Archives of Pulmonology and Respiratory Care
Micropapillary Adenocarcinoma of the lung: Recent updates and literature review
Paloma del C Monroig-Bosque1, Ahmed N Shehabeldin1, and Jae Y Ro2*
2Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Weill Medical College of Cornell University, Houston, TX, USA
Cite this as
Monroig-Bosque PDC, Shehabeldin AN, Ro JY (2019) Micropapillary Adenocarcinoma of the lung: Recent updates and literature review. Arch Pulmonol Respir Care 5(1): 001-011. DOI: 10.17352/aprc.000035Background: Lung cancer is the most frequent cause of cancer-related death worldwide. For adenocarcinomas, histological subtyping is typically performed (e.g. lepidic, acinar, papillary, micropapillary, and solid), as suggested by the World Health Organization. Among these, micropapillary carcinoma has been a focus of significant research in recent years, as it is the newest subtype and has the worst prognosis. Herein, we summarize the most relevant findings of this morphological variant in detail with anecdotal experience and a review of the published literature.
Results: A PubMed query for “adenocarcinoma of the lung” resulted in 177 full-length articles. The reviewed articles were grouped by category as follows: 1) history (2002-2008), 2) morphologic/diagnostic features, 3) prognostic implications, 4) molecular features and targeted therapies, and 5) radiologic/imaging findings. Our results consistently showed that a micropapillary carcinoma component is associated with poor prognosis in patients with lung adenocarcinoma. Additionally, our investigation highlighted the fact that there have been no studies validating the diagnostic/morphologic criteria that should be used to achieve an accurate diagnosis of micropapillary lung adenocarcinoma. Nonetheless, we present that molecular studies continue to emerge, and there is significant opportunity for targeted therapeutic options that remains to be explored in depth.
Conclusion: Our review offers insight into novel studies focused on micropapillary lung adenocarcinoma. We present the potential for new research opportunities, particularly molecular studies aimed at determining targeted treatment options.
Abbreviations
IASLC/ATS/ERS: International Association for the Study of Lung Cancer, American Thoracic Society and the European Respiratory Society; WHO: World Health Organization; STAS: Spread Through Air Spaces; HR: Hazar Ratio; RFS: Recurrence Free Survival; MFS: Metastasis Free Survival; OS: Overall Survival; DFS: Disease-Free Survival; PD-1: Programmed Death 1; PDL-1: Programmed Death Ligand 1; GLUT1: Glucose Transporter 1; MMP9: Matrix Metalloproteinase 9; TTF1: Thyroid Transcription Factor 1; CEA: Carcinoembryonic Antigen; SP-A: Surfactant Apoprotein A; EGFR: Epidermal Growth Factor Receptor; IQGAP1: Ras GTPase-Activating-like Protein; SCLC: Small Cell Lung Cancer; NSCLC: Non- Small Cell Lung Cancer; LADC: Lung Adenocarcinoma; EML4: Echinoderm Microtubule Associated Protein-Like 4; ALK: Anaplastic Lymphoma Kinase; HER2: Human Epidermal Receptor 2; PET: Positron Emission Tomography; CT: Computed Tomography; HRCT: High-Resolution Computed Tomography; TDR: Tumor-Shadow Disappearance Ratio; SUVmax: Maximum Standardized Uptake Value; DSS: Disease specific survival.
Background
Lung adenocarcinoma has been the most common cause of cancer-related death in both men and women for many years [1]. Recently, diagnostic accuracy has improved with the use of imaging techniques that can efficiently identify tumor lesions. This allows for faster time to treatment and more conservative, less invasive surgical options with decreased morbidity [2]. Morphology continues to be the most immediately crucial information that allows tumor grading and subtyping as it has implications for patient prognosis. In 2011, the International Association for the Study of Lung Cancer, American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS) revised the classification of lung adenocarcinoma by proposing new morphological criteria to provide a uniform diagnostic terminology for multidisciplinary patient management. This classification delivers histological subtyping based on pattern recognition and a semi-quantitative assessment of each pattern in 5% increments [3]. This new classification system has been tested in hundreds of cases in clinical datasets, and has shown to accurately define prognostically significant subgroups of lung adenocarcinoma [4].
