Archives of Pulmonology and Respiratory Care
A Post-Mortem Examination of COVID-19 Pulmonary Pathology in 9 Cases
Alexis Bloom1, Austyn Colter1, Max Jacobsen1, Domnique Battles2, Tamara Albertson2 and George Sandusky1
2Marion County Coroner’s Office, Indianapolis, IN, USA
Cite this as
Bloom A, Colter A, Jacobsen M, Battles D, Sandusky G, et al. (2020) A Post-Mortem Examination of COVID-19 Pulmonary Pathology in 9 Cases. Arch Pulmonol Respir Care 6(1): 045-047. DOI: 10.17352/aprc.000051A new novel virus called SARS-CoV-2 has expanded into a pandemic in the past several months. The virus is an acute respiratory RNA virus that has symptoms in three clinical groups: asymptomatic, suspicious, and COVID-19 positive. The clinical lab testsused for diagnosis are Nasalpharyngeal swabs, with further testing done with sputum or BAL samples. Serological samples are collected for diagnosis in deceased patients using RT-PCR. The clinical symptoms usually occur 2 to 14 days after exposure and include fever, dry cough, and fatigue. In some cases, symptoms can progress and cause multiple organ failure due to adult respiratory distress syndrome (ARDS). The virus is in the family of coronaviruses and has also been identified in lung tissue using transmission electron microscopy. Gross and microscopic lung pathology was examined in five positive cases and four negative cases by hematoxylin and eosin (H&E) and Masson’s Trichrome stains. Of the collected, the age range was 28 to 76. The ethnicities were six Caucasians and three minorities, with a male to female ratio of 7:2. The salient histology features seen in the study were multifocal to diffuse alveolar necrosis, bronchiolar epithelial necrosis, and interstitial mononuclear lymphocytic infiltrates. Other features were perivascular and peribronchiolar lymphoid infiltrates and marked congestion. Scattered fibroplasia was found in the damaged alveoli and the alveolar septae in the more severe cases. These pathologic features are similar to other coronaviruses.
Introduction
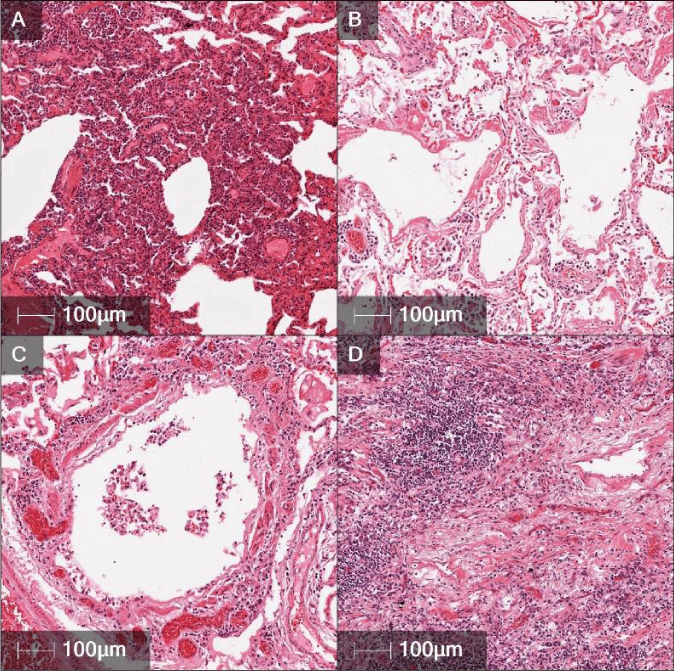
Since December 2019, the infections disease agent SARS-CoV-2 has spread across the globe affecting people on every continent except Antarctica. In general, the virus can cause acute respiratory disease with severe symptoms that can lead to death [1-3]. The clinical lab tests used for diagnosis are Nasalpharyngeal swabs, with further testing done with sputum or BAL samples [4-8]. As of now, COVID-19 has infected over 4.4 million people worldwide, but this number is increasing constantly. The death rate is approximately 2% [1]. Severe symptoms causing death is due to severe alveolar and small bronchiolar damage with subacute to chronic inflammation [1-3,9]. Recent studies have shown SARS-CoV-2 to have a high affinity for the ACE2 receptor found in ciliated bronchial epithelium [10]. The ACE2 receptor triggers a protective effect in the bronchial epithelium against ARDS [11]. With this pathway compromised by SARS-CoV-2, there is severe damage to the bronchiolar epithelium and an increase of ARDS (Figure 1).
In this paper, the lung pathology of 5 patients who had died from severe COVID-19 symptoms were analyzed along with 4 negative COVID-19 cases (Table 1). These samples were collected post-mortem, and patients were not seen in the clinical setting. The pathology findings from these cases will add to the pathogenesis of this novel infectious viral disease.
