Archives of Pulmonology and Respiratory Care
Is there a relationship between COVID-19 and sarcoidosis? A case report
Yasar Kucukardali1, Arzu Gunturk2, Mehmet Akif Ozturk1, Şenay Acikel1, Hatice Zeynep Ceylan1*, Pınar Fırat3 and Banu Salepci4
2Department of Internal Medicine, Bilim University Florance Nightingale Hospital, Istanbul, Turkey
3Department of Pathology, Koç University Hospital, Istanbul, Turkey
4Department of Chest Diseases, Yeditepe University Hospital, Istanbul, Turkey
Cite this as
Kucukardali Y, Gunturk A, Ozturk MA, Acikel Ş, Ceylan HZ, et al. (2022) Is there a relationship between COVID-19 and sarcoidosis? A case report. Arch Pulmonol Respir Care 8(1): 023-027. DOI: 10.17352/aprc.000080Copyright License
© 2022 Kucukardali Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Mediastinal lymphadenopathy is rare in Coronavirus Disease-2019 (COVID-19) patients with mild clinical course. The frequency of lymphadenopathy increases in COVID-19 patients who develop Acute Respiratory Distress Syndrome (ARDS). In a 38-year-old male patient, mediastinal lymphadenopathy and asymptomatic pulmonary embolism were detected during the third week of COVID-19 infection at home. Sarcoidosis was diagnosed with a finding of non-caseating granulomas. Even if it is asymptomatic, pulmonary embolism should be considered, especially in COVID-19 patients with high C - Reactive Protein (CRP) and D-dimer levels. If mediastinal lymphadenopathy is detected in mild COVID-19 cases, systemic diseases should be investigated. In severe COVID-19 cases, if lymphadenopathy continues despite a COVID-19 recovery, further investigation is required.
Introduction
