Archives of Pulmonology and Respiratory Care
Achieving humidification of inspired gases in the delivery room for very preterm infants: Rationale and set up
Michael P Meyer1,2* and Jonathan Barrett1
2Department of Pediatrics, Child and Youth Health, University of Auckland, Auckland, New Zealand
Cite this as
Meyer MP, Barrett J (2022) Achieving humidification of inspired gases in the delivery room for very preterm infants: Rationale and set up. Arch Pulmonol Respir Care 8(1): 016-019. DOI: 10.17352/aprc.000078Copyright License
© 2022 Meyer MP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Nearly all very preterm infants will require some form of respiratory support in the neonatal unit. It is standard practice to humidify the inspired gases. It appears logical to bring this practice of humidification forward to the time immediately after birth. There is an evidence base for early provision of heated humidified gases in the delivery room, but because several forms of respiratory support may be needed (eg binasal prong CPAP and use of a T-piece resuscitator) this may be difficult to achieve. We describe a setup using a radiant warmer and humidification circuits to make this possible.
Introduction
Heating and humidification of inspired gases in very preterm infants in the delivery room are increasingly used. According to a recent survey over 60% of neonatal intensive care units (NICUs) in high-income settings have adopted the procedure [1]. There is a modest evidence base for the practice, particularly for achieving target admission temperatures between 36.5 °C and 37.5 °C. There have been two randomized controlled trials (RCTs) and an observational study [2-4]. A meta-analysis of a total of 476 preterm infants < 32 weeks Postmenstrual Age (PMA) enrolled in the randomized studies indicated a significant improvement in admission temperature for infants < 32 weeks and in addition significant improvement for the subgroup < 28 weeks PMA [5]. The observational study showed similar results [4]. Heating and humidification appeared to have an additional effect on admission temperatures, even with the comprehensive use of other measures to prevent heat loss such as a thermal wrap, the use of radiant warmers in the delivery room, utilizing powered warmers or incubators for transport to NICU, maintaining optimal environmental temperatures in theatre and delivery room (25 °C or above) and providing head covering. GRADE score indicated a high score for the prevention of hypothermia and low heterogeneity [5]. In one of the studies, there was an increase in the number of infants with admission temperatures above 37.5°C [3] but this was not seen in the study where servo control was used [2]. The improvement in admission temperature with heating and humidification was in keeping with earlier studies indicating a 1.4 °C drop of core temp for every hour non-heated humidified gas was used in neonatal anesthesia [6].
Respiratory outcomes in the RCT heating and humidification studies were not significantly different between groups, although there were trends toward improvements in early intubation rates, and surfactant use [5]. There was a significant reduction in severe intraventricular haemorrhage although the GRADE evidence for this finding was weak [5]. As the studies to date have not been powered for these outcomes, it follows that further study is indicated.
Delivery room transition
Heating and humidification of inspired gases need to be viewed in the context of other important events taking place soon after delivery.
Deferred Cord Clamping (DCC) and heated and humidified gases
DCC has become standard practice with a recent systematic review indicating improved survival in preterm infants < 34 weeks PMA [7]. Maintaining normothermia (36.5 °C to 37.5 °C) is facilitated by using plastic wrap and maintaining environmental temperatures. Several studies have reported no increase in admission hypothermia when DCC is performed, but nevertheless, in the largest study to date comparing DCC to immediate clamping, 30% - 40% of preterm infants with PMA less than 32 weeks had sub-optimal admission temperatures [8]. Heating and humidification of inspired gases are not usually attempted while DCC is taking place. In most cases, the cord is clamped after 30 to 60 sec. providing heated humidified gases is certainly possible as several studies have reported the use of T-piece resuscitation prior to cord clamping and a humidifier could be added to the circuit [9-11]. If the cord is not clamped for a longer period eg in the setting of physiologic cord clamping (where 5 min or longer may elapse prior to clamping), heating and humidification might be indicated [12]. However, these possibilities do not appear to have been studied.