Micropapillary lung adenocarcinoma is an important histological variant consisting of micropapillary morphology. It is similar to variants seen in other organs such as the breast, urinary bladder, and ovary. Micropapillary adenocarcinoma has been recently included in the World Health Organization (WHO) updated classification. Micropapillary adenocarcinoma is considered equivalent to poorly differentiated adenocarcinoma and resembles a morphology associated with a high-grade lesions [5,6]. Herein we aim to review studies published within the past ten years, that allude to the possibility of lung adenocarcinoma. Additionally, we reviewed updates from the past 3-5 years (including up to four years ago for molecular studies), and summarized the most relevant morphological/diagnostic features, prognostic implications, molecular changes, treatment modalities, and radiological/imaging findings.
Methods
A review of literature was performed as follows:
* Inclusion criteria
- A broad PubMed search was performed using “micropapillary lung adenocarcinoma” in the title/abstract of any article.
- We narrowed the article types to include case reports, clinical conference, clinical study, clinical trial (including phases I-IV), comparative study, controlled clinical trial, editorial, guideline, meta-analysis, multicenter study, observational study, randomized controlled trial, review, and systematic review.
- Of the searched articles, only the ones that were written in English, from human studies with adults of ≥19 years, and with full text available were reviewed.
* Exclusion criteria
- Articles in languages other than English, animal studies, and articles with only abstracts available for review were excluded.
* Withdrawal criteria
- Studies of micropapillary variants of adenocarcinomas in other organs (e.g. colon)
- Articles focused on other tumor types, or other morphologies (e.g. adenoid cystic carcinoma, lepidic lung adenocarcinoma)
- Manuscripts for which the PubMed link was not accessible
- Non-relevant case reports
- Articles that referred to non-targeted/cytotoxic therapeutic modalities
* Analysis
Results
Using our research criteria, we found 177 full-length articles available for review. The articles were classified as follows: 1) initial description of micropapillary histology in lung adenocarcinoma from more than 10 years ago (2002-2008), 14 articles; 2) morphologic/diagnostic features, 1 article; 3) prognostic implications, 28 articles; 4) molecular features and targeted therapies, 20 articles; and 5) radiology/imaging, 5 articles. Table 1 contains the articles explored in detail, as well as those cited for future review.
Discussion
A. History (2002-2008).
The first report found with our literature query was published in 2002 by Amin et al. [6]. In their study, the authors acknowledged that micropapillary carcinoma in lung had been recognized, but no importance had yet been given to it. However, the authors were aware that micropapillary architecture had prognostic relevance in other organs such as breast, urinary bladder, and ovary. They studied 35 cases of lung adenocarcinoma with a micropapillary component. They observed that the primary tumors metastasized in 94% of the cases, most with a predominant micropapillary component in the metastatic site. This study suggested for the first time that a micropapillary morphology could be observed in primary lung tumors. Furthermore, this study asserted that recognizing the micropapillary component was important because this morphology could be correlated with aggressive tumor progression [6].
Miyoshi et al. were the first to describe the morphologic features of the micropapillary pattern in lung primary tumors. They simply described it as “small papillary tufts lacking a central fibrovascular core” [7]. This criterion continued to be used in subsequent years [8,9]. Additionally, a handful of research groups evaluated the prognostic implications of the histological component, and published the details of how their patients showed higher propensity to develop lymph node metastasis and had an overall decreased survival compared to those without a micropapillary component [7-12].
Approximately two years after the first proposal of micropapillary components in lung primary tumors, preoperative detection of a micropapillary component was desired for making treatment decisions, given that this structure was associated with worse patient prognosis. Thus, cytology became an important tool for diagnosis. Hoshi et al. defined the cytological features important in the diagnosis of micropapillary components as: round, 3-dimensional, cohesive clusters of neoplastic cells (consisting of >3 and <20 cells) with a pseudopapillary configuration. In their study, patients with these features in early stage I disease had worse prognosis compared to controls [13]. Furthermore, a case report by Duncan et al. described the features seen in a primary lung cancer with significant micropapillary components as highly cellular malignant cells arranged in tight three-dimensional clusters, papillae without fibrovascular cores, and discohesive sheets of cells. Their avascular papillary structures and cell clusters were composed of overlapping atypical nuclei with irregular nuclear membranes and prominent nucleoli. Additionally, they described many discohesive individual cells, and clear spaces at the periphery of the cell balls, accentuated with Romanowsky stain [14]. These features were to diagnosis micropapillary carcinoma at the time, and some are still used.