Lymphadenopathy in COVID-19 infections is a rare finding. The frequency is 3.38% in a meta-analysis [1]. This finding should prompt investigation for other diseases [2]. In the early stages of the disease, lymphadenectasis will not be seen in the mediastinum and hilum. Very few cases present lymphadenectasis in progressive stages [3] (Figure 1 A-D).
Case
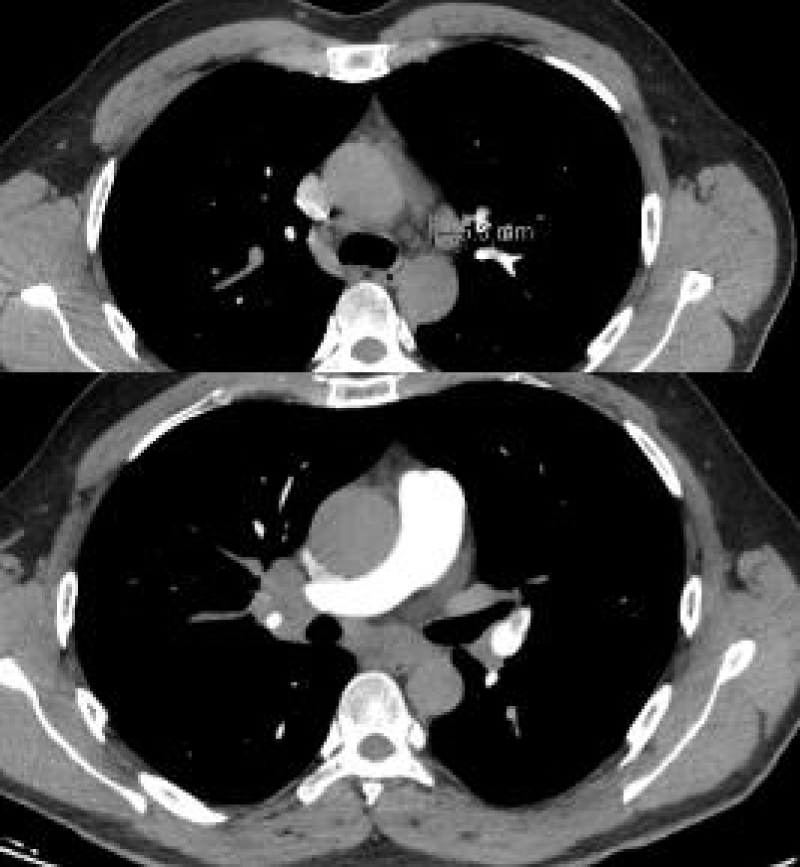
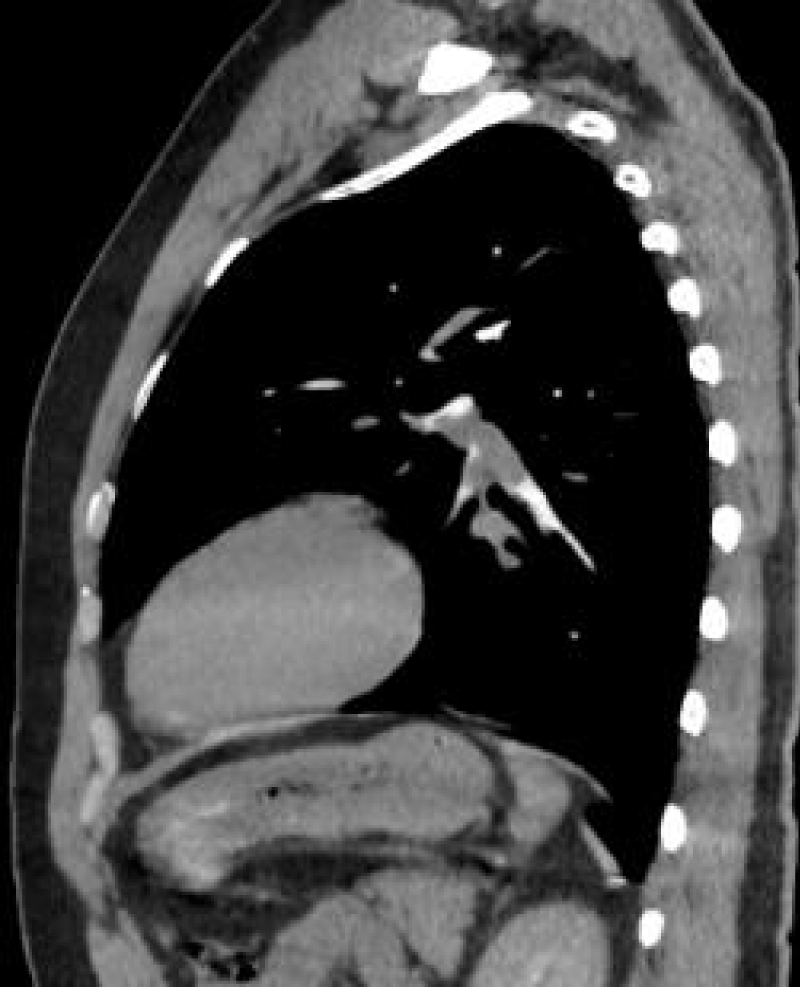
A 38-year-old male patient was admitted to the hospital with a complaint of occasional cough. Two weeks before, a nasal swab test with polymerase chain reaction confirmed a SARS-CoV-2 infection which the patient gave due to fever, weakness, headache, and loss of taste and smell. He used favipiravir, paracetamol, vitamin C for five days. His complaints have decreased. He applied for a COVID-19 Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) test to be able to resume his work because he had a cough complaint. He does not smoke or drink alcohol. The mother has a history of sudden cardiac death at the age of 40. On physical examination, the blood pressure was 130/110mmHg, the pulse was 77 beats/min, and rhythmic, and the body temperature was 36.5 C. Varicose enlargement in the legs was noted. There was no abnormality in the laboratory findings except for an Aspartate Aminotransferase (AST) level of 87 U/L [<48 IU/L], CRP of 63 mg/dl, [<5 mg/dl] D-dimer of 1.28 ug/ml [0,50 µg/mL]. The right hilus looked plump on chest radiography. There was no hilar fullness in the chest radiography taken in 2017. Thoracic tomography revealed multiple lymphadenomegalies in the mediastinum and conglomerated lymphadenomegalies of approximately 3x2 cm in the right hilus, and 3.5x4 cm subcarinal area (Picture 1-A). The serum calcium level [8.5-10.2 mmol/L] and Angiotensin Converting Enzyme (ACE) level [<40 micrograms/L] were found to be within the expected values.
