Archives of Pulmonology and Respiratory Care
Mortality and survival of patients with rheumatoid arthritis and symptomatic diffuse interstitial lung disease
M Gema Bonilla-Hernán1*, Luis Gómez-Carrera2, María Fernández-Velilla Peña3, Chamaida Plasencia-Rodríguez1, Pilar Aguado1, Rodolfo Álvarez-Sala Walther2 and Alejandro Balsa1
2Medicine Department, Pulmonology Service, La Paz University Hospital, Madrid, España, Idipaz, Autonoma University, Madrid, Spain
3Medicine Department, Radiodiagnostic Service, La Paz University Hospital, Madrid, España, Idipaz, Autonoma University, Madrid, Spain
Cite this as
Bonilla-Hernán MG, Gómez-Carrera L, Fernández-Velilla Peña M, Plasencia-Rodríguez C, Aguado P, et al. (2022) Mortality and survival of patients with rheumatoid arthritis and symptomatic diffuse interstitial lung disease. Arch Pulmonol Respir Care 8(1): 003-009. DOI: 10.17352/aprc.000075Copyright License
© 2022 Bonilla-Hernán MG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: In Spain, few data have been reported on mortality and survival in rheumatoid arthritis with diffuse interstitial lung disease.
Objectives: To estimate mortality and survival for patients with symptomatic diffuse interstitial lung disease and rheumatoid arthritis and to analyze the effect of clinical factors.
Methods: We performed an observational study between 2007 and 2018 at the Interdisciplinary Rheumatology and Pulmonology Clinic, from a tertiary Hospital. Patients with rheumatoid arthritis and symptomatic of diffuse interstitial lung disease confirmed by high-resolution computed tomography were included. Causes of death and clinical factors were reported.
Results: We identified 90 patients with rheumatoid arthritis and symptomatic interstitial lung disease. Twenty-six patients died and diffuse interstitial lung disease was the most frequent cause (50%). The overall mortality rate was 19.7 per 1000 patient-years (95% CI: 13.4 - 29). The multivariate model revealed the predictors of mortality to be a long time between diagnosis of rheumatoid arthritis and lung involvement (HR = 1.17; p = 0.003) and low forced vital capacity (HR = 0.02; p = 0.018). The probability of survival was 50% at 10.2 years from diagnosis of interstitial lung disease. Comparison of survival did not reveal significant differences by type of radiologic pattern (p = 0.823).
Conclusions: The fact that almost one-third of patients died and that survival is 50% at 10 years highlights the important role of diffuse interstitial lung disease in rheumatoid arthritis. The radiologic pattern does not seem to be as important for survival as forced vital capacity at diagnosis and the time between diagnosis of rheumatoid arthritis and lung involvement.
Key points
1. DILD is associated with shorter survival in patients with RA.
2. The radiologic pattern does not seem to influence the survival in patients with RA and DILD.
3. The FVC at diagnosis is an important factor that influences the prognosis of patients with RA and DILD.
Introduction
Patients with Rheumatoid Arthritis (RA) often experience extra-articular manifestations, which play a role in the morbidity and excess mortality reported for this disease [1]. The lung is the site most commonly affected by extra-articular RA, particularly in the form of Diffuse Interstitial Lung Disease (DILD) [2].
DILD is the second most frequent cause of early death in patients with RA after cardiovascular disease [3]. Lung involvement is very often asymptomatic, although it accounts for 10% - 20% of deaths in patients with RA [4] and most of these are due to DILD [5-8]. In the case of early RA, DILD is responsible for 6% - 7% of deaths [9]; in fact, overall, DILD accounts for 6% - 13% of excess mortality in patients with RA [3].
Current treatments for RA are very effective and have made it possible to control the disease and, therefore, reduce the mortality of RA. However, it seems that the burden and mortality of RA associated with DILD are increasing [10]. Consequently, in recent years, interest in DILD in patients with RA has grown exponentially, with numerous studies being performed. Nevertheless, many aspects of this disease remain unknown.
