Archives of Pulmonology and Respiratory Care
Measurement of maximal respiratory static pressure in stable COPD patients and their co-rrelation with various physiological anthropometric and spirometry parpmeters and arterial blood gas tension
Pradeep Kumar Vyas1*, Priyanka S Bindroo2, Madhavi Gondha3, Rajeev S Mathur3, Gaurav Ghtawat4 and Vidyadhara Lakkappan1
2Department of Critical care and Respiratory medicine, Rajan Babu T.B. Hospital New Delhi, India 3Director, Senior consultant, Head of Department and D.N.B. Teacher, Department of Pulmonology, Jaslok Hospital and Research Centre, Mumbai India
4Department of Pulmonology, Bhatia Hospital Mumbai, India
Cite this as
Vyas PK, Bindroo PS, Gondha M, Mathur RS, Ghtawat G, et al. (2020) Measurement of maximal respiratory static pressure in stable COPD patients and their co-rrelation with various physiological anthropometric and spirometry parpmeters and arterial blood gas tension. Arch Pulmonol Respir Care 6(1): 033-042. DOI: 10.17352/aprc.000049The strength of respiratory muscles can be evaluated from static measurement (PImax and PEmax) or inferred from dynamic measurement such as maximal voluntary ventilation. Maximal inspiratory pressure and Maximal expiratory pressure are simple, convenient and non-invasive measurement of respiratory muscle strength. Respiratory muscle strength decreases in COPD patients due to multiple factors. The primary aim of this study is to obtain mean PImax and PEmax values in Indian COPD patients and its co-rrelation with anthropomatric, physiological, spirometric parameters and arterial blood gas tension.
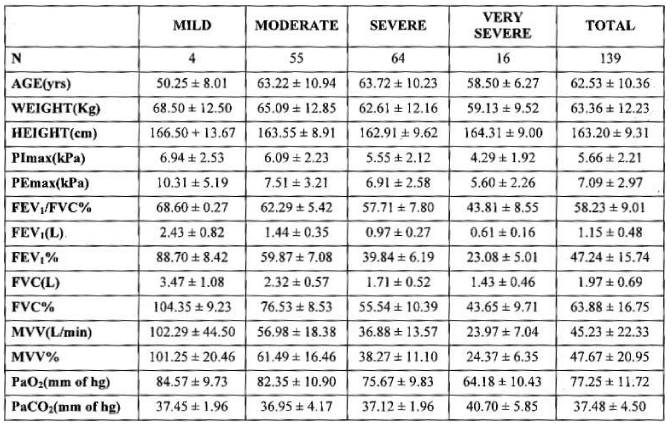
Subjects and methods: Retrospective data were collected between 2007to 2010.139 stable COPD patients of different stages according to GOLD classification were selected for this study with mean age 62.53 ± 10.36 years, out of 139 patients 104 were male and 35 were female.
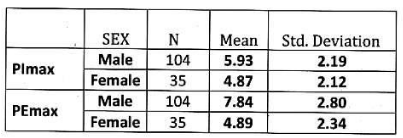
Results: In our study mean PImax values in male and female COPD patients were 5.93 ± 2.19 kPa and 4.87 ±2.12 kPa respectively. Mean PEmax value in male and female COPD patients were 7.84 ± 2.80 kPa and 4.89 ±2.34 kPa respectively.
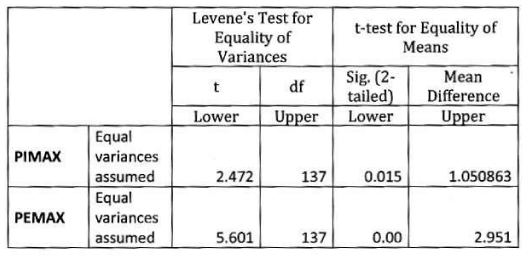
Conclusion: In our study PImax, PEmax were reduced in COPD patients in comparison to normal populations and significantly lower in patients with severe airflow limitations than mild airflow limitations. This can help in assessment and monitoring of the disease burden, severity of functional impairment and response to treatment. It had significant positive or negative correlation with anthropometric and spirometry parameters and arterial blood gas tension.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases [1,2]. The chronic airflow limitation that is characteristics of COPD is caused by a mixture of small airways disease (e.g. Obstructive bronchiolitis) and parenchymal destruction (Emphysema), the relative contribution of which vary from person to person. COPD produces significant systemic consequences [2]. COPD is a leading cause of morbidity and mortality in the world which continues to cause disabling symptoms, impair the functional status and quality of life. COPD ranked fourth as the cause of death currently in the world which will become the third leading cause of death by 2020 [1]. More than 3 million people died due to COPD in 2012 about 6% of all deaths. It is important public health problem which is both preventable and treatable. The burden of COPD will increase progressively due to its risk factors and aging of the populations. In India prevalence of COPD is around 4.1% [3]. Effects of COPD do not remain localized to lungs only but also produce several extra-pulmonary effects. Systemic features include chronic low grade systemic inflammation and altered regulation of protein metabolism, result in muscle atrophy at an early stage [4,5], that leads to cachexia at later stage, which attribute to skeletal muscle dysfunction [6-8]. Respiratory muscle weakness in COPD is multi-factorial which includes (a) Age (b) Smoking (c) Malnutrition related to biochemical, anatomical and physiological changes (d) Chronic inactivity (e) Muscular atrophy (f) Steroid induced myopathy (g) Pulmonary hyperinflation with increased residual volume (h) Decreased blood flow to respiratory muscle. (i) Hypoxemia (j) Oxidative stress and superoxide anion production [7-16]. Maximal respiratory static pressures reflect respiratory muscle strength. PImax measures strength of the diaphragm while PEmax measures strength of abdominal and intercostal muscles. The measurement of PImax and PEmax is easy, non-invasive, rapid, convenient and reproducible [17,18]. In advance stage respiratory muscle weakness leads to respiratory failure. Inspiratory muscle weakness explains dyspnoea and expiratory muscle weakness leads to mucus retention due to impaired cough efficacy. Respiratory muscle strength is a strong predictor for survival in COPD patients [19]. Hence measurement of maximal respiratory static pressure is very useful in diagnosing respiratory muscle dysfunction and helpful in assessment of impact of chronic disease. Serial measurement allows to monitor progression of muscle dysfunction and response to treatment. Various studies show that maximal respiratory static pressures are reduced in COPD patients compared to normal populations. Some of these studies also show PImax and PEmax correlate positively with spirometry parameters, arterial blood gas tensions and anthropometric measurements [14,20-25]. Till present no data exists for COPD patients in India that prompted us to do this study with the following objectives: (a) To obtain mean values of PImax and PEmax (b)To study correlation of MRP with spirometry, physiological parameters and ABG.