The pulmonary CT (Computer Tomography) angiography revealed a filling defect consistent with pulmonary thromboembolism, which completely filled the branch of the left pulmonary artery leading to the lower lobe and partially obliterated the branch leading to the lingula (Picture 1-B).
Venous Doppler examination of the lower extremity revealed a varicous widening. Fine needle aspiration biopsy was performed under the guidance of endobronchial ultrasound. A diagnosis of non-necrotizing granulomatosis was made. In the microbiological examination of the biopsy material, Acid Resistant Bacteria (ARB) was found negative, and the Quantiferon test was also negative. The patient was diagnosed with sarcoidosis. Since there were no activity criteria and organ involvement, follow-up without treatment was decided. For pulmonary embolism, the patient was started on enoxaparin 8000 U twice a day, warfarin 10 mg on the first day, and 5 mg after the second day. Heparin toxicity developed on the 9th day of treatment. Alanine Aminotransferase (ALT) increased to 291 U/L [<46 IU/L] and AST 74 U/L [<48 IU/L]. There was no history of alcohol consumption, and the viral serology, autoimmune panel, and portal venous Doppler ultrasonography were normal. Echocardiography, cardiac biomarkers, abdominal USG, and cholestasis tests were also found to be normal. Heparin was discontinued and Apixaban 5 mg twice a day was initiated. The liver enzymes were within normal values after ten days.
Discussion
Viral infections have long been associated with the emergence of autoimmune diseases such as systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), Sjögren’s Syndrome (SS), systemic vasculitis, celiac disease, and multiple sclerosis. With the onset of the SARS-CoV-2 pandemic, de novo autoimmune diseases emerge in the infected patients, including sarcoidosis, in at least 4 cases according to a 2021 study by Gracia and coworkers. They reported that the mean age of the patients with sarcoidosis was 52.5 ± 16.4 years, all were females, and the mean onset of days from COVID-19 to sarcoidosis was 17 ± 9.5. In their cases, three patients had cutaneous involvement and two lymphadenopathies [4]. The incidence peaks in persons aged 25-35 years. A second peak occurs for women aged 45-65 years [5]. Kobak. et al. reported that young-onset sarcoidosis was diagnosed in most patients (84.7%), while older-onset sarcoidosis was diagnosed in 15.3% of patients (75% of whom were females) in 131 sarcoidosis cases from our country, Turkey [6]. Our report is about another case who had sarcoidosis after a SARS-CoV-2 infection and we believe it will be useful in shedding light on clinical manifestations of immune responses in persons infected with this virus.
Even if the Coronavirus disease is not severe in a patient, we believe that if there is lymphadenopathy, it should be shown that there are no other diseases. Hilar lymphadenopathy is more common in fungal, and mycobacterial infections, lymphoma, and sarcoidosis. Rarely, it also occurs in viral pneumonia. In the literature review in the early period of the COVID-19 pandemic, bilateral hilar lymphadenopathy was not reported [7,8].
The common CT manifestation of COVID-19 includes multiple segmental ground glass opacities distributed dominantly in subpleural zones and interlobular septal thickening and consolidation. Pleural effusion or mediastinal lymphadenopathy is rarely seen [6,9]. Bao, et al. reported that the frequency of lymphadenopathy was found to be 3.38% in the general COVID-19 disease group [10]. In the study of Zhu, et al. the rate of lymphadenopathy among 4100 COVID-19 patients was found to be 5.4% [11]. In a study conducted on COVID-19 patients brought to the emergency department in Italy, the rate of mediastinal lymphadenopathy larger than 1 cm was found to be 19% [12]. In a study reported from France, mediastinal lymphadenopathy was reported in 66% of 15 severe COVID-19 patients who developed ARDS while hospitalized in intensive care. Lymphadenomegaly size reaches up to 3 cm, especially in subcarinal ones. Fungal, bacterial, neoplastic, and systemic diseases were excluded in all of these cases [13]. This data shows that as the severity of the disease increases, the frequency of lymphadenopathy increases in COVID-19 patients. If there is mediastinal lymphadenopathy in COVID-19 patients with ARDS, it should be associated with COVID-19 first, and it is necessary to wait for recovery before proceeding with further tests. If lymphadenopathy continues despite a COVID-19 recovery, further investigations are required.