In 2007, an Interdisciplinary Rheumatology and Pulmonology Clinic was created at Hospital Universitario La Paz (HULP), Madrid, Spain, with the aim of assessing lung involvement in patients with systemic immune-mediated diseases. The objective of the present study was to estimate mortality and survival in patients with RA and symptomatic DILD in our area and to ascertain the potential underlying clinical and serological factors.
Methods
We performed a longitudinal study between 2007 and 2018 in the Interdisciplinary Rheumatology and Pulmonology Clinic of HULP. The protocol was approved by the local Research Ethics Committee for Medications. All procedures were carried out according to the recommendations of good clinical practice, and data confidentiality regulations were adhered to. The selection of the included patients was made from the total of patients evaluated at the interdisciplinary Rheumatology and Pulmonology Clinic of HULP (Figure S1. with the flow diagram).
Population
The study population comprised patients with RA seen in the Rheumatology Department. Patients with DILD were characterized by selecting those diagnosed with RA according to the 1987 criteria of the American Rheumatism Association or 2010 criteria of the American College of Rheumatology/European League Against Rheumatism [11,12] and who had a cough, dyspnea, or crackles on chest auscultation and were diagnosed with DILD by means of high-resolution computed tomography (HRCT). We excluded patients with overlap syndromes, patients who had been exposed to environmental agents that could potentially cause DILD (eg, inhalation of inorganic dust [pneumoconyosis] or organic dust [extrinsic allergic alveolitis]), and patients who had received treatment with amiodarone, nitrofurantoin, cytostatic agents, or radiotherapy. Data on vital signs were unavailable for 6 patients at the time of the study. The idiopathic interstitial pneumonia classification of the European Respiratory Society (ERS) and American Thoracic Society (ATS) for 2013 [13] was used, as were the joint recommendations on idiopathic lung fibrosis of the American Thoracic Society, the European Respiratory Society, the Japanese Respiratory Society, and the Latin American Thoracic Society for 2018 [14]. We analyzed the association between mortality and radiologic pattern, classifying patients as having Usual Interstitial Pneumonia (UIP) plus probable UIP, Nonspecific Interstitial Pneumonia (NSIP), and Combined Pulmonary Fibrosis And Emphysema (CPFE).
Measurements and data collection
We collected the following variables: sociodemographic data, smoking history, baseline clinical characteristics, treatment received for RA, respiratory symptoms (cough and dyspnea), crackles on pulmonary auscultation, positivity and titer of Rheumatoid Factor (RF) and Anti-Cyclic Citrullinated Protein Antibodies (ACPA), Pulmonary Function Test (PFT) results, and radiologic patterns by HRCT. In addition, the date and cause of death were collected.
PFTs were performed using a spirometry module included in the MasterLab-body 6.0 device (Viasys, Wuerzburg, Alemania), following the recommendations of the ATS/ERS for calibration and measurement procedures [15]. Flows were calibrated daily using a 3-liter syringe, according to current guidelines. The procedure followed both for slow and forced spirometry was that reported by SEPAR [16]. At least 3 acceptable maneuvers were performed for forced vital capacity (FVC), with a maximum of 8. The result was adjusted for body temperature and pressure (saturated); the reference values applied were those proposed by the Global Lung Initiative [17]. The diffusing capacity of the lung for carbon monoxide (DLCO) was measured using the same system with the single-breath technique. DLCO was adjusted for hemoglobin based on the reference values proposed by Cotes [18]. Pulmonary function was considered abnormal when FVC was <80% predicted and/or DLCO was <80% [19].
DILD was diagnosed based on HRCT. The protocol included a program to determine the limits of the volume to be acquired in maximum inspiration in the cranial-caudal direction. Two slices were also taken during expiration at the level of the aortic arch and the entry to the pulmonary veins to evaluate air trapping. The investigations were performed using a 16-slice CT scanner (SOMATON EMOTION 16, Siemens Medical Solutions, Erlangen, Germany). The radiation parameters were adjusted as standard with respect to the morphological characteristics of each patient as follows: 120 KV, with a current of 160 mA for the tube, slice thickness of 0.75 mm, with a reconstruction increment of 0.5 mm, a pitch of 0.8, and a collimation width of 0.6 mm. Images were reconstructed using a soft-tissue kernel (B41f) and a lung kernel (B90f) for routine diagnostic practice.