Few studies utilizing additional diagnostic tools such as immunohistochemical profiles and/or molecular diagnostics were described early on. In our review of literature, Amin et al. demonstrated that among 15 cases, they found the following immunostain results: 80% were TTF1 (thyroid transcription factor 1) positive, 93% CK7 positive, and 13% CK20 positive [6]. Kawakami et al. showed that MUC1 was positive in the outer surface of micropapillary tufts, while non-micropapillary components showed reactivity limited to the luminal surface of carcinoma cells, suggestive of what has later been described as inverted nuclear polarity [11]. In a case report, Kuroda et al. performed an immunohistochemical workup in an autopsy case of a patient who had metastatic lung adenocarcinoma with micropapillary features. Their results determined that the micropapillary component was positive for cytokeratin CK7, CK19, TTF1, carcinoembryonic antigen (CEA), and surfactant apoprotein A (SP-A), but negative for CK20, estrogen receptor, progesterone receptor, uroplakin III, and CA125 [15]. Additionally, a study by Sanchez-Mora et al. evaluated the prognostic significance of various markers in five autopsy cases. They found p53, Ki67, and c-myc were elevated in more than half of their cases. Cyclin D1 (43%), epidermal growth factor receptor (EGFR) (36%), and Bax (43%) were also found to be expressed. Only cyclin D1 and Bax expression were associated with significantly worse survival [16].
In relation to molecular diagnostics, two studies were found from 10 years ago. In the first, Motoi et al. performed gene expression and KRAS and EGFR mutation analysis on 100 cases. This study from 2008 demonstrated that micropapillary subtype correlated strongly with EGFR mutation, but did not correlate with clusters of genes seen in other histological subtypes [17]. Similarly, a year later Ninomiya et al. studied 63 cases of lung adenocarcinoma and found that EGFR mutations were significantly associated with micropapillary pattern [18].
More than 10 years ago, Miyoshi T, et al. hypothesized a possible mechanism for the metastatic potential of tumors with micropapillary morphology. They speculated that the metastatic potential was mediated by a deficiency in Ras GTPase-activating-like protein (IQGAP1), which is ubiquitously expressed in humans. In the micropapillary component, deficiency in IQGAP1 causes the integrity of the entire cadherin-catenin-actin network to be compromised and the cellular adherent junction disassembled, resulting in the release of carcinoma cells organizing in a micropapillary pattern [19]. Similarly, Kamiya et al. in 2008 found that laminin was identified in the basement membrane of normal alveolar cells and neoplastic cells of the main tumor, but was not found in any cell with micropapillary tufts. They also suggested that cells with the micropapillary pattern are likely to have acquired anchorage-independent growth and a potential for high malignancy [9].