Review of literature
COPD: International guidelines for diagnosis of COPD staging is still based on severity of airway obstruction. Patients are homebound and depressed; they avoid dyspnoea which everyday activities produce. Respiratory muscles are essential for alveolar ventilation. Inspiratory muscle function is abnormal in COPD patients [26]. Expiratory muscle weakness is also seen, but its clinical importance is not well understood Table 1 Figure 1 [27,28].
Respiratory muscle dysfunction: Byrd, et al. [20], demonstrated that MRP is reduced in COPD [14,20, 25,27,28]. Polkey, et al. [29], assessed diaphragm strength in 20 COPD patients and 7 control subjects by measuring maximal sniff trans-diaphragmatic pressure and twitch trans-diaphragmatic pressure. Tw Pdi, sniff Pdi, twitch oesophageal pressure and sniff Pes in COPD patients were reduced. The ability of diaphragm to generate trans-diaphragmatic and a negative intra-thoracic pressure is reduced in COPD and these changes are exaggerated with acute on chronic hyperinflation.
Causes of Diaphragm dysfunction [26]: Mechanical disadvantage Hyperinflation is the main feature in COPD. In hyperinflation the area of diaphragm immediately apposed to rib cage is reduced at FRC [30]. Alteration in diaphragm geometry impairs three critical actions of diaphragm (1) Piston like axial displacement of the diaphragmatic dome, which is main mechanism of the contribution of the diaphragm to tidal volume in normal subjects.(2) Appositional action of the diaphragm which is the lower rib cage expanding action of the diaphragm that depends on the rise in abdominal pressure acting through the zone of apposition to expand the lower rib cage and (3)Its insertion action which is the lower rib cage expanding action of the diaphragm exerted by virtue of its insertion into the lower ribs. It causes lifting and rotating of lower ribs outward. Hyperinflation could change the mechanical arrangement between the crural and costal parts of the diaphragm from a parallel to a series arrangement, leading to a further reduction of the force-generating capacity [31-33].
Muscle length: Muscle fibre length is an important determinant of force-generating capacity. Cassart, et al. [30], demonstrated that diaphragm length reduced in COPD patients. Shortening of diaphragm impair its force-generating capacity. Inspiratory pressures generated by these patients are reduced in COPD. Acute dynamic hyperinflation develops once expiratory flow develops .This interposition of acute on chronic hyperinflation decreases the force capacity of the diaphragm.
Muscle structure: Severe COPD increases the slow-twitch characteristics of the muscle fibres in diaphragm, an adaption that increases resistance to fatigue. Levine, et al. [34], studied 6 COPD patient’s diaphragm biopsies, which showed increased proportion of type-I fibres essentially at the expense of type-IIb fibres. Biopsy specimens from the patients had higher percentages of slow myosin heavy chain I and lower percentages of fast myosin heavy chains IIa and IIb than the diaphragm of the controls.
Muscle environment: A variety of electrolyte disturbances have been shown to alter skeletal muscle function. Acute hypercapnia reduces the diaphragm contractility because breathing CO2 was associated with lower Pdi [35]. Acute respiratory acidosis decreases the contractility and endurance time of diaphragm. Aging COPD develops with advancing age. Changes occur in structure and function of muscles in elderly persons [36]. Atrophy of type-II fibres causes decline in muscle strength. Pdimax, sniff Pdi and twitch Pdi were 25%, 13% and 23% lower in elderly people [37,38].
Body weight and malnutrition: Malnutrition in COPD patients is the result of an imbalance between energy intake and energy expenditure. Reduced dietary intake in these patients attributed to symptoms of postprandial dyspnoea, early satiety, fatigue and loss of appetite. Elevated energy expenditure has been attributed to increased work of breathing and systemic inflammation. Malnutrition reduces respiratory muscle mass and contractile force through atrophy of Type II fibres and impaired energy metabolism [9].
Steroid induced changes: Steroids are prescribed for acute exacerbation and chronic symptoms. Long term therapy with steroids elicits significant reduction in strength of ventilator and limb muscles [11]. Steroid myopathy is due to reduction in protein synthesis and increased glycogen accumulation. An elevation of urinary creatinine excretion and selective type-IIb fibre atrophy may be observed [39]. Corticosteroids may down regulate IGF-1, and protein synthesis and increases intracellular proteolysis. Protein synthesis is inhibited in type-II fibres by down regulating peptide initiation on the ribosomes. Increased cytoplasmic protease activity may lead to myofibrillar destruction. Therefore steroids inhibit protein synthesis and accelerate myofibrillar and soluble protein degradation in muscle. Carbohydrate metabolism is altered in steroid myopathy. Reduced muscle glycogen phosphorylase activity and increased glycogen synthatase activity lead to an increased intramuscular concentration of glycogen, reduction in creatine kinase activity. This impairment in glycolytic activity may in part be compensated by increasing oxidative metabolism. It has been postulated that steroids may impair diaphragm function by reducing myofibrillar density and/or by slowing cross-bridge kinetics [8].