Statistical analysis
The number and causes of death were reported, and baseline clinical characteristics were compared between patients who died and those who did not. Patients who did not die or for whom insufficient data on vital signs were available were censored on the date of their last visit.
Mortality was evaluated using Kaplan-Meier survival analysis, which was performed considering the most frequent radiologic patterns (UIP, CPFE and NISP). The minor radiologic patterns were excluded.
The observation period for mortality was defined as that running from the date of onset of the disease (joint or lung involvement) to death or the date of the last available information for the patient (censoring). The overall mortality rate was calculated as the quotient of the number of deaths recorded and cumulative survival (expressed in patient years). The survival curves were compared using the Mantel-Haenszel statistic (log-rank test).
Lastly, Cox proportional hazards regression models were constructed to identify baseline characteristics associated with mortality. Models were calculated for crude values and after adjustment for confounders. Theoretical meaningful variables and those with a p-value below 0.250 in the bivariate analysis were entered into the multivariate analysis. The final model was the most parsimonious (smallest number of variables) and that with lower information criteria (Akaike and Bayesian). The predictive capacity of the adjusted model was evaluated using Harrell’s C statistic.
Results
Information was available for 84 patients of the 90 (93.3%) diagnosed with RA and DILD (RA-DILD). Most patients (74) did not have DILD at RA diagnosis, only 10 patients (11.9%) had this condition at the time of RA diagnosis. In total, 26 out of 84 patients (31%) died during the study follow-up. The cause of death was known in 16 cases (61,5%), being the most frequent DILD (8 cases; 50%), followed by respiratory infection (3; 18.8%), cancer (3; 18.8% [lung cancer in 1 case]), intestinal perforation (1; 6%), and sepsis (1; 6%).
DILD progression was considered a cause of death when clinical worsening and respiratory function decline or radiologic progression, in absence of other causes.
The comparison of baseline clinical and serological characteristics between patients who died and those who did not reveal a series of findings that were more frequent in those who died, as follows: crackles [85% (22/26) vs. 57 % (31/58) p = 0.022], higher RF titers (median 630 vs. 162; p = 0.005), higher ACPA titers (1200 vs. 380; p = 0.034), and lower FVC at the first visit (0.81 vs. 0.96; p = 0.005) (Table 1).
Total follow-up time after diagnosis of DILD was 437.5 patient-years, with a maximum of 19 years and a probability of survival of 50% at 10.2 years after diagnosis of DILD. The overall mortality rate was 19.9 per 1,000 patient-years (95% CI: 13.4 - 29.9) (Table 2, Figure S2).
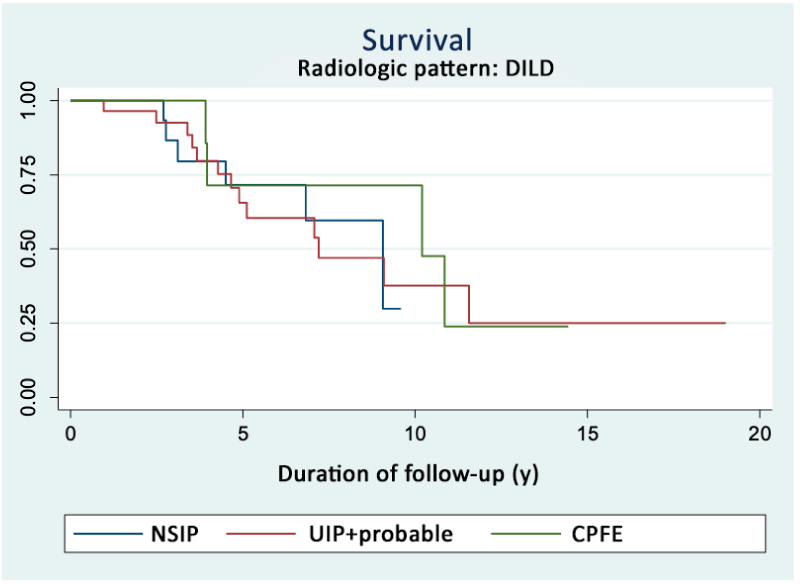
The comparison of survival according to the radiologic pattern of DILD was performed among the 61 patients with the most frequent patterns (31 with UIP + probable UIP, 11 with CPFE, and 19 with NISP). No statistical differences were observed among radiologic patterns by the log-rank test (p = 0.823). Similarly, no differences were found depending on the initial manifestation of joint or lung disease (0.245) (Table 3, Figures 1 and S3).