B. Morphologic/diagnostic features
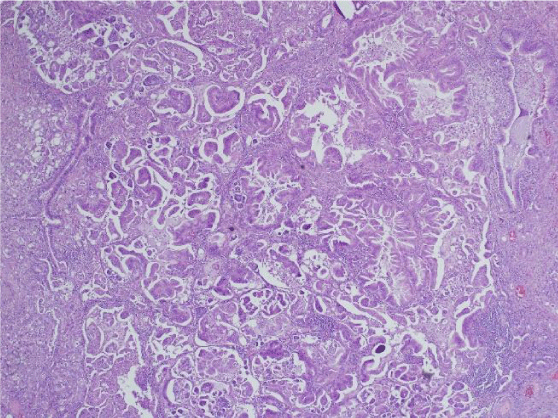
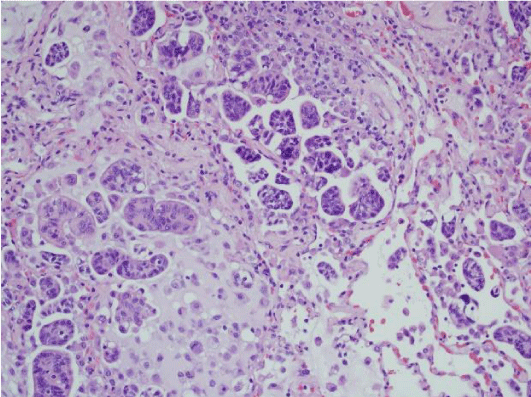
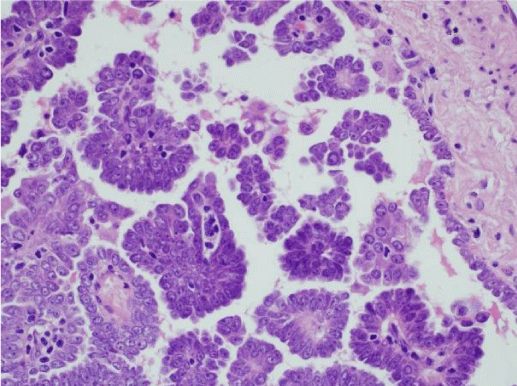
We did not find any recent publications within the last three years where the main focus was assessing the criteria used to diagnose micropapillary lung adenocarcinoma. There have not been any consensus studies among experts to determine which features are unique in lung, and which are similar compared to micropapillary tumors in other organs. A recent review article by Cao et al. summarized some of the criteria that have been suggested in the literature for the past years [20]. They concluded that the cellular tufts with absence of fibrovascular core (Figure 1) tended to be a hallmark for this entity. This was very different from other tumor types in which vessels and neovascularization are important. In the lung, it is though that tumor cells nourish from surrounding fluids in the alveolar surfaces. Micropapillary tumor cells are generally small and cuboidal with minimal nuclear atypia, detaching and/or connecting to alveolar walls (Figure 2). Cell matrix can be absent, and polarity is usually inverted with peripheral nuclear polarization (Figure 3). The clusters are usually located at the periphery of the tumor. The disordered micropapillary structures are thought to facilitate spread to lymphatics and other tissues [20].
C. Prognostic implications
General update
The majority of recently published research articles regarding lung adenocarcinomas were based on the architectural grading system established in the classification system by IASLC/ATS/ERS in 2011. The established classification system was the most effective system out of three proposed models (architectural, Kadota, and Sica;) because it made the best distinction between the outcome of low-grade, intermediate-grade, and high-grade stage I adenocarcinomas (for a detailed review of the systems please refer to the publication by Sica et al from 2010 [21]). These findings have already been validated in older and more recent studies [22-24]. Thus, the IASLC/ATS/ERS grading system was used for this entity. Morphologies are defined as follows: lepidic as well differentiated, acinar and papillary as moderately differentiated, and solid and micropapillary as poorly differentiated.
The two poorly differentiated morphologies—solid and the more recently described micropapillary—have been the focus of significant research throughout the years. A study by Zombori et al. showed that both solid and micropapillary patterns are more frequently seen in patients with recurrence, and that predominance of these patterns is associated with unfavorable prognosis, particularly for micropapillary. Zombori et al. demonstrated that among tumors with the same main morphologic pattern, the presence of micropapillary as a secondarily predominant pattern correlated with worse prognosis. Their results highlighted the significance of describing both a predominant and a secondary predominant pattern in lung adenocarcinomas, similar to what the Sica grading system suggests [21,24,25]. Additional studies have confirmed these findings. For example, Zhao et al. showed that adenocarcinomas harboring micropapillary and/or solid components as a secondary, non-predominant pattern had higher rates of metastatic lymph nodes and shorter median recurrence-free survival (RFS) and overall survival (OS) [26].
In multivariate analysis, micropapillary and solid morphologies together have the worst prognosis compared to well- and moderately-differentiated morphologies (i.e. lepidic, acinar, and papillary). This demonstrates that subtyping is a significantly independent prognostic factor [27]. Similarly, several studies have shown that harboring micropapillary morphology has the equivalent clinical recurrence and survival outcome as tumors with solid morphology [28,29].