Hypoxemia: COPD patients develop chronic hypoxemia or repeated episodes of hypoxemia during exacerbation. Maximum diaphragm force and endurance are both significantly reduced in COPD patients with chronic hypoxemia although inhalation of oxygen for 15 minutes elicits an increase in strength and endurance [40]. Jakobsson, et al. [41], reported that ATP, glycogen and creatine phosphate levels in quadriceps muscle fibres are reduced in COPD patients with chronic respiratory failure and found a significant co-relation between muscle metabolites and PaO2. Chronic hypoxemic COPD patients show greater levels of exercise induced lipid peroxidation and oxidized proteins in their quadriceps muscle than do non hypoxemic [42-44]. Hypoxemia can elicit skeletal muscle dysfunctions through direct and indirect actions on contractile processes. Indirect effects mediated through release of pro-inflammatory cytokines TNF-α and IL-44.
Systemic inflammation is a feature of COPD, elevated levels of CRP, fibrinogen, circulating leukocytes and pro-inflammatory cytokines: TNF-α, interleukins IL8, IL6, IL18, S TNF-R55 and 72(s TNF-R75) have been observed [45-47]. No direct correlations have been observed between sputum and plasma concentration of these in patients with mild to moderate COPD suggesting that organs other than the lungs(diaphragm and intercostal muscle where the work of breathing is elevated) contribute to elevated levels of systemic inflammatory mediators. Casadevall, et al. found elevated levels of TNF-α and IL-6 levels in intercostal muscles of COPD patients [48]. TNF-α promotes muscle wasting by enhancing the activity of the ubiquitin proteasome pathway, apoptosis, and altered protein metabolism. Apoptosis cause loss of nuclei, alter myonuclear domain size resulting in muscle atrophy Table 2 [6,7].
Oxidative stress: Hypoxemia and systemic inflammation contribute to oxidative stress in COPD patients. Production of ROS and NO within skeletal muscle fibres is regulated by strong muscle contractions, severe COPD patients are exposed to respiratory overloads, their diaphragm fibres generate greater levels of oxidants than those normally neutralized by intracellular antioxidants defences, thus leading to the development of oxidative stress. Barreiro, et al. [15], showed that diaphragm muscle exhibits increased levels of oxidative stress, and such levels are associated with impairment of both pulmonary and respiratory muscle functions. Marrin-Corral, et al. [16], has demonstrated oxidation of diaphragm proteins and increased superoxide anion level in diaphragm specimens of COPD patients. Oxidation of diaphragm proteins involved in energy production and contractile performance contribute to respiratory muscle dysfunction. Rochester, et al. [21], studied 32 COPD patients and 22 normal subjects. Half had normal and half had low values of PEmax. PImax was normal in patients with normal PEmax, but lower in patients with low PEmax. PaCO2 was elevated in 13 of 18 patients whose PImax was less than 55 cm H2O and inversely correlated with PImax. Morrision, et al. [22], studied respiratory muscle performance in elderly subjects. PImax, but not PEmax was less in COPD patients. Heijidra, et al. [14], studied 30 male COPD patients, PImax and PEmax were low. Significant correlation was found between PImax and FRC, RV%, TLC, BMI, FEV1, PaCO2. Nishimura, et al. [13], studied respiratory muscle strength, pulmonary function and body composition in 24 Japanese male COPD patients (group A < 80% ideal body weight, group B > 80% compared to group C controls). PImax and PEmax in group A were lower than in group B and C. Both PImax and PEmax were correlated with lean body mass.Positive correlations amongst PImax and PEmax and VC, VC% and FEV1.No correlation with TLC, RV, RV/TLC, PaO2 and PCO2. Peter, et al. [23], studied PImax in 34 COPD patients and 149 healthy subjects. PImax was lower in both sex. Kbitz, et al. [24], studied PImax in 33 patients and control PImax was low in COPD patients. Terzano, et al. [25], compared PImax and PEmax value of 110 COPD patients with 21 control. Both were low in COPD. Significant positive correlation found amongst MIP and FEV1, FVC, PEF, TLC and height, MEP and functional parameters. No correlation found with RV, RV/TLC, weight and age. As severity of COPD increases, muscle strength reduces and quality of life deteriorates. Above mentioned reasons prompted us to do this study to derive PImax and PEmax values COPD patients India.
Materials and methods
Aims and objectives
(1) To obtain average values of PImax and PEmax (2) To study correlation between MRP and various anthropometric, spirometry parameters and ABG.
Materials and methods
Study was conducted in retrospective manner after taking approval from hospital ethical committee and patient’s informed written consent were obtained. Data were collected from pulmonary laboratory of Jaslok Hospital Mumbai. All Patient’s medical records, clinical history, diagnosis, checked.
Inclusion criteria
Age > 18 years.
Exclusion criteria
Patients with AECOPD within last 3 months, acute pulmonary illness, co-existing chronic pulmonary and co-existent illness impairing respiratory muscle function.