In a recent study of preterm infants < 31 weeks PMA who had 50 sec DCC, we noted that 26/113 (23%) of infants had axillary temperatures < 36.5 °C (hypothermia) on admission to NICU. In addition, 29/113 had axillary temperature < 36.5 °C at 5 min of age in DR (11). In an analysis of data (previously unpublished) from this study, we found that hypothermia at 5 min was the variable most strongly associated with hypothermia at admission (p < 0.001 using logistic regression; odds ratio 28.5 with 95% confidence interval 6-145). Other factors entered into the model for hypothermia on admission were gestation, birth weight, sex, multiple births, birth weight Z score, intubation in the delivery room, Apgar scores at 1 and 5 min, and caesarean delivery. To determine which factors were associated with 5 min hypothermia we explored the same variables and found that significant factors for 5 min hypothermia were gestation, birth weight, and birth weight Z score. Although this was a relatively small single-center study, our observation appears to confirm findings from the much larger Brazilian Neonatal Network study (13). This study of over 1700 preterm infants < 34 weeks PMA noted the strong association between the temperature at 5 min of age and admission temperature below 36 °C (odds ratio of 7.5; 95% CI 5.7 - 9.7) [13]. This is in spite of admission occurring some 15 to 20 min later, and it might be expected that the use of radiant heat would overcome this initial hypothermia. It does raise the question of whether early heating and humidification could reduce hypothermia within 5 min of delivery.
Providing heated humidified respiratory support in the delivery room
The majority of very preterm infants can be managed on early CPAP in the Delivery Room (DR) with several studies noting that for infants with a PMA of below 32 weeks, 70% to 80% will not require endotracheal tube placement in the DR [2,3,11]. For those infants on CPAP, it is becoming common practice to provide heating and humidification to the CPAP circuit in the DR. For binasal prong bubble CPAP, we provide heated and humidified gases using the F&P 950 series humidifier (Fisher&Paykel 950 Respiratory Humidifier, Fisher&Paykel Healthcare, Auckland, NZ). However, data from a recent study performed in our unit indicated that approximately 50% of infants < 31 weeks PMA will need Positive Pressure Ventilation (PPV) in the DR [11], and this is usually provided via a T-piece resuscitator. For the majority of infants we noted this period of PPV was short-lived; however, some infants, especially at lower gestations, required intubation. As the F&P 950 humidifier CPAP circuit has a pressure-relief valve set at 15 cm - 17cm water, this makes the circuit unsuitable for the provision of PPV as higher inspiratory pressures are usually used (25 cm water or more). A “dry” T-piece circuit (without humidity) can be used but as 20 to 30 min may elapse before reaching NICU it could be important to heat and humidify the T-piece circuit as well. For this we propose the use of 2 circuits set up on the radiant warmer (as described in more detail below); one circuit for heated humidified binasal prong CPAP with an F&P 950 humidifier and one using the MR850 humidifier (Fisher&Paykel, Auckland, NZ) for the T piece circuit. The MR850 humidifier T-piece circuit does not have a pressure relief valve (pressure is set on the radiant warmer pressure dial), making it suitable for T-piece resuscitation and this MR850 setup was used in the 3 published studies to date [2-4]. Whilst the MR850 could be used for both T piece and nasal prong CPAP, different circuits are required, and changing these over in the stress of DR events may be inadvisable. The T piece and MR850 circuit could also be used to provide T piece CPAP. However, the use of a face mask in DR has been associated with apnea in over 50% of preterm infants <32 weeks who were spontaneously breathing [14]. For this reason, our practice is to provide CPAP using binasal prongs to spontaneously breathing infants and not a face mask in DR. Whether the use of warmed humidified gas in this setting is helpful in reducing apnea has not been studied. Controlling the oxygen concentration of inspired gas is also crucial, both to maintain optimal oxygen saturations and potentially to open a closed glottis [15], so it is important to be able to blend oxygen and heated humidified gas.
Skin-to-skin contact
The provision of early skin-to-skin contact and closeness between parents and preterm babies is increasingly recognized as an important aspect of care. A recent study noted that providing 60 min of skin-to-skin care in the delivery room for extremely preterm infants was not only feasible but had a number of benefits including a reduction in early postnatal depression and improved bonding [16]. It is likely that the use of heated humidified gases in this setting, particularly if longer periods of time elapse before admission to the nursery, would be important to prevent hypothermia, but there are no studies that have specifically investigated this.