As for the mortality rate according to the radiologic DILD pattern, we found the highest mortality in CPFE (30.1 x 1,000 person-years), followed by UIP plus probable UIP (29.5 x 1,000 person-years), compared with the NSIP group (17.5 x 1,000 person-years), although the difference was not statistically significant (Table S1; Figure 1).
The bivariate Cox regression analysis revealed some baseline characteristics to be associated with mortality. This association was defined by the hazard ratio (HR). Mortality increased with the presence of crackles (HR = 3.03; p = 0.042) and greater time between diagnosis of joint and lung involvement (HR = 1.09; p < 0.0001). In contrast, mortality decreased with greater FVC at the baseline visit (HR = 0.06, p = 0.050), although the difference in this parameter was at the limit of statistical significance. No association was identified with a radiologic pattern, smoking, or the remainder of the characteristics analyzed (Table 4).
The multivariate analysis revealed the predictors of mortality to be greater time between joint and lung involvement (HR = 1.17; p = 0.003) and low FVC values (HR = 0.02; p = 0.018). These effects were independent of smoking and age. The predictive ability of the model was 76% (Harrell C statistic) (Table 4).
Discussion
In our study, 30% of patients died during follow-up, half from respiratory involvement. We found an overall mortality rate of 19.9 per 1,000 patient-years (95% CI: 13.4-29.9) and no significant differences with respect to survival between the different radiologic patterns. FVC at diagnosis and longer time to onset of joint and lung disease were associated with increased mortality.
Extra-articular manifestations, especially pulmonary manifestations, are one of the main causes of death in patients with RA [20] and the mean survival of patients with RA-DILD ranges from 3 to 10 years depending on the series; the differences found in the literature reflect the heterogeneous nature of this disease [8,21,22].
In a population-based cohort study, Hyldgaard, et al. [23] found that mortality at 5 years in patients with RA-DILD was 19% in those with stable disease and 55% in those with progressive disease. A previous study from the same authors revealed that mortality was 14% at 1 year, 39% at 5 years, and 60% at 10 years [24]. These data are similar to those reported in our series, where we calculated the probability of survival to be 50% at 10 years.
A systematic review of the medical literature with a meta-analysis of the association between DILD and RA and the risk of mortality showed that most deaths were caused by respiratory disease, namely, the progression of DILD, acute exacerbation of DILD, or pneumonia [25]. In our study, of the 26 patients who died, almost half died because of lung involvement, and of these, 31% because of the progression of DILD, thus confirming the importance of this manifestation in the prognosis of patients with RA.
Several authors have identified predictors of mortality in patients with RA-DILD [25-28]. Some showed that patients with clinical manifestations such as cough and dyspnea die early [29]. Owing to our inclusion criteria, we found that all patients had a cough, dyspnea, or crackles and that crackles were more frequent in patients who died (p = 0.022).
A 2014 systematic review of the literature on predictors of mortality in RA-DILD found the predictors of mortality to be male sex, advanced age at diagnosis of DILD, reduced DLCO, the UIP radiologic pattern, and extension of fibrosis [26]. The duration of RA at diagnosis of DILD has also been observed to be associated with mortality in patients with RA-DILD [30]. In patients with RA-DILD and the UIP pattern, FVC and the change in FVC or DLCO at 6 months have been shown to be significant predictors of mortality [31].