Measurements
For all patients, demographic data were obtained. Anthropometry was done by measuring weight in kilogram (kg) and height with a stadiometer and Body Mass Index (BMI) was calculated according to the formula kg/m2 . Spirometry test was performed on jaeger ms PFT analysis unit, which was calibrated daily, all tests were conducted by same technicians, as per ATS/ERS statement [18]. Persons were explained about procedure and motivated. For measurement of PImax the subject was asked to make a maximum inspiratory effort starting from the residual volume, whereas for PEmax a maximal expiratory effort starting from total lung capacity was elicited [7]. At least three reproducible manoeuvres each maintained at least one second until three technically adequate efforts were selected and highest value was recorded.
MVV is the largest volume that can be breathed into and out of the lungs during a period of 10-15 seconds interval with maximal voluntary efforts.
Arterial blood gases analysed.
Stastistical data analysis
Was performed for all measured parameters using SPSS software. Patients were divided according to severity of COPD as per GOLD classification [1]. Standard descriptive statistics based on mean and standard deviation for quantitative variables were used. Pearson’s correlation co-efficient test was performed to assess possible correlation between PImax and PEmax values and anthropometric spirometry parameters and Arterial blood gas measurement.
Results
Mean age, weight and height of subjects were 62.53± 10.36 years, 63.36 ± 12.23 kg and 163.20 ± 9.31 cm. Mean FEV1/FVC% 58.23± 9.10%, mean FEV1(L) and FVC(L) were 1.15±0.48 and 1.97±0.69 L. Mean FEV1% 47.24±15.74% and mean FVC% 63.88± 16.75%. Mean MVV (L/min) and MVV% 45.23 ±22.33 and 47.67± 20.95 % . Mean PaO2 and PaCO2 were 77.25 ±11.72 and 37.48 ±4.50 mm of Hg.
Entire group results of MRP
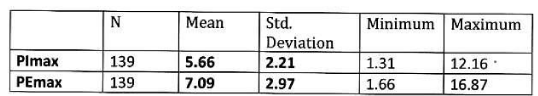
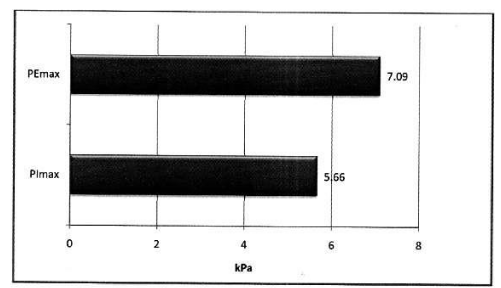
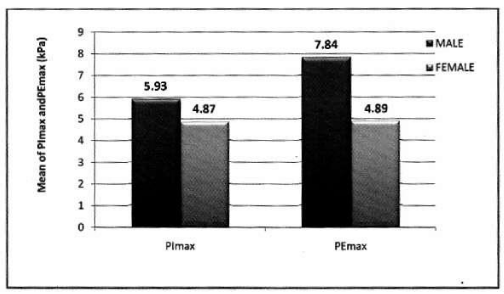
Mean PImax 5.66 kPa ±2.21 kPa, mean PEmax 7.09 kPa± 2.97 kPa. Table 3 Figure 2.
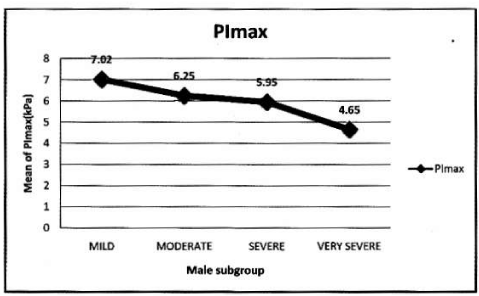
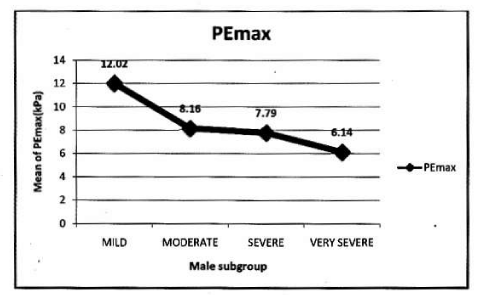
In male: Mean PImax 5.93 kPa ±2.19 kPa. Mean PEmax 7.84 kPa±2.80 kPa. Tables 4,5 Figure 3.
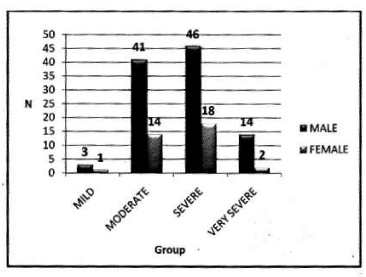
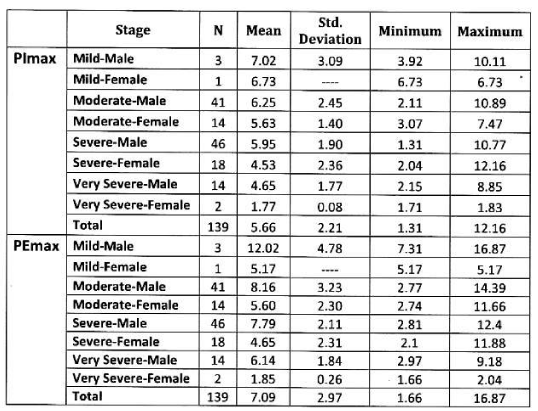
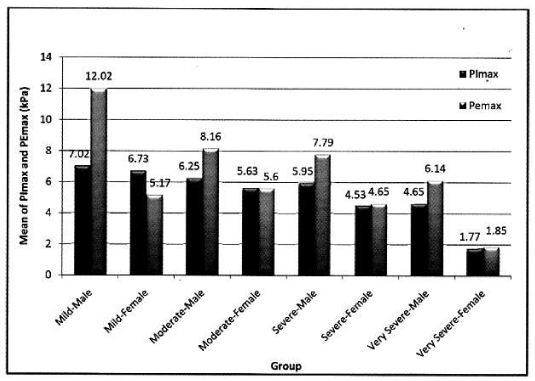
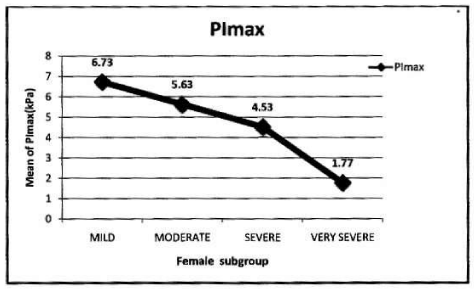
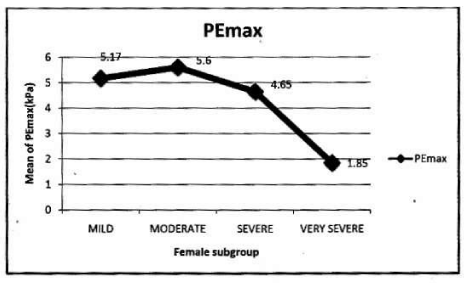
In female: Mean PImax 4.87 kPa±2.12 kPa. Mean PEmax 4.89 kPa±2.34 kPa. In female both values were lower than male. There was significant difference between both sexes. PImax and PEmax in different stages of COPD are given in Table 6 and Figure 4.