Currently, certain assumptions that can be made about tumors with micropapillary carcinoma components. The presence of a micropapillary carcinoma is sufficient to predict nodal upstaging and decreased recurrence free survival (RFS) [30]. Compared to the other adenocarcinomas, micropapillary carcinoma has been shown to have the lowest overall survival (OS) and disease-free survival (DFS), sometimes even lower than the only other poorly differentiated diagnostic category, solid adenocarcinoma [24,31,32]. Similarly, it has been shown that the presence of the micropapillary pattern with confirmed micrometastasis confers significantly worse RFS and OS [33]. Finally, several studies have confirmed that the presence of micropapillary pattern is an independent predictor of occult mediastinal lymph node metastasis. Taken together, these observations have potential therapeutic implications for the management of early-stage lung adenocarcinoma [34].
Additional studies contributed other interesting insights about micropapillary carcinoma. Hung et al. evaluated the prognostic value of clinicopathological variables for specific organ site MFS in a cohort of 182 patients with lung adenocarcinoma and distant metastasis. The metastatic sites identified during follow up included: contralateral lung metastasis (51.1%), brain metastasis (44.5%), bone metastasis (39.0%), and liver metastasis (8.9%). The micropapillary variant in particular was strongly associated with brain metastasis (hazard ratio (HR), 2.686). Micropapillary predominant subtype (HR, 2.186) was a significant prognostic factor for decreased brain metastasis free survival (MFS). Overall, this study underscores that there are significant differences in the metastatic behavior between predominant pathological subtypes of lung adenocarcinoma, and that these findings should encourage clinicians to focus on detailed follow up strategies in these cases. Further studies are needed for complete validation [35].
Regarding treatment options, a recent study by Luo et al. in 2016 focused on patients with stage IB invasive adenocarcinomas. This study showed that adjuvant chemotherapy was associated with a better DFS [36]. In patients with micropapillary or solid patterns, adjuvant chemotherapy significantly improved DFS, but not OS [36]. The significant association between micropapillary and solid patterns and occult N2 lymph node metastasis in lung adenocarcinoma, has led researchers to hypothesize that radical mediastinal lymph node dissection may help to identify occult lymph node metastasis in these patients.
Factors correlated with poor prognosis
STAS: Tumor spread through air spaces (STAS) has been reported as a novel poor prognostic factor in the latest WHO classification. Two recent studies regarding the prognostic significance of STAS have been recently published. In one study, Morimoto et al. retrospectively reviewed the clinicopathological characteristics of 444 patients with pulmonary adenocarcinoma who underwent surgery and had tumors with micropapillary pattern and surrounding free tumor clusters in the periphery (i.e. STAS, although they used the terminology “free tumor clusters”). Morimoto et al. defined the “free tumor clusters” or STAS as three small clusters containing <20 nonintegrated micropapillary tumor cells that were within air spaces and >3 mm apart from the main tumor. In their study, a total of 31/67 patients with micropapillary tumors also had free tumor clusters. Locoregional recurrences were more common in these patients, and they had a lower five-year RFS rate compared to patients with micropapillary adenocarcinoma but no free tumor clusters [37]. In another study, Masai et. al found that STAS-positive patients commonly had tumors with the micropapillary components and with lymphovascular and pleural invasion. Notably, the presence of STAS and tumor margins less than 1.0 cm are significant risk factors for local recurrence in early disease stage [38].
Podoplanin: Tumor microenvironment has also been shown to predict disease outcome. For example, Podoplanin is a well-conserved, mucin-type transmembrane protein that has recently been shown to be positive in cancer associated fibroblasts, and is associated with lymphatic invasion and high-grade solid and/or micropapillary components constituting ≥1% of the entire tumor. Positive podoplanin was also associated with decreased DFS [39].
Stereotactic body radiation therapy (SBRT): The outcomes after stereotactic body radiation therapy (SBRT) for early-stage adenocarcinoma of the lung correlate highly with histologic subtype. Micropapillary and solid tumors portend significantly higher rates of locoregional and metastatic progression. The histologic subtype determined from core biopsies after SBRT is a prognostic factor and could have important implications for patient selection, adjuvant treatment, biopsy methods, and clinical trial design [40].