RF and ACPA positivity and titers are known risk factors for RA-DILD, although, to date, no data have been reported to confirm them as predictors of mortality. However, a 2019 study did reveal that the ACPA titer was a predictor of the progression of DILD [32]. In our study, we found that patients who died had higher RF and ACPA titers than those who did not die during follow-up, although, as in other studies, they were not predictors of mortality.
In terms of lung function, FVC and DLCO, both at diagnosis and in terms of change over time, have been associated with prognosis. Solomon, et al. [28] showed that lung function, age, and smoking can account for differences in survival. Zamora-Legoff, et al. [30] found that lower baseline DLCO, age, and duration of RA were predictors of mortality. We found that patients who died had a lower FVC at the baseline visit.
DILD is classified into patterns based on radiologic or histopathological findings, with UIP and NSIP being the most common patterns in RA [33]. Various studies have shown that the prognosis for RA-DILD varies significantly depending on the pattern [21,34] and that survival rates are better for patients with RA-DILD and a radiologic pattern other than UIP [25,28,35,36]. However, while many articles report a poorer prognosis among patients with RA-DILD and a UIP pattern, this is not a universal finding. Zamora, et al. [30] did not find an association between DILD pattern and mortality. The multivariate analysis of their cohort revealed that low baseline DLCO at the onset of the disease, age, and time with RA—but not radiologic pattern—were predictors of mortality [30].
The multivariate analyses of survival in the study of Solomon, et al. [21] indicated that lung function, age, and smoking accounted for the differences in survival, whereas the radiologic pattern in the baseline HRCT was not an independent predictor of mortality. Similarly, Hyldgaard, et al. [23] found that the impact of the HRCT pattern was significant when adjusted for age, whereas DLCO was confirmed as a predictor of mortality, thus supporting that longitudinal changes in PFT results are an independent predictor of mortality. This suggests that patients with greater pathophysiologic abnormalities (evaluated according to the expected percentages of FVC and DLCO) and patients with evidence of progression according to PFT findings are at greater risk of dying, independently of the radiologic pattern [28]. In fact, the meta-analysis by Singh, et al. [25] from 2019, which revealed the UIP pattern to be associated with a greater risk of death than other patterns, stresses the importance of lung function and degree of lung involvement as a significant predictor of mortality, as opposed to the radiologic pattern.
In our series, we included patients with UIP and probable UIP in the same group based on the report by Yunt, et al. [37], who did not report differences with respect to mortality between both patterns. We compared survival and mortality between 3 groups of patients according to the radiologic pattern, namely, UIP plus probable UIP, NSIP, and CPFE. While it is true that survival was poorer and mortality greater in patients with CPFE and probable UIP, the differences were not statistically significant. Therefore, the radiologic pattern was not a predictor of mortality. These findings lead us and other authors to believe that PFT results are a more important predictor of mortality than radiologic patterns [21,23]. In a study performed in 2017 and consistent with our findings, Rojas-Serrano, et al. [38] concluded that depending on the extension of ground glass opacities in HRCT and the difficulty performing PFT, the factors associated with mortality were age and severity of DILD, but not HRCT pattern.
The time between the onset of RA and diagnosis of DILD is also reported to be a predictor of mortality [30]. We found the time between the onset of RA and diagnosis of DILD to be a key factor in and a very important predictor of mortality, probably because DILD was defined as symptomatic; therefore, the longer the time between the appearance of both manifestations, the higher the possibility that lung involvement is more advanced and prognosis poorer. This observation, together with the greater probability of onset of DILD during the first 10 years after diagnosis of RA, highlights the importance of screening for lung involvement in patients with RA and risk factors for DILD, as well as regular re-evaluation during follow-up [39].
Our study is limited by the size of the cohort and the fact that the cause of death was not known for all patients. It is also a study of patients from an interdisciplinary clinic at a single hospital. Our results should be interpreted with caution owing to the number of patients included in the multivariate model. Also, comorbidities (diabetes, hypertension, atherosclerosis, and cardiac disease) were not available and then, were not included in the multivariate analysis. In contrast, none of the known causes of death was due to a cardiovascular event.