Correlation of PImax to variables in male
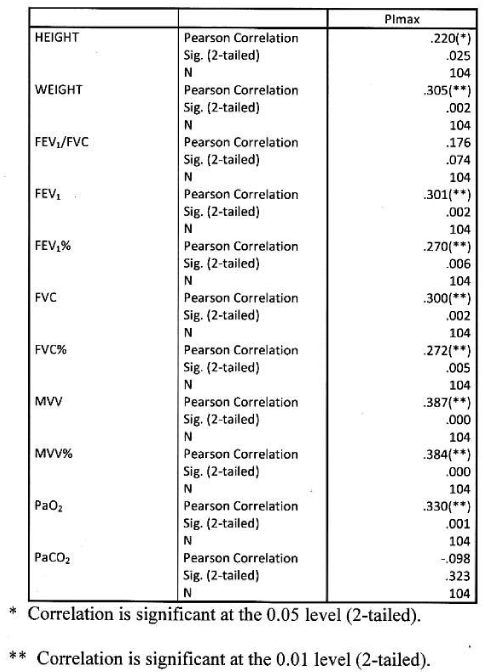
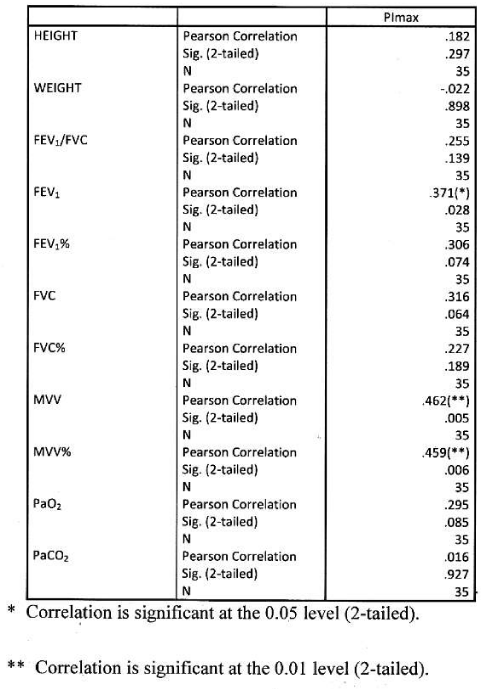
Significant positive correlation found between PImax and height and weight. Significant positive correlation found between PImax and FEV1, FEV1%, FVC, FVC%, MVV, MVV and PaO2. Correlation were more significant with absolute value than percentage predicted values. PImax had no significant correlation with FEV1/FVC and PaCO2 Table 7 Figure 5.
Correlation of PEmax to variables in male
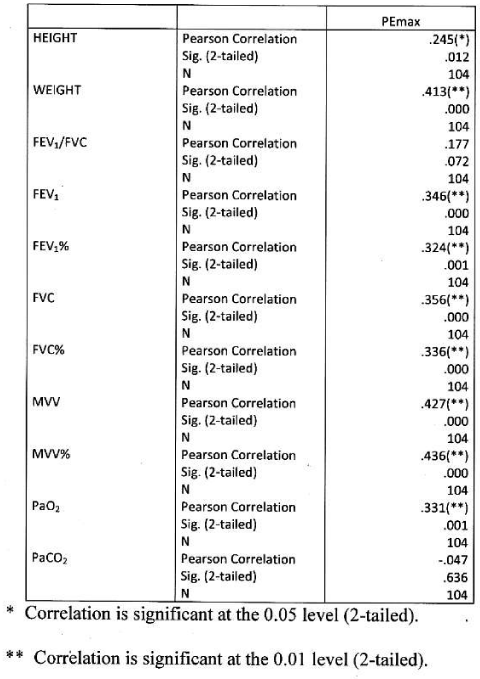
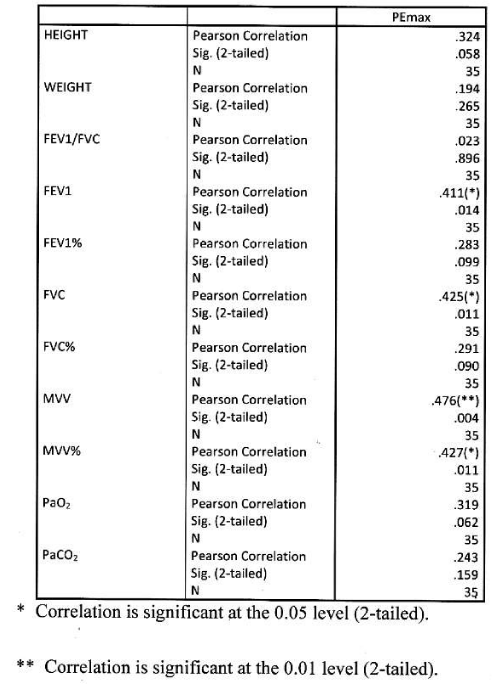
Significant positive correlation found between PEmax and height and weight, between PEmax and FEV1, FEV1%, FVC% MVV, MVV% and PaO2. Correlation was more significant with MVV amongst all parameters. PEmax had no significant correlation with FEV1/FVC and PaCO2 Table 8 Figure 6.