Our case did not have severe COVID-19 disease, ARDS did not develop in the patient, and the home treatment process was completed. Different SARS-CoV-2 variants are known to cause different clinical presentations. However, our case was detected in the early part of the pandemic. At that time, we did not perform the variant analysis due to that being an uncommon practice. Moreover, we could not find data on sarcoidosis and SARS-CoV-2 variants in the literature.
The respiratory system is the most important target organ for SARS-CoV-2 infections. Sarcoidosis is a granulomatous disease that can affect all organs and systems, especially the lungs. Pulmonary involvement occurs in more than 90% of the cases [14]. Anemia or other cell line deficiencies, elevated liver enzymes, hypercalciuria, and ECG abnormalities may also be present. Angiotensin Converting Enzyme levels may be elevated but are not diagnostic. Histopathological confirmation of noncaseating granulomas is essential for diagnosis [15].
Symptomatic patients with sarcoidosis use immunosuppressive drugs that are considered a risk for SARS-CoV-2 infection. Therefore, we can argue that sarcoidosis patients get more SARS-CoV-2. However, there is not much evidence in the literature to support this prediction. In Xiani’s letter to the editor, in the COVID-19 series of 300 cases, it was reported that 10 cases were registered to the Sarcoidosis outpatient clinic, and all of these cases were using cortisone, and none of these cases died [16]. In a study from Italy, it was reported that only two of 859 patients who received Disease Modifying Anti-Rheumatismal Drug (DMARD) treatment were diagnosed with SARS-CoV-2 infection [17]. The low rates may be attributed to sarcoidosis patients employing better self-protection. In addition, the use of some drugs used in the treatment of sarcoidosis in SARS-CoV-2 infections may lead to a less severe clinical presentation.
However, different results have also been reported. In the study of Baughman, et al. SARS-CoV-2 infection was found more in sarcoidosis patients than in the general population. In the USA, 1060 patients per million in the general population had COVID-19, while 2.23% of 5200 cases with sarcoidosis were found to have COVID-19 in the same population. It corresponds to 22308 cases per million for the sarcoidosis population [18]. In a recent retrospective study, the rate of hospitalization in 37 patients with sarcoidosis with COVID-19 infections was similar to patients without sarcoidosis. However, the intubation rate and mortality rate were found to be higher in sarcoidosis cases [19]. It has been shown that diseases such as diabetes, hypertension, heart disease, and Chronic Obstructive Pulmonary Disease (COPD) do not pose an additional risk in terms of COVID-19 infections in sarcoidosis patients, but the use of Rituximab leads to an increased risk [20-22].
Our case was not diagnosed with sarcoidosis before hospitalization and was not using immunosuppressive drugs. When the medical records of the patient were examined, it was observed that there was no hilar fullness in the chest radiography in 2017.
Recent publications have shown that excessive inflammation, hypoxia, and immobilization in COVID-19 patients lead to intravascular coagulation by creating a prothrombotic state [23,24]. Obesity, high CRP, and D-dimer levels have also been shown as risk factors for pulmonary embolism in COVID-19 patients [25]. Our patient contracted his illness at home, and there were no initial inflammation and D-dimer tests. He was not receiving anticoagulant and antiaggregant treatment at home. Although he was asymptomatic when he came for his control, pulmonary CT angiography was performed because of high levels of CRP and D-dimer, and a pulmonary embolism was observed. Pulmonary Embolism (PE) and deep vein thrombosis (DVT) occurred in 16.5% and 14.8% of patients with coronavirus disease 2019 (COVID-19) [26]. Léonard-Lorant, et al. reported that 30% of 106 COVID-19 patients who had pulmonary CT angiography were found to have an acute pulmonary embolism. The D-dimer level was found above 2660ug / L in all patients with pulmonary embolism. However, in 49 of 74 patients without embolism, the D-dimer level was found above 2,660 ug/L. [27] Therefore, in cases of pulmonary embolism, the D-dimer test has high sensitivity but low specificity. In the study of Ruaro, et al. pulmonary embolism was found in 15 of 256 sarcoidosis cases. The development of pulmonary emboli was found to be correlated with antiphospholipid antibodies in sarcoidosis [28]. However, to our knowledge, there is no study that investigates the incidence of pulmonary embolism in patients that developed sarcoidosis after a COVID-19 infection.