Pathologic index predicting survival:. Lee et al. aimed to determine the prognostic significance of a pathologic index designed to take into account morphological subtypes and overall tumor heterogeneity. This study used a cohort of patients and determined the HR of each pattern individually. Based on the HR of each subtype, four indices were developed [41]. The validation group consisted of 148 patients with completely resected adenocarcinomas. One of their indices enabled significant patient stratification in the validation cohort according to DFS, and showed the highest Harrell’s C index (a measure of how well binary outcomes in a logistic regression model fit). This novel pathologic index reflects tumor heterogeneity in lung adenocarcinomas and has good prognostic ability to predict survival [41].
MMP9: In a cohort of 104 cases, Yu et al. analyzed the activity of matrix metalloproteinase 9 (MMP9). Their results showed that levels of MMP9 were the highest in micropapillary and solid predominant subtypes of lung adenocarcinomas. In contrast, MMP9 levels were low in acinar and papillary subtypes, and even lower in the lepidic subtype. Multivariate analysis revealed that pathological subtype and activity of MMP9 were independent prognostic factors for DFS [42].
Mucinous variant of micropapillary carcinoma: In survival analyses, mucinous variant of micropapillary carcinoma tended to be more aggressive compared with non-mucinous micropapillary cacrcinoma. However, the prognostic value of the mucinous variant was not shown to be statistically significant in a study by Kamata et al [43].
Prognostic factors by tumor size
Tumor size ≤1 cm: Zhao et al. studied small invasive lung adenocarcinomas and found that the tumors with high heterogeneity and the presence of micropapillary component in 5% or more of the adenocarcinomas was significantly correlated with lymph node involvement and tumor recurrence. Stage IA patients who underwent limited resection had a higher risk of recurrence than did those treated by lobectomy [44].
Tumor size ≤2 cm: Yoshida et al. evaluated 21 cases and found that a micropapillary component was associated with a higher frequency of lymphatic invasion, vascular invasion, and lymph node metastasis. Additional correlation with findings on imaging techniques were performed (for detailed results please refer to the Radiology/Imaging section) [45].
Tumor size ≤3 cm: Hung et al. investigated the prognostic factors in patients with node-negative lung adenocarcinoma 3 cm or smaller to find potential candidates for adjuvant chemotherapy. Their study showed that patients with the micropapillary and/or solid predominant patterns had significantly higher risk for recurrence. This study also suggested that these patients could benefit from adjuvant therapy [46]. In completely resected lung adenocarcinoma cases of less than 3 cm, micropapillary pattern was a significant predictor of occult N2 lymph node metastasis in a study including 471 patients performed by Hung et al. [47]. Additionally, a more detailed study by Yu et al. evaluated 2,268 cases of lung adenocarcinoma (≤3 cm) and found that lymph node involvement (pN1+ pN2) was present in 3.2% patients with tumor size ≤ 1.0 cm, 14.5% patients with tumor size >1.0 cm but ≤2.0 cm, and 31.1% patients with tumor size > 2.0 cm but ≤3.0 cm. Among these histotypes, lymph node involvement was the highest in tumors with solid and micropapillary patterns (47.6% and 47.2%, respectively) [48]. Finally, Matsuoka et al. evaluated 86 patients with acinar and papillary-predominant tumors and found that solid or micropapillary components had the most significant effect on DFS and disease specific survival (DSS) in multivariate analysis [49].
Molecular features and targeted therapies: In the 1980’s, primary lung cancers were crudely divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). This distinction was largely based on the response of these two categories to different cytotoxic chemotherapeutic agents [50]. In the 2000’s, the differential response of patients with NSCLC to newer therapies such as anti-angiogenic agents and tyrosine kinase inhibitors prompted further subclassification of NSCLC [51,52]. Since then, driver mutations of lung adenocarcinoma (LADC) have been identified, and in the current era of precision medicine, detection of molecular changes is crucial to guide targeted therapies. Moreover, in patients with actionable molecular changes, the addition of selective inhibitors improves outcomes compared to standard cytotoxic chemotherapy [53-55].