Correlation of PImax to variables in female
Significant positive correlation found between Pimax and FEV1, MVV, MVV%. Correlation was more significant with absolute MVV than percentage predicted. PImax had no significant correlation with height, weight, FEV1/FVC, FEV1%, FVC, FVC%, PaO2 and PaCO2. Table 9 Figure 7.
Correlation of PEmax to variables in female
Significant positive correlation found between PEmax and FEV1, FVC, MVV, MVV%. Correlation was more significant with MVV amongst all functional parameters. PEmax had no significant correlation with height, weight, FEV1/FVC, FEV1%, FVC%, PaO2 and PaCO2 Table 10, Figure 8.
Discussion
This is the first Indian study that analyses PImax and PEmax values and their correlation with anthropometric and spirometry parameters in of COPD patients in Indian population. In our study PImax and PEmax in COPD patients were 5.66±2.19 kPa and 7.84± 2.80 kPa amongst 139 COPD patients. Mean PImax in COPD patients described in previous studies were 7.1±2.3kPa [14], 7.1±2.4 kPa [20], 5.6±2.5 kPa [21], 6.0±1.9 kPa [22], 7.3±3.0 kPa [24] and 7.5±2.7 kPa [25]. Mean PEmax in COPD patients described in previous studies were 9.3±3.0kPa [14], 20.6±4.1 kPa [20], 13.7±4.8 kPa [21], 13.8±6.5 kPa [22] and 8.7± 3.0 kPa [25]. Different studies showed wide variations in PEmax values. Marked difference in result of above studies can be explained on the basis that all studies had different characteristics of patients. Compared to previous study, our mean PImax and PEmax were 16.5% and 30.7% lower than western populations. In our study mean PImax in male and female were 5.93±2.19kPa and 4.87±2.12 kPa respectively. Mean PEmax in male and female were 7.84 ±2.80 kPa and 4.89 ±2.34 kPa respectively. Johan et al derived values of MRP in Indian population. Mean PImax in male and female were 8.6± 2.9kPa and 4.9± 1.4 kPa respectively. Mean PEmax in male and female were 9.6±2.8 kPa and 5.9 ± 2.0kPa respectively. Devasahayam J, et al. [49,50], reported mean PImax in male and female 10.59 kPa and 7.65 kPa respectively. Mean PEmax in male and female were 12.94 kPa and 8.86 kPa respectively. Priyanka Chaudhary, et al. [51-53], reported mean PImax 59.8951c m±24.49023 (male and female 66.7600 ±24.48717and 50.6900±21.50713 cm respectively). Mean PEmax was 67.3636 c m±23.63529, (male and female 75.8012± 23.27 025cm and 56.0496±19.132337 cm respectively). Mean MVV 97.6900 L/min±31.04196 (male and female 115.6236±24.93948 and 73.6427± 20.34229 L/min respectively). Respiratory muscle strength was reduced in Indian COPD population when compared to normal Indian population [49]. As disease severity increases respiratory muscle strength decreases. Female had lower values than male. In male PImax and PEmax showed significant positive correlation with weight. Nishimura, et al. [13], reported that respiratory muscle is closely associated with body weight and lean body mass. Undernourished patients with COPD have lower muscle strength than well-nourished because prolong malnutrition can lead to skeletal and respiratory muscle wasting with severe effects on the contractile properties of diaphragm [26]. This suggests that nutrition supplementation should be a primary intervention. Our study suggests strong correlation between PImax and PEmax and FEV1 and FVC. Other studies [13-25], showed similar results. The relation between PImax and FEV1 might suggest that the impaired muscle function results in lower FEV1. In our study absolute values of FEV1 and FVC had better correlation with PImax and PEmax than percentage predictive values. Study also highlights relation between MVV and PImax and PEmax, amongst all parameters MVV showed highest positive correlation with respiratory muscle strength, absolute MVV showed better correlation than percentage predicted values reflect the respiratory muscle endurance and fatigue. The lack of cardiopulmonary fitness in patients with COPD may cause reduced respiratory muscle endurance. Nishimura, et al. [13], did not show significant correlation between respiratory muscle strength and PaO2. But our study showed significant positive correlation with PaO2. This finding suggests that chronic hypoxemia does cause impairment. But effect of long term oxygen therapy on respiratory muscle strength is not known. A negative correlation between PImax and PaCO2 found in previous studies [14,21], our study does not show such results.
Conclusion
COPD is a leading cause of morbidity and mortality. Respiratory muscle strength is a strong predictor of survival in COPD patients. Its weakness is multi-factorial that leads to respiratory failure. Hence measurement of maximal respiratory static pressure is extremely useful in diagnosing respiratory muscle dysfunction and helpful in assessment of impact of chronic disease and plan for exercise and rehabilitation programme. In our study PImax and PEmax in COPD patients were lower than western studies. PImax and PEmax showed significant correlation with weight and MVV amongst all parameters.