Set up humidified circuits for the delivery room
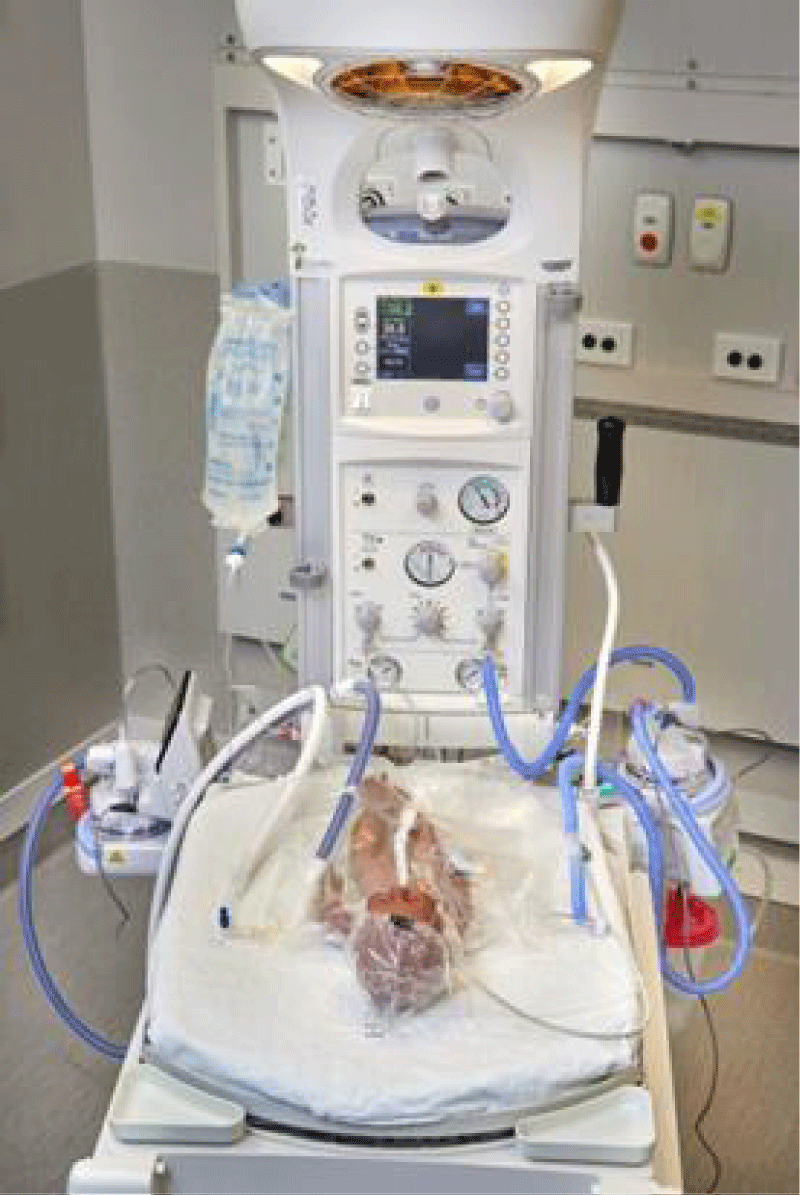
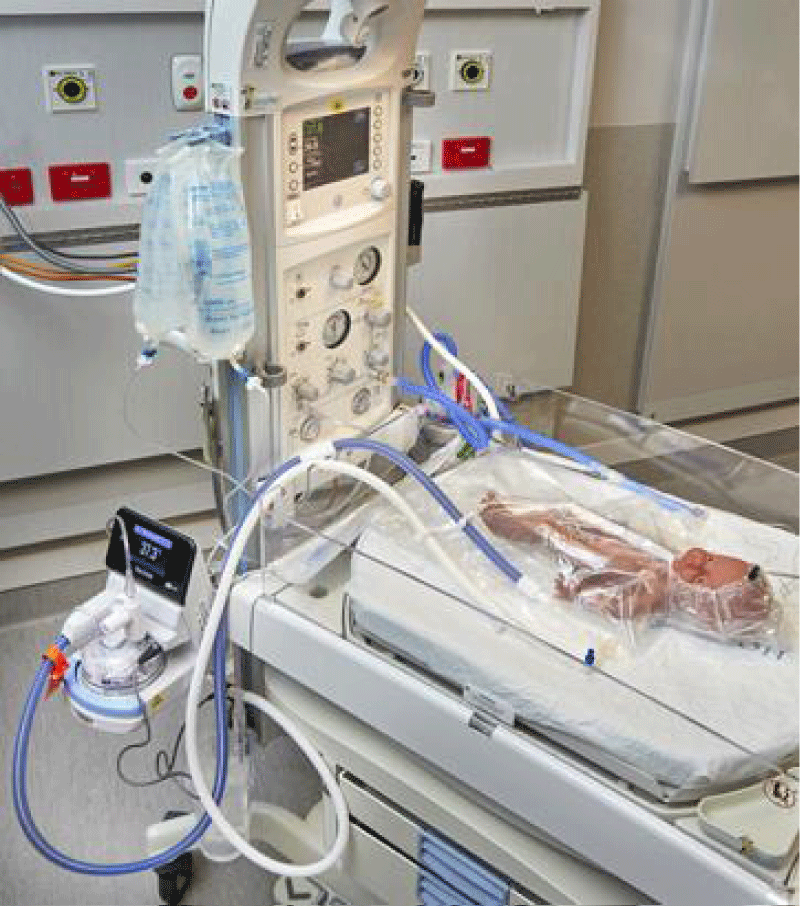
The Giraffe warmer (GE Healthcare, Australia and New Zealand) has 2 gas outlet ports and an oxygen blender (see Figure 1). A gas outlet port is used to supply each humidifier. The F&P 950 circuit on the left of the Figure is used to provide heated humidified CPAP and a close-up is shown in Figure 2.
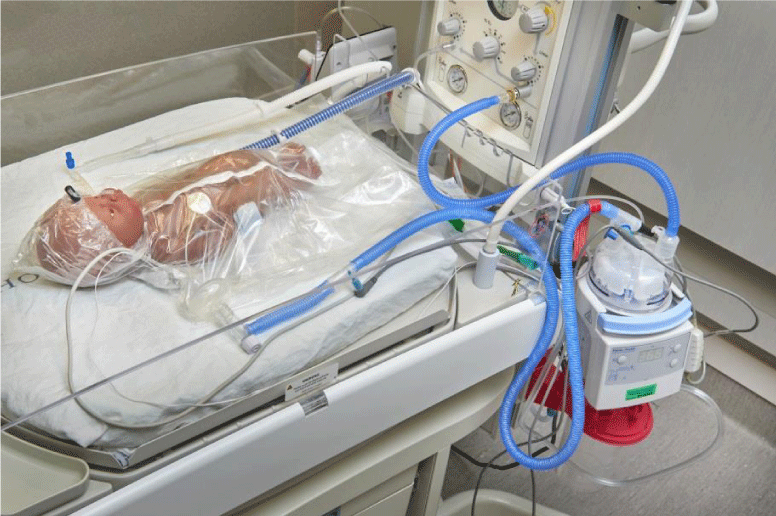
The MR850 humidifier on the right (Figure 1) is used to heat and humidify the T-piece circuit and a close-up of this setup is shown in Figure 3.
The T-piece circuit with humidification is only used in DR and for transport to NICU, a small volume of water (20 ml) is placed in the chamber with the humidifier set to 37 °C [2,3] and it has been shown that this water heats up rapidly (within a few minutes). Each circuit requires a humidification chamber top and is designed for single-patient use. The T-piece heated humidification circuit (Fisher&Paykel 900RD110 circuit and humidifier dome MR225X) in our practice is indicated for infants < 28 weeks PMA. The heated humidified CPAP circuit (950N62) with F&P 950 humidifier (set to 37 °C) is standard in our NICU for all infants requiring respiratory support and the same circuit is used once the infant is admitted to NICU Hence, the extra cost is for the heated humidified T piece circuit. It should be noted that power should continue to be supplied to the warmer and humidifiers during the transfer from DR to NICU.