In summary, mediastinal lymphadenopathy is not a common radiological finding in COVID-19 cases unless ARDS is present. Evaluating patients for lymphadenopathy after an acute period, namely one month of COVID-19 infection is crucial, because if mediastinal lymphadenopathy is detected in mild-moderate COVID-19 cases, sarcoidosis should also be considered, and necessary tests should be performed. In severe COVID-19 cases too, if lymphadenopathy continues despite the recovery, further investigation is required. Even if there are no symptoms pulmonary embolism should be considered, especially in COVID-19 patients with high CRP and D-dimer levels.
- Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J Am Coll Radiol. 2020 Jun;17(6):701-709. doi: 10.1016/j.jacr.2020.03.006. Epub 2020 Mar 25. PMID: 32283052; PMCID: PMC7151282..
- Plesner LL, Dyrberg E, Hansen IV, Abild A, Andersen MB. [Diagnostic imaging findings in COVID-19]. Ugeskr Laeger. 2020 Apr 6;182(15):V03200191. Danish. PMID: 32286216..
- Zheng Q, Lu Y, Lure F, Jaeger S, Lu P. Clinical and radiological features of novel coronavirus pneumonia. J Xray Sci Technol. 2020;28(3):391-404. doi: 10.3233/XST-200687. PMID: 32538893; PMCID: PMC7369043.
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells. 2021 Dec 20;10(12):3592. doi: 10.3390/cells10123592. PMID: 34944099; PMCID: PMC8700122.
- Nader Kamangar, MD, FACP,FCCP,FCCM. Sarcoidosis. Updated: Feb 09, 2022)
- Kobak S, Yildiz F, Semiz H, Orman M. Elderly-onset sarcoidosis: A single center comparative study. Reumatol Clin (Engl Ed). 2020 May-Jun;16(3):235-238. English, Spanish. doi: 10.1016/j.reuma.2018.06.004. Epub 2018 Jul 24. PMID: 30054252.
- Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020 Apr;295(1):202-207. doi: 10.1148/radiol.2020200230. Epub 2020 Feb 4. PMID: 32017661; PMCID: PMC7194022..
- Ng MY, Lee EYP, Yang J, Yang F, Li X, Wang H, Lui MM, Lo CS, Leung B, Khong PL, Hui CK, Yuen KY, Kuo MD. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol Cardiothorac Imaging. 2020 Feb 13;2(1):e200034. doi: 10.1148/ryct.2020200034. PMID: 33778547; PMCID: PMC7233595.
- Zheng Q, Lu Y, Lure F, Jaeger S, Lu P. Clinical and radiological features of novel coronavirus pneumonia. J Xray Sci Technol. 2020;28(3):391-404. doi: 10.3233/XST-200687. PMID: 32538893; PMCID: PMC7369043.
- Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J Am Coll Radiol. 2020 Jun;17(6):701-709. doi: 10.1016/j.jacr.2020.03.006. Epub 2020 Mar 25. PMID: 32283052; PMCID: PMC7151282..
- Zhu J, Zhong Z, Li H, Ji P, Pang J, Li B, Zhang J. CT imaging features of 4121 patients with COVID-19: A meta-analysis. J Med Virol. 2020 Jul;92(7):891-902. doi: 10.1002/jmv.25910. Epub 2020 Apr 29. PMID: 32314805; PMCID: PMC7264580..
- Sardanelli F, Cozzi A, Monfardini L, Bnà C, Foà RA, Spinazzola A, Tresoldi S, Cariati M, Secchi F, Schiaffino S. Association of mediastinal lymphadenopathy with COVID-19 prognosis. Lancet Infect Dis. 2020 Nov;20(11):1230-1231. doi: 10.1016/S1473-3099(20)30521-1. Epub 2020 Jun 19. PMID: 32569623; PMCID: PMC7304961..
- Valette X, du Cheyron D, Goursaud S. Mediastinal lymphadenopathy in patients with severe COVID-19. Lancet Infect Dis. 2020 Nov;20(11):1230. doi: 10.1016/S1473-3099(20)30310-8. Epub 2020 Apr 21. PMID: 32330440; PMCID: PMC7173806.
