Biological Chemistry. 1994; 269(36):22459–62.
- Minaev BF. Spin effects in reductive activation of O2 by oxidase enzymes. RIKEN Review. Tokyo. 2002; 44: 147-149.
- Minaev BF. Electronic mechanisms of molecular oxygen activation. Russian Chemical Review. 2007; 76(11): 988-1010. PMID: 15040259. DOI: 10.1038/nrmicro821.
- Siegbahn PEM. Structures and Energetics for O2 Formation in Photosystem II. Acc Chem Res. 2009; 42(10): 1871−1880.
- Minaev BF. How cofactor–free oxygenases can overcome spin prohibition in substrates oxygenation by dioxygen. Chem Physics. 2019; 251(1): 61–68. DOI: 10.1016/j.chemphys.2019.01.021.
- Minaev BF, Minaeva VA. Spin-dependent binding of dioxygen to heme and charge transfer mechanism of spin-orbit coupling enhancement. Ukrainica Bioorganica Acta. 2008; 2(1): 56-64.
- Khudyakov IV, Minaev BF. Molecular Terms of Dioxygen and Nitric Oxide. Physchem. 2021; 1(2): 121-132.
- Romero E, Gómez Castellanos JR, Gadda G, Fraaije MW, Mattevi A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem Rev. 2018 Feb 28;118(4):1742-1769. doi: 10.1021/acs.chemrev.7b00650. Epub 2018 Jan 11. PMID: 29323892.
- Minaev BF. Magnetic torque inside the superoxide radical is the driving force for oxygen activation by dioxygenases. Advances in Chemistry Research. Nova Science Publishers, Inc. 2022; 75.
- Fetzner S, Steiner RA. Cofactor-independent oxidases and oxygenases. Appl Microbiol Biotechnol. 2010 Apr;86(3):791-804. doi: 10.1007/s00253-010-2455-0. Epub 2010 Feb 16. PMID: 20157809.
- Baas BJ, Poddar H, Geertsema EM, Rozeboom HJ, Poelarends GJ. Functional and Structural Characterization of an Unusual Cofactor-Independent Oxygenase. Biochemistry. 2019; 50(5): 2889-2899.
- Minaev BF, Minaeva VA, Agren H. Spin-orbit coupling in enzymatic reactions and the role of spin in biochemistry. In: Leszczynski J, editor. Handbook of Computational Chemistry: Springer Berlin/ Heidelberg. 2012; 1067–93. DOI: 10.1007/978–94-007-0711–5_29.
- Mittler R. ROS Are Good. Trends Plant Sci. 2017 Jan;22(1):11-19. doi: 10.1016/j.tplants.2016.08.002. Epub 2016 Sep 23. PMID: 27666517.
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049-55. PMID: 5389100.
- Zavhorodnia VO, Kovalenko SO, Minaev BF. Interaction of myoglobin model with ligands of gas exchange. Cherkasy University Bulletin: Biological Sciences Series. 2019; 1: 13-23. DOI: 10.31651/2076-5835-2018-1-2019-1-13-23
- Salikhov KM, Molin YN, Sagdeev R, Buchachenko A. Spin polarization and magnetic effects in radical reactions. New York, NY: Elsevier. 1984.
- Prabhakar R, Siegbahn PE, Minaev BF. A theoretical study of the dioxygen activation by glucose oxidase and copper amine oxidase. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):173-8. doi: 10.1016/s1570-9639(03)00090-6. PMID: 12686129.
- Minaev BF, Panchenko AA. Spin–catalysis of Unsaturated Substrates Oxidation by Cofactor–free Mono– and Di–oxygenases. How Triplet Oxygen Can Overcome Spin Prohibition. Ukr. J. Medicine, Biology, and Sport. 2019; 4(6): 329-343. DOI: 10.26693/jmbs04.06.329.
- Bathellier C, Yu LJ, Farquhar GD, Coote ML, Lorimer GH, Tcherkez G. Ribulose 1,5-bisphosphate carboxylase/oxygenase activates O2 by electron transfer. Proc Natl Acad Sci U S A. 2020 Sep 29;117(39):24234-24242. doi: 10.1073/pnas.2008824117. Epub 2020 Sep 15. PMID: 32934141; PMCID: PMC7533879.