Clearly, in the future, it would be desirable to change the circuit design, so that the same humidified circuit could be used for different applications and this is likely to take place in the future.
Conclusion
Early use of heated humidified inspired gases in the delivery room after birth is likely to be important. As very preterm infants are likely to require more than one type of respiratory support eg nasal CPAP and positive pressure ventilation during early stabilization and transition, there are technical challenges to achieving this humidification. As a result of the current limitations of circuit design, the use of 2 separate circuits may be required. We anticipate carrying out further studies on this type of setup.
Both authors report receiving consulting fees from Fisher & Paykel. However, the authors report that none of the product manufacturers mentioned in this article had any role in the design, writing, review, or decision to publish this article and the work and opinions expressed are those of the authors.
- Jani P, Mishra U, Buchmayer J, Walker K, Gözen D, Maheshwari R, D'Çruz D, Lowe K, Wright A, Marceau J, Culcer M, Priyadarshi A, Kirby A, Moore JE, Oei JL, Shah V, Vaidya U, Khashana A, Godambe S, Cheah FC, Zhou W, Xiaojing H, Satardien M. Thermoregulation and golden hour practices in extremely preterm infants: an international survey. Pediatr Res. 2022 Sep 8:1–9. doi: 10.1038/s41390-022-02297-0. Epub ahead of print. PMID: 36075989; PMCID: PMC9453708.
- Meyer MP, Hou D, Ishrar NN, Dito I, te Pas AB. Initial respiratory support with cold, dry gas versus heated humidified gas and admission temperature of preterm infants. J Pediatr. 2015 Feb;166(2):245-50.e1. doi: 10.1016/j.jpeds.2014.09.049. Epub 2014 Oct 28. PMID: 25449225.
- McGrory L, Owen LS, Thio M, Dawson JA, Rafferty AR, Malhotra A, Davis PG, Kamlin COF. A Randomized Trial of Conditioned or Unconditioned Gases for Stabilizing Preterm Infants at Birth. J Pediatr. 2018 Feb;193:47-53. doi: 10.1016/j.jpeds.2017.09.006. Epub 2017 Nov 6. PMID: 29106924.
- te Pas AB, Lopriore E, Dito I, Morley CJ, Walther FJ. Humidified and heated air during stabilization at birth improves temperature in preterm infants. Pediatrics. 2010 Jun;125(6):e1427-32. doi: 10.1542/peds.2009-2656. Epub 2010 May 10. PMID: 20457686.
- Meyer MP, Owen LS, Te Pas AB. Use of Heated Humidified Gases for Early Stabilization of Preterm Infants: A Meta-Analysis. Front Pediatr. 2018 Oct 25;6:319. doi: 10.3389/fped.2018.00319. PMID: 30410876; PMCID: PMC6209662.
- Fonkalsrud EW, Calmes S, Barcliff LT, Barrett CT. Reduction of operative heat loss and pulmonary secretions in neonates by use of heated and humidified anesthetic gases. J Thorac Cardiovasc Surg. 1980 Nov;80(5):718-23. PMID: 7431968.
- Seidler AL, Gyte GML, Rabe H, Díaz-Rossello JL, Duley L, Aziz K, Testoni Costa-Nobre D, Davis PG, Schmölzer GM, Ovelman C, Askie LM, Soll R; INTERNATIONAL LIAISON COMMITTEE ON RESUSCITATION NEONATAL LIFE SUPPORT TASK FORCE. Umbilical Cord Management for Newborns <34 Weeks' Gestation: A Meta-analysis. Pediatrics. 2021 Mar;147(3):e20200576. doi: 10.1542/peds.2020-0576. PMID: 33632931; PMCID: PMC7924139.
- Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, Evans N, Finlayson S, Fogarty M, Gebski V, Ghadge A, Hague W, Isaacs D, Jeffery M, Keech A, Kluckow M, Popat H, Sebastian L, Aagaard K, Belfort M, Pammi M, Abdel-Latif M, Reynolds G, Ariff S, Sheikh L, Chen Y, Colditz P, Liley H, Pritchard M, de Luca D, de Waal K, Forder P, Duley L, El-Naggar W, Gill A, Newnham J, Simmer K, Groom K, Weston P, Gullam J, Patel H, Koh G, Lui K, Marlow N, Morris S, Sehgal A, Wallace E, Soll R, Young L, Sweet D, Walker S, Watkins A, Wright I, Osborn D, Simes J; Australian Placental Transfusion Study Collaborative Group. Delayed versus Immediate Cord Clamping in Preterm Infants. N Engl J Med. 2017 Dec 21;377(25):2445-2455. doi: 10.1056/NEJMoa1711281. Epub 2017 Oct 29. PMID: 29081267.
- Katheria A, Poeltler D, Durham J, Steen J, Rich W, Arnell K, Maldonado M, Cousins L, Finer N. Neonatal Resuscitation with an Intact Cord: A Randomized Clinical Trial. J Pediatr. 2016 Nov;178:75-80.e3. doi: 10.1016/j.jpeds.2016.07.053. Epub 2016 Aug 26. PMID: 27574999; PMCID: PMC5527831.
- Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, Bradshaw L, Mitchell EJ, Fawke JA; Cord Pilot Trial Collaborative Group. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed. 2018 Jan;103(1):F6-F14. doi: 10.1136/archdischild-2016-312567. Epub 2017 Sep 18. PMID: 28923985; PMCID: PMC5750367.
- Nevill E, Mildenhall LFJ, Meyer MP. Effect of breathing support in very preterm infants not breathing during deferred cord clamping: A randomized controlled trial (The ABC study). J Pediatr. 2022 Sep 22:S0022-3476(22)00847-2. doi: 10.1016/j.jpeds.2022.09.025. Epub ahead of print. PMID: 36152686.
- Knol R, Brouwer E, van den Akker T, DeKoninck P, van Geloven N, Polglase GR, Lopriore E, Herkert E, Reiss IKM, Hooper SB, Te Pas AB. Physiological-based cord clamping in very preterm infants - Randomised controlled trial on effectiveness of stabilisation. Resuscitation. 2020 Feb 1;147:26-33. doi: 10.1016/j.resuscitation.2019.12.007. Epub 2019 Dec 23. PMID: 31874212.
- de Almeida MF, Guinsburg R, Sancho GA, Rosa IR, Lamy ZC, Martinez FE, da Silva RP, Ferrari LS, de Souza Rugolo LM, Abdallah VO, Silveira Rde C; Brazilian Network on Neonatal Research. Hypothermia and early neonatal mortality in preterm infants. J Pediatr. 2014 Feb;164(2):271-5.e1. doi: 10.1016/j.jpeds.2013.09.049. Epub 2013 Nov 6. PMID: 24210925.
- Kuypers KLAM, Lamberska T, Martherus T, Dekker J, Böhringer S, Hooper SB, Plavka R, Te Pas AB. The effect of a face mask for respiratory support on breathing in preterm infants at birth. Resuscitation. 2019 Nov;144:178-184. doi: 10.1016/j.resuscitation.2019.08.043. Epub 2019 Sep 12. PMID: 31521774.
- Te Pas AB, Hooper SB, Dekker J. The Changing Landscape in Supporting Preterm Infants at Birth. Neonatology. 2019;115(4):392-397. doi: 10.1159/000497421. Epub 2019 Apr 11. PMID: 30974440; PMCID: PMC6604262.
- Mehler K, Hucklenbruch-Rother E, Trautmann-Villalba P, Becker I, Roth B, Kribs A. Delivery room skin-to-skin contact for preterm infants-A randomized clinical trial. Acta Paediatr. 2020 Mar;109(3):518-526. doi: 10.1111/apa.14975. Epub 2019 Sep 16. PMID: 31423649.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