Materials and methods
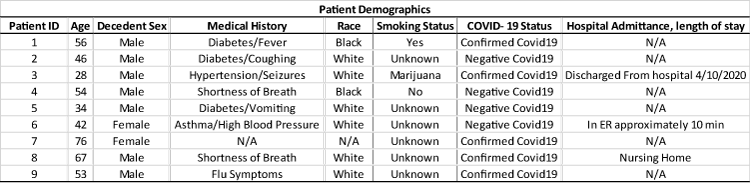
Informed consent and IRB approval was obtained for this decedent study. All lung and blood specimens were collected from the Marion County Coroner’s Office and private funeral homes. These were collected from partial autopsies over the period of April 9th to May 7th. The cases collected were deemed COVID-19 suspicious or COVID-19 positive based on the medical forensic coroner’s decision at the scene of death. All cases were then tested by the Indiana Department of Health Lab using a CDC approved RT-PCR method and then were classified into COVID-19 positive and COVID-19 negative.Of the collected, the age range was 28 to 76. The ethnicities were six Caucasians and three minorities, with a male to female ratio of 7:2.
Most COVID-19 patients that died in hospitals in the central Indiana area were sent directly to funeral homes. The collected cases were found dead in their homes, with one in the nursing home. Cases are designated as a coroner’s case based on dying in their homes and not in a hospital setting. Therefore, medical history is limited and only fragments can be collected from next of kin.
The blood was drawn from the heart using an autopsy needle and sent to the Indiana Department of Health Laboratories. This testing is required by the state on COVID-19 suspicious cases, and the test results were used to classify each patient as COVID-19 positive or COVID-19 negative. Next, a four centimeter incision was made in the chest between the 4th and 5th ribs on the right side. A section of lung approximately 3cm by 2cm was removed and placed in 10% NBF.
The fixation time in 10% NBF was extended to 48 hours due to the infectious nature of the disease. Usual fixation time for most tissues is approximately 24 hours. When the tissues were fixed, they were trimmed and placed into tissue cassettes. Fourteen tissue cassettes were prepared for each case. The tissues were then transferred to 70% ethanol before processing into a paraffin block. Five micron sections were microtomed and stained with H&E and Masson’s Trichrome stains.
Results
Histology from the 5 positive cases revealed multifocal to diffuse alveolar necrosis and bronchiolar respiratory epithelial necrosis. Interstitial mononuclear inflammatory infiltrates, mainly lymphocytes, were seen in a multifocal pattern throughout the biopsies. Perivascular and peribronchiolar lymphoid infiltrates were also seen throughout the biopsies, along with marked congestion. Scattered fibroplasia can also be seen in the severe cases, extending into alveolar spaces and thickening the alveolar septum (Figure 1a,b,c,d). In one case, there was mild hyaline membrane formation and slight micro thrombi formations in small pulmonary vasculature. Another case was found to have aspiration pneumonia. This was characterized by plant fiber and other foreign material, along with numerous neutrophils in the lumen of the bronchioles.
The 4 negative cases were characterized by diffuse pulmonary edema and marked congestion. There was no evidence of pneumonia or inflammation in the airways or alveoli.
Discussion
The lung pathological features of COVID-19 found in this paper are similar to SARS, MERS, and a recent published COVID-19 clinical case with pathology [1,8,12,13]. Based on the consistent findings in these cases, the main pathology change seen in all diseases listed above was diffuse alveolar damage [12,13]. In addition, this study found a complete loss of the tertiary bronchiolar epithelial layers also associated with peribronchiolar lymphoid hyperplasia in severe cases. Recent studies have shown SARS-CoV-2 to have a high affinity for the ACE2 receptor found in ciliated bronchial epithelium, and when induced by SARS-CoV-2 there is an increase of tissue damage [10,11].
- Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, et al. (2020) Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. Link: https://bit.ly/2BC7aH9
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, et al. (2020) Covid-19 Does Not Lead to a “Typical” Acute Respiratory Disease Syndrom. Am J Respir Crit Care Med 201: 1299-1300. Link: https://bit.ly/2BKRAsZ
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420-422. Link: https://bit.ly/3dKj1kO
- Lippi G, Mattiuzzi C, Bovo C, Plebani M (2020) Current laboratory diagnostics of coronavirus disease 2019 (COVID-19). Acta Biomed. Link: https://bit.ly/2BHSDtz
- Pan Y, Zhang D, Yang P, Poon LLM, Wang Q (2020) Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 20: P411-P412. Link: https://bit.ly/2UmqT4p
- Tang YW, Schmitz JE, Persing DH, Stratton CW (2020) The Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J Clin Microbiol. Link: https://bit.ly/2UiIqKA
- Torrego A, Pajares V, Fernández-Arias C, Vera P, Mancebo J (2020) Bronchoscopy in COVID-19 Patients with Invasive Mechanical Ventilation: A Center Experience. Am J RespirCrit Care Med. Link: https://bit.ly/2XLazfD
- Venter M, Richter K (2020) Towards effective diagnostic assays for COVID-19: a review. J ClinPathol. Link: https://bit.ly/2XG9kye
- Sufang T, Weidong H, Li N, Liu H, Xu H, et al. (2020) Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J Thoracic Oncology 15: 700-704. Link: https://bit.ly/2Yh4TsA
- Alanagreh L, Alzoughool F, Atoum M (2020) The Human Coronavirus Disease COVID-19: Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. Pathogens 9: 331. Link: https://bit.ly/3dIYCN5
- Bourgonje A, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, et al. (2020) Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol Link: https://bit.ly/37dkJIX
- Ding Y, Wang H, Shen H, Li Z, Geng J, et al. (2003) The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 200: 282-289. Link: https://bit.ly/2ASxRHu
- Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, et al. (2014) Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates. Am J Pathol 186: 652-658. Link: https://bit.ly/2BLSLbF
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