Our sincere thanks to Late Dr. Jayant R.Shah and Late Dr. A.S. Chitnis Senior pulmonologist and D.N.B. Teacher for their technical guidance and help provided in preparing this manuscript. We are also thankful to the staff of pulmonary department, to the management of Jaslok Hospital to allow us to conduct this study and to all the participants.
a). Conflict of interest: None to declare.
b). Funders: No funders available for this disease.
c). Patient consent taken.
- Global initiate for COPD. Global strategy for diagnosis management and prevention of COPD. Link: https://bit.ly/2ysp0Lw
- Celli BR, MacNee A, Agusti A, Anzueto B, Berg AS, et al. (2004) Standards for the diagnosis and treatment of COPD patients summary of ATS/ERS position paper. Eur Respir J 23: 932-946. Link: https://bit.ly/3bX5apr
- Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D'Souza GA, et al. (2006) Asthma Epidemiology study group. A multicentric study on epidemiology of COPD and its relationship with tobacco smoking and environmental tobacco smoke exposure. Indian J Chest Dis Allied Sci 48: 23-29. Link: https://bit.ly/3bZqpXG
- Agusti AG (2005) Systemic effects of COPD. Proc Am Thorac SOC 2: 367-372.
- Balasubramanian VP, Vaerkey B (2006) COPD: effects beyond the lungs. Curre Opin Pulm Med 12:106-112.
- Remels AH, Gosker HR, der Velden J, Langen RC, Schols AM (2007) Systemic inflammation and skeletal muscle dysfunction in COPD: State of the Art and Novel Insight in Regulation of muscle Plasticity. Clin Chest Med 28: 537-552. Link: https://bit.ly/2A8W9w9
- Kim HC, Mofarrahi M, Hussain SNA (2008) Skeletal muscle dysfunction in patients with COPD. Int J of COPD 3: 637-658. Link: https://bit.ly/2ZyhMkd
- ATS/ERS (1999) Dysfunction of skeletal muscle in patients with COPD. Am J Respir Crit Med 159: 1-40.
- Rochester DF (1986) Malnutrition and respiratory muscles. Clin Chest Med 91-99.
- Openbrier DR, Irwin MM, Rogers RM, Gottlieb GP, Dauber JH, et al. (1983) Nutritional status and lung function in patients with emphysema and chronic bronchitis. Chest 83: 17-22.
- Decramer M, Stas KJ (1992) Corticosteroids induced myopathy involving respiratory muscles in patients with COPD and Asthma. A Rev Respir Dis 146: 800-802. Link: https://bit.ly/2A2nS1I
- Van Balkom RH, Zhan WH, Prakash YS, Dekhuijzen, PN, Sieck GC (1997) Corticosteroids effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol 83: 1062-1067. Link: https://bit.ly/3eexMMl
- Nishimura Y, Tsutsumi M, Nakata H, Tsunenari T, Maeda H, et al. (1995) Relationship between respiratory muscle strength and lean body mass in men with COPD. Chest 107: 1232-1236. Link: https://bit.ly/2XoGSQ8
- Heijdra YF, Dekhuijzen PN, van Herwaarden CL, Folgering HT (1994) Effects of body position, hyperinflation and blood gas tension on maximal respiratory static pressures in patients with chronic obstructive pulmonary disease. Thorax 49: 453-458. Link: https://bit.ly/2WXYByz
- Barreiro E, Puente BDl, Minguella J, Corominas JM, Serrano S, et al. (2005) Oxidative stress and respiratory muscle dysfunction in severe COPD. Am J Respir Crit Care Med 17: 1116-1124. Link: https://bit.ly/3c0Jkl9
- Marin-Corral J, Minguella J, Ramírez-Sarmiento AL, Hussain SNA, Gea J, et al. (2009) Oxidized proteins and superoxide anion production in diaphragm of severe COPD patients. Eur Resspir J 33: 1309-1319. Link: https://bit.ly/2Ab1tz9
- Epstein SK (1994) An overview on respiratory muscle function. Clin Chest Med 15: 619-639. Link: https://bit.ly/3d3B4SK
- American Thoracic Society/European Respiratory Society ATS/ERS (2002) Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med 166: 518-624. Link: https://bit.ly/2LWZGQY
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG (1996) Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respire Crit Care Med 153: 961-966. Link: https://bit.ly/3gj5y4V
- Byrd RB, Hyatt RE (1968) Maximal Respiratory in chronic obstructive lung disease. Am Rev Respire Dis 98: 848-856.