In conclusion, the fact that almost one-third of patients in follow-up at our unit died and survival at 10 years was 50% highlights the importance of DILD in RA and, therefore, the need for early diagnosis and expert management; hence the importance of interdisciplinary clinics. The radiologic pattern does not seem to be important for survival, in contrast with FVC at diagnosis and time between joint and lung involvement.
Musculoskeletal Health Institute for conducting the statistical analysis and critical review of the manuscript.
Spanish Society of Rheumatology for their contribution to the translation of the manuscript.
The specific author contributions are as follows
Mª Gema Bonilla-Hernán (Rheumatology Department): 1) Data acquisition, analysis, and interpretation; 2) drafting of the article; 3) final approval of the version presented.
Luis Gómez-Carrera (Pulmonology Department): Data acquisition, analysis, and interpretation; 2) review of intellectual content; 3) final approval of the version presented.
María Fernández-Velilla Peña (Radiodiagnostics Department): 1) data acquisition, analysis, and interpretation; 2) review of intellectual content; 3) final approval of the version presented.
Chamaida Plasencia-Rodríguez (Rheumatology Department): 1) data acquisition, analysis, and interpretation; 2) review of intellectual content; 3) final approval of the version presented.
Pilar Aguado (Rheumatology Department): 1) data acquisition, analysis, and interpretation; 2) review of intellectual content; 3) final approval of the version presented
Rodolfo Álvarez-Sala Walther (Pulmonology Department): 1) conception and design of the study; 2) review of the intellectual content; 3) final approval of the version presented.
Alejandro Balsa (Rheumatology Department): 1) conception and design of the study; 2) review of the intellectual content; 3) final approval of the version presented.
- Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33(2):65-72. doi: 10.1080/03009740310004621. PMID: 15163106.
- Alunno A, Gerli R, Giacomelli R, Carubbi F. Clinical, Epidemiological, and Histopathological Features of Respiratory Involvement in Rheumatoid Arthritis. Biomed Res Int. 2017;2017:7915340. doi: 10.1155/2017/7915340. Epub 2017 Nov 7. PMID: 29238722; PMCID: PMC5697381.
- Kelly CA, Nisar M, Arthanari S, Carty S, Woodhead FA, Price-Forbes A, Middleton D, Dempsey O, Miller D, Basu N, Dawson J, Sathi N, Ahmad Y, Palmer E, Iqbal K, Janakiraman G, Koduri G, Young A. Rheumatoid arthritis related interstitial lung disease - improving outcomes over 25 years: a large multicentre UK study. Rheumatology (Oxford). 2021 Apr 6;60(4):1882-1890. doi: 10.1093/rheumatology/keaa577. PMID: 33150434.
- Esposito AJ, Chu SG, Madan R, Doyle TJ, Dellaripa PF. Thoracic Manifestations of Rheumatoid Arthritis. Clin Chest Med. 2019 Sep;40(3):545-560. doi: 10.1016/j.ccm.2019.05.003. Epub 2019 Jul 6. PMID: 31376890; PMCID: PMC6994971.
- Suzuki A, Ohosone Y, Obana M, Mita S, Matsuoka Y, Irimajiri S, Fukuda J. Cause of death in 81 autopsied patients with rheumatoid arthritis. J Rheumatol. 1994 Jan;21(1):33-6. PMID: 8151583.
- Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33(4):221-7. doi: 10.1080/03009740410005845. Erratum in: Scand J Rheumatol. 2006 Jul-Aug;35(4):332. PMID: 15370716.
- Turesson C, Jacobsson L, Bergström U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford). 1999 Jul;38(7):668-74. doi: 10.1093/rheumatology/38.7.668. PMID: 10461483.
- Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Gabriel SE, Matteson EL. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010 Jun;62(6):1583-91. doi: 10.1002/art.27405. PMID: 20155830; PMCID: PMC4028137.
- Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, Dixey J; Early Rheumatoid Arthritis Study (ERAS) group. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford). 2007 Feb;46(2):350-7. doi: 10.1093/rheumatology/kel253. Epub 2006 Aug 14. PMID: 16908509.