- Caruana LB, Redwine GD, Rohde RE, Russian CJ. A prospective study of patients diagnosed with sarcoidosis: factors - environmental exposure, health assessment, and genetic outlooks. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36(3):228-242. doi: 10.36141/svdld.v36i3.7112. Epub 2019 May 1. PMID: 32476958; PMCID: PMC7247080.
- Heinle R, Chang C. Diagnostic criteria for sarcoidosis. Autoimmun Rev. 2014 Apr-May;13(4-5):383-7. doi: 10.1016/j.autrev.2014.01.035. Epub 2014 Jan 11. PMID: 24424172..
- (CDC) USCfDCaP. Coronavirus disease 2019 (COVID-2019). Accessed April 20, 2020
- Conticini E, Bargagli E, Bardelli M, Rana GD, Baldi C, Cameli P, Gentileschi S, Bennett D, Falsetti P, Lanzarone N, Bellisai F, Barreca C, D'Alessandro R, Cantarini L, Frediani B. COVID-19 pneumonia in a large cohort of patients treated with biological and targeted synthetic antirheumatic drugs. Ann Rheum Dis. 2021 Feb;80(2):e14. doi: 10.1136/annrheumdis-2020-217681. Epub 2020 May 15. PMID: 32414804..
- Baughman RP, Lower EE, Buchanan M, Rottoli P, Drent M, Sellares J, Terwiel M, Elfferich M, Francesqui J, Barriuso Cabrerizo MR, Sweiss N, Martone F, Al-Hakim T, Judson MA. Risk and outcome of COVID-19 infection in sarcoidosis patients: results of a self-reporting questionnaire. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37(4):e2020009. doi: 10.36141/svdld.v37i4.10726. Epub 2020 Dec 16. PMID: 33597796; PMCID: PMC7883514.
- Morgenthau AS, Levin MA, Freeman R, Reich DL, Klang E. Moderate or Severe Impairment in Pulmonary Function is Associated with Mortality in Sarcoidosis Patients Infected with SARS‑CoV‑2. Lung. 2020 Oct;198(5):771-775. doi: 10.1007/s00408-020-00392-9. Epub 2020 Sep 11. PMID: 32915271; PMCID: PMC7484928..
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020 Apr 8;12(7):6049-6057. doi: 10.18632/aging.103000. Epub 2020 Apr 8. PMID: 32267833; PMCID: PMC7185114..
- Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020 Jun 22;62:e43. doi: 10.1590/S1678-9946202062043. PMID: 32578683; PMCID: PMC7310609..
- Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020 May 14;55(5):2000547. doi: 10.1183/13993003.00547-2020. PMID: 32217650; PMCID: PMC7098485..
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [PMC free article] [PubMed] [Google Scholar]
- Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VEA, Meijers JCM. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012;84(10):1680-1696. [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N, Cormier P, Patel PY, Hadied MO, Bhargava P, Khanna K, Nadig J, Keimig T, Spizarny D, Reeser N, Klochko C, Peterson EL, Song T. Acute Pulmonary Embolism and COVID-19. Radiology. 2020 Dec;297(3):E335-E338. doi: 10.1148/radiol.2020201955. Epub 2020 May 14. PMID: 32407256; PMCID: PMC7706099.
- Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, Gervaise A, Poissy J, Susen S, Hékimian G, Artifoni M, Periard D, Contou D, Delaloye J, Sanchez B, Fang C, Garzillo G, Robbie H, Yoon SH. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology. 2021 Feb;298(2):E70-E80. doi: 10.1148/radiol.2020203557. Epub 2020 Dec 15. PMID: 33320063; PMCID: PMC7745997.
- Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Molière S, Leyendecker P, Roy C, Ohana M. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020 Sep;296(3):E189-E191. doi: 10.1148/radiol.2020201561. Epub 2020 Apr 23. PMID: 32324102; PMCID: PMC7233397.
- Ruaro B, Confalonieri P, Santagiuliana M, Wade B, Baratella E, Kodric M, Berria M, Jaber M, Torregiani C, Bruni C, Confalonieri M, Salton F. Correlation between Potential Risk Factors and Pulmonary Embolism in Sarcoidosis Patients Timely Treated. J Clin Med. 2021 Jun 2;10(11):2462. doi: 10.3390/jcm10112462. PMID: 34199396; PMCID: PMC8199598.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