- Pagliara P, Lanubile R, Dwikat M, Abbro L, Dini L. Differentiation of monocytic U937 cells under static magnetic field exposure. Eur J Histochem. 2009; 49: 75. (doi:10.4081/930).
- Player TC, Hore PJ. Viability of superoxide-containing radical pairs as magnetoreceptors. J Chem Phys. 2019 Dec 14;151(22):225101. doi: 10.1063/1.5129608. PMID: 31837685.
- Zadeh-Haghighi H, Simon C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J R Soc Interface. 2022 Aug;19(193):20220325. doi: 10.1098/rsif.2022.0325. Epub 2022 Aug 3. PMID: 35919980; PMCID: PMC9346374.
- Panchenko OO, Minaev BF. Enzymatic spin-catalysis in flavin-containing oxidases and magnetic orientation of birds. Cherkasy University Bulletin: Biological Sciences Series. 2018; 1: 114-120.
- Smith J, Zadeh-Haghighi H, Salahub D, Simon C. Radical pairs may play a role in xenon-induced general anesthesia. Sci Rep. 2021; 11: 1–13.
- Turin L, Skoulakis EM, Horsfield AP. Electron spin changes during general anesthesia in Drosophila. Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):E3524-33. doi: 10.1073/pnas.1404387111. Epub 2014 Aug 11. PMID: 25114249; PMCID: PMC4151765.
- Zheng L, Zhang L, Chen L, Jiang J, Zhou X, Wang M, Fan Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J Tissue Eng Regen Med. 2018 Oct;12(10):2029-2040. doi: 10.1002/term.2737. Epub 2018 Sep 2. PMID: 30058115.
- Land JE, Raith W. Fine Structure of O2− Measured by Electron Time-of-Flight Spectroscopy. Phys Rev Lett. 1973; 30: 349-352.
- Saito K, Watabe Y, Fujihara T, Takayanagi T, Hasegawa JY. Spin-inversion mechanisms in O2 binding to a model heme complex revisited by density function theory calculations. J Comput Chem. 2020 Apr 30;41(11):1130-1138. doi: 10.1002/jcc.26159. Epub 2020 Feb 5. PMID: 32020659.
- Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969 Sep 10;36(6):891-7. doi: 10.1016/0006-291x(69)90287-3. PMID: 5388670.
- Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol Lett. 2012 Dec;4(6):1151-1157. doi: 10.3892/ol.2012.928. Epub 2012 Sep 20. PMID: 23226794; PMCID: PMC3506713.
- Minaev B. Magnetc torque in superoxide ion is the main driving force of dioxygen activation in aerobic life. Biomed. J Sci Tech Res. 2021; 38(4): 121-131. DOI: 10.27717. BJSTR.MS.ID.006171.
- Minaev BF, Valiev RR. Spin-orbit coupling effects in O2 activation by cofactor–independent 2, 4–dioxygenase. Ukrainian Biochem J. 2019; 91(1): 38–46. DOI: 10.15407/ubj91.01.038.
- Minaev BF. Spin-orbit coupling of charge-transfer states and the mechanism for quenching singlet oxygen by amines. Theoretical and Experimental Chemistry. 1984; 20(2): 209-212.
- Papkovsky DB, Zhdanov AV. Phosphorescence based O2 sensors - Essential tools for monitoring cell and tissue oxygenation and its impact on metabolism. Free Radic Biol Med. 2016 Dec;101:202-210. doi: 10.1016/j.freeradbiomed.2016.09.018. Epub 2016 Oct 24. PMID: 27789291.
- Skuodis E, Leitonas K, Panchenko A. Very sensitive probes for quantitative and organoleptic detection of oxygen based on conformer-induced room-temperature phosphorescence enhancement of the derivative. Sensors and Actuators B: Chemical. 2022; 373: 132727. Doi: 10.1016/j.snb.2022.132727.
- Ligrone R. The Great Oxygenation Event, Springer International Publishing, Cham. 2019: 129–154.
- Zakharov II, Kudjukov KY, Bondar VV, Tyupalo NF, Minaev BF. DFT-based thermodynamics of fenton reactions rejects the ‘pure’aquacomplex models. Comput Theor Chemistry. 2011; 964(1-3): 94-99.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