- Suda T. Up-to-Date Information on Rheumatoid Arthritis-Associated Interstitial Lung Disease. Clin Med Insights Circ Respir Pulm Med. 2016 May 31;9(Suppl 1):155-62. doi: 10.4137/CCRPM.S23289. PMID: 27279757; PMCID: PMC4888751.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315-24. doi: 10.1002/art.1780310302. PMID: 3358796.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569-81. doi: 10.1002/art.27584. PMID: 20872595.
- Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013 Sep 15;188(6):733-48. doi: 10.1164/rccm.201308-1483ST. PMID: 24032382; PMCID: PMC5803655.
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, Azuma A, Bice TJ, Bouros D, Brown KK, Collard HR, Duggal A, Galvin L, Inoue Y, Jenkins RG, Johkoh T, Kazerooni EA, Kitaichi M, Knight SL, Mansour G, Nicholson AG, Pipavath SNJ, Buendía-Roldán I, Selman M, Travis WD, Walsh S, Wilson KC; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018 Sep 1;198(5):e44-e68. doi: 10.1164/rccm.201807-1255ST. PMID: 30168753.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319-38. doi: 10.1183/09031936.05.00034805. PMID: 16055882.
- García-Río F, Calle M, Burgos F, Casan P, Del Campo F, Galdiz JB, Giner J, González-Mangado N, Ortega F, Puente Maestu L; Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch Bronconeumol. 2013 Sep;49(9):388-401. English, Spanish. doi: 10.1016/j.arbres.2013.04.001. Epub 2013 May 28. PMID: 23726118.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J; ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012 Dec;40(6):1324-43. doi: 10.1183/09031936.00080312. Epub 2012 Jun 27. PMID: 22743675; PMCID: PMC3786581.
- Cotes J. Lung function: physiology, measurement and application in medicine. Blackwell Scientific Publishing Ltd 2009:
- Ooi GC, Mok MY, Tsang KW, Wong Y, Khong PL, Fung PC, Chan S, Tse HF, Wong RW, Lam WK, Lau CS. Interstitial lung disease in systemic sclerosis. Acta Radiol. 2003 May;44(3):258-64. doi: 10.1080/j.1600-0455.2003.00058.x. PMID: 12751995.
- Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002 Jan;29(1):62-7. PMID: 11824973.
- 21. Solomon JJ, Ryu JH, Tazelaar HD, Myers JL, Tuder R, Cool CD, Curran-Everett D, Fischer A, Swigris JJ, Brown KK. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med. 2013 Aug;107(8):1247-52. doi: 10.1016/j.rmed.2013.05.002. Epub 2013 Jun 19. PMID: 23791462.
- Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, Dixey J, Gough A, Prouse P, Winfield J, Williams P; ERAS (Early Rheumatoid Arthritis Study). Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford). 2010 Aug;49(8):1483-9. doi: 10.1093/rheumatology/keq035. Epub 2010 Mar 11. PMID: 20223814.
- Hyldgaard C, Ellingsen T, Hilberg O, Bendstrup E. Rheumatoid Arthritis-Associated Interstitial Lung Disease: Clinical Characteristics and Predictors of Mortality. Respiration. 2019;98(5):455-460. doi: 10.1159/000502551. Epub 2019 Oct 9. PMID: 31597131.
- Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, Ellingsen T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017 Oct;76(10):1700-1706. doi: 10.1136/annrheumdis-2017-211138. Epub 2017 Jun 13. PMID: 28611082.
- Singh N, Varghese J, England BR, Solomon JJ, Michaud K, Mikuls TR, Healy HS, Kimpston EM, Schweizer ML. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: A systematic literature review and meta-analysis. Semin Arthritis Rheum. 2019 Dec;49(3):358-365. doi: 10.1016/j.semarthrit.2019.04.005. Epub 2019 May 15. PMID: 31153706.
- Assayag D, Lubin M, Lee JS, King TE, Collard HR, Ryerson CJ. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology. 2014 May;19(4):493-500. doi: 10.1111/resp.12234. Epub 2013 Dec 26. PMID: 24372981.
- Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, Young A; British Rheumatoid Interstitial Lung (BRILL) Network. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology (Oxford). 2014 Sep;53(9):1676-82. doi: 10.1093/rheumatology/keu165. Epub 2014 Apr 23. PMID: 24758887.
- Solomon JJ, Chung JH, Cosgrove GP, Demoruelle MK, Fernandez-Perez ER, Fischer A, Frankel SK, Hobbs SB, Huie TJ, Ketzer J, Mannina A, Olson AL, Russell G, Tsuchiya Y, Yunt ZX, Zelarney PT, Brown KK, Swigris JJ. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016 Feb;47(2):588-96. doi: 10.1183/13993003.00357-2015. Epub 2015 Nov 19. PMID: 26585429.
- Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, Siegelman S, Connors G, Robinson WH, Bathon JM. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2014 Aug;73(8):1487-94. doi: 10.1136/annrheumdis-2012-203160. Epub 2013 May 28. PMID: 23716070; PMCID: PMC3883892.
- Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology (Oxford). 2017 Mar 1;56(3):344-350. doi: 10.1093/rheumatology/kew391. PMID: 27940586; PMCID: PMC6251572.
- Song JW, Lee HK, Lee CK, Chae EJ, Jang SJ, Colby TV, Kim DS. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2013 Aug 1;30(2):103-12. PMID: 24071881.
- Fu Q, Wang L, Li L, Li Y, Liu R, Zheng Y. Risk factors for progression and prognosis of rheumatoid arthritis-associated interstitial lung disease: single center study with a large sample of Chinese population. Clin Rheumatol. 2019 Apr;38(4):1109-1116. doi: 10.1007/s10067-018-4382-x. Epub 2018 Dec 7. PMID: 30535993.
- Tanaka N, Kim JS, Newell JD, Brown KK, Cool CD, Meehan R, Emoto T, Matsumoto T, Lynch DA. Rheumatoid arthritis-related lung diseases: CT findings. Radiology. 2004 Jul;232(1):81-91. doi: 10.1148/radiol.2321030174. Epub 2004 May 27. PMID: 15166329.
- Johnson C. Recent advances in the pathogenesis, prediction, and management of rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol. 2017 May;29(3):254-259. doi: 10.1097/BOR.0000000000000380. PMID: 28207496.
- Nurmi HM, Purokivi MK, Kärkkäinen MS, Kettunen HP, Selander TA, Kaarteenaho RL. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med. 2016 Jul 27;16(1):107. doi: 10.1186/s12890-016-0269-2. PMID: 27461264; PMCID: PMC4962382.
- Yang JA, Lee JS, Park JK, Lee EB, Song YW, Lee EY. Clinical characteristics associated with occurrence and poor prognosis of interstitial lung disease in rheumatoid arthritis. Korean J Intern Med. 2019 Mar;34(2):434-441. doi: 10.3904/kjim.2016.349. Epub 2017 Mar 28. PMID: 28352064; PMCID: PMC6406107.
- Yunt ZX, Chung JH, Hobbs S, Fernandez-Perez ER, Olson AL, Huie TJ, Keith RC, Janssen WJ, Goldstein BL, Lynch DA, Brown KK, Swigris JJ, Solomon JJ. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: Relationship to survival. Respir Med. 2017 May;126:100-104. doi: 10.1016/j.rmed.2017.03.027. Epub 2017 Mar 30. PMID: 28427540.
- Rojas-Serrano J, Herrera-Bringas D, Pérez-Román DI, Pérez-Dorame R, Mateos-Toledo H, Mejía M. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol. 2017 Jul;36(7):1493-1500. doi: 10.1007/s10067-017-3707-5. Epub 2017 Jun 6. PMID: 28585060.
- Zhang Y, Li H, Wu N, Dong X, Zheng Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2017 Apr;36(4):817-823. doi: 10.1007/s10067-017-3561-5. Epub 2017 Feb 12. PMID: 28191607.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