- Rochester DF, Braun NMT (1985) Determinants of maximal inspiratory pressure in chronic obstructive pulmonary disease. Am Rev Respire Dis 132: 42-47. Link: https://bit.ly/2WWFTHM
- Morrison NJ, Richardson R, Dunn L, Pardy RL (1989) Respiratory muscle performance in normal elderly subject and patients with COPD. Chest 95: 90-94. Link: https://bit.ly/3dcLdg1
- Wijkstra PJ, van der Mark TW, Boezen M, van Altena R, Postma DS, et al. (1995) Inspiratory Mouth Pressure in Health Subject and in Patients with COPD. Chest 107: 652-656. Link: https://pubmed.ncbi.nlm.nih.gov/7874932/
- Kabitz HJ, Walterspacher S, Walker D, Windisch W (2007) Inspiratory muscle strength in chronic obstructive pulmonary disease depending on disease severity. Clin Sci 113: 243-249. Link: https://bit.ly/3gg5xyZ
- Terzano C, Ceccarelli D, Conti V, Graziani E, Ricci A, et al. (2008) Maximal respiratory static pressure in patients with different stage of COPD severity. Respire Res 9: 8. Link: https://bit.ly/2LSsU3D
- Marchand E, Decramer M (2000) Respiratory Muscle Function and Drive in Chronic Obstructive Pulmonary Disease. Clin Chest Med 21: 679-692. Link: https://bit.ly/2TCVQ3E
- Ramirez-Sarmiento A, Orozco-Levi M, Barreiro E, Méndez R, Ferrer A, et al. (2002) Expiratory muscle endurance in chronic obstructive pulmonary disease. Thorax 57: 132-136. Link: https://bit.ly/3d20XlA
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N (2003) Specific expiratory muscle training in COPD. Chest 124: 468-473. Link: https://bit.ly/2zjyJVb
- Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, et al. (1996) Diaphragm strength in chronic obstructive pulmonary disease. Am J Respire Crit Care Med 154: 1310-1317. Link: https://bit.ly/3gkeBCL
- Cassart M, Pettiaux N, Gevenois PA, Paiva M, Estenne M (1997) Effect of chronic hyperinflation on diaphragm length and surface area. Am J Respire Crit Care Med 156: 504-508. Link: https://bit.ly/3edJp6n
- Macklem PT, Macklem DM, De Troyer A (1983) A model of inspiratory muscle mechanics. J Appl Physiol 55: 547-557. Link: https://bit.ly/2TA8wZ3
- Braun NM, Arora NS, Rochester DF (1982) Force-length relationship of the normal human diaphragm. J Appl Physiol Respir Environ Exerc Physiol 53: 405-412. Link: https://bit.ly/2LT6q2k
- Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F et al. (1991) Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 325: 917-923. Link: https://bit.ly/2Zzd0mw
- Levin S, Kaiser L, Leferovich J, Tikunov B (1997) Cellular adaptation in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med 337: 1799-1806. Link: https://bit.ly/3d3154r
- Juan G, Calverley P, Talamo C, Schnader J, Roussos C (1984) Effect of carbon dioxide on diaphragmatic function in human beings. N Engl J Med 310: 874-879. Link: https://bit.ly/3bZ2nw5
- Tolep K, Kelsen SG (1993) Effect of aging on respiratory skeletal muscle. Clin Chest med 14: 363-378. Link: https://bit.ly/2A2qrku
- Tolep K, Higgins N, Muza S, Criner G, Kelsen SG (1995) Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respire Crit Care Med 152: 677-682. Link: https://bit.ly/2WYlxh8
- Polkey MI, Harris ML, Hughes PD, Hamnegärd CH, Lyons D, et al. (1997) The contractile properties of elderly human diaphragm. Am J Respire Crit Care Med 155: 1560-1564. Link: https://bit.ly/2WXOrhu
- van Balkom RH, van der Heijden HF, van Herwaarden CL, Dekhuijzen PN (1994) Corticosteroid-induced myopathy of the respiratory muscles. Neth J Med 45: 114-122. Link: https://bit.ly/2TDsG4F
- Zattara-Hartmann MC, Badier M, Guillot C, Tomei C, Jammes Y (1995) Maximal force and endurance to fatigue of respiratory and skeletal muscles in chronic hypoxemic patients: the effect of oxygen breathing. Muscle Nerve 18: 495-502. Link: https://bit.ly/3estQYP
- Jakobsson P, Jorfeldt L, Brundin A (1990) Skeletal muscle metabolites and fibre types in patients with advance chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respire J 3: 192-196. Link: https://bit.ly/36sQNbi
- Koechlin C, Maltais F, Saey D, Michaud A, LeBlanc P, et al. (2005) Hypoxaemia enhance peripheral muscle oxidative stress in chronic obstructive pulmonary disease. Thorax 60: 834-841. Link: https://bit.ly/2WY1pM7
- Shee CD, Cameron IR (1990) The effect of pH and hypoxia on function and intracellular pH of the rat diaphragm. Respire Physiol 79: 57-68. Link: https://bit.ly/3gn31qr
- Ghezzi P (1991) Hypoxia increase production of interleukin-1 and tumor necrosis by human mononuclear cells. Cytokine 3: 189-194. Link: https://bit.ly/3c0U9mU
- Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF (1996) Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 51: 819-824. Link: https://bit.ly/2Aajf5y
- Gan W, Man S, Senthilselvan A, Sin D (2004) Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a mate-analysis. Thorax 59: 574-580. Link: https://bit.ly/3ef0ZXB
- Petersen AM, Penkowa M, Iversen M, Frydelund-Larsen L, Andersen JL, et al. (2007) Elevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary disease. Lung 185: 161-171. Ink: https://bit.ly/3eiVvei
- Casadevall C, Coronell C, Ramírez-Sarmiento AL, Martínez-Llorens J, Barreiro E, et al. (2007) Upregulation of pro-inflammatory cytokine in the intercostals muscle of COPD patients. Eur Respire J 30: 701-707. Link: https://bit.ly/3bZUxCf
- Johan A, Chan CC, Chia HP, Chan OY, Wang YT (1997) Maximal respiratory pressure in adult Chinese, Malays and Indians. Eur Respire J 10: 2825-2828. Link: https://bit.ly/2XpJnBI
- Devasahayam J (2006) Prediction equations for maximal respiratory static pressures in Indian adults. Chest.
- Priyanka C, Vyas PK (2018) Measurement of maximal respiratory static pressure in healthy Indian a retrospective study IJOPID Nov.
- Veen k (2018) Muscle pressure in male with COPD a cross sectional study IJORC 7.
- Khalil M, Waih R (2014) evaluation of respiratory muscle function in COPD patients Egypt chest disease. Egypt chest diseases.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