Most Common Mutations and Translocations: Variable rates of EGFR, KRAS, and BRAF mutations and ALK translocations in micropapillary LADC have been reported. KRAS, EGFR, and HER2 mutations are mutually exclusive [58-61]. According to a study performed on 425 micropapillary LADC cases from a Western European cohort, the most common alterations detected, in descending order, were KRAS, EGFR, BRAF, and ALK [56]. However, a meta-analysis performed with 48 studies and 19,502 cases of LADC with an incidence rate of micropapillary carcinoma of 0.101 showed that the most common molecular alterations were EGFR mutations, followed by KRAS mutations and ALK translocations [57].
While KRAS mutation is found more commonly in male smokers [56], EGFR mutations are found more commonly in LADC in female non-smokers [55,56]. EGFR is a transmembrane glycoprotein receptor that when activated triggers the activation of a signaling cascade that drives cellular proliferation. Overexpression of EGFR in cancer cells is associated with a poor prognosis and resistance to chemotherapy and radiation. Therapeutics such as erlotinib, gefitinib, afatinib, and osimertinib, which consist of either monoclonal antibodies that target the extracellular binding domain of the EGFR receptor or small-molecule inhibitors that function by inhibiting intracellular signal transduction, have been developed to combat the proliferation effects of EGFR overexpression in cancer cells [62].
In recent studies, Matsumura et al. presented a hypothesis of the carcinogenetic pathways for the development of wild-type and mutated EGFR in LADC. Their investigation was conducted on 337 samples of resected LADC and 177 biopsy samples of surgically unresectable advanced tumors from a Japanese cohort. Their findings suggested that EGFR-mutated LADC may develop from terminal respiratory units, shown as a lepidic pattern with hobnail or spheroid cellular features, and may progress to form predominantly papillary and micropapillary patterns. In contrast, EGFR wild-type LADC may develop from central airway compartments, and shows lepidic pattern with a more columnar morphology, and progresses to form predominantly acinar and solid patterns of invasive LADC. Although papillary and micropapillary components were rarely seen in EGFR wild-type tumors, they represented a minor component and their association with the malignancy grade in EGFR wild-type tumors was not found to be statistically significant [63].
Similarly, a study performed on 107 cases from a South Korean population showed a correlation between EGFR mutation, micropapillary predominant subtype of LADC, and the presence of any amount of lepidic subtype [64]. However, similar studies done on cases from a Western cohort showed results that are not consistent with those found in East Asian cohorts. De Oliveira, et al. studied 15 cases of micropapillary LADC patients from a Western cohort and found that EGFR mutations were only present in three of the 15 cases; KRAS and BRAF mutations detected in five and three cases, respectively [60]. In another study, Suda et al. [65], investigated the heterogeneity of expression of mutant EGFR protein and EGFR gene copy number in an autopsy case. In their study, they sampled 15 tumor specimens from different sites from a patient who had extensive metastatic disease. Their results suggested that tumor progression does not lead to EGFR mutant protein and EGFR gene copy number alterations. Due to the high fidelity of the metastases with the primary tumor, they advocated for using biopsy specimens from metastatic lesions as surrogates for the primary tumor. They also documented the higher mutant-specific EGFR protein in the micropapillary components of the examined lesions compared to the non-micropapillary components.
Regarding molecular studies and clinicopathological features, Wang et al. [66], studied lung adenocarcinoma correlated with echinoderm microtubule associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion and EGFR mutations. In their study, tumors with predominantly micropapillary pattern (n = 5) contained EGFR mutation, but no EML4-ALK mutations. Additionally, another study of 100 LADC cases showed a correlation between micropapillary architecture and EGFR mutation [67]. LADC with the presence of a solid or micropapillary growth pattern and high CpG island methylation, but no EGFR mutations was associated with a lower OS [68].
Human Epidermal Receptor 2 (HER2), also known as ERBB2, is another member of the EGFR family of receptors that has no ligand receptor, but does tyrosine kinase activity [61,69]. In LADC, HER2 mutation is more common in females [70]. Micropapillary LADC, particularly mucinous type, showed higher incidences of HER2 and ALK mutations with lower incidences of EGFR mutation in two studies [58,71]. Patients with tumors harboring HER2 mutations have been shown in clinical trials to respond to several types of targeted therapies (i.e. trastuzumab, afatinib, and ado-trastuzumab, either as single agent or in combination with a cytotoxic chemotherapy) [72].
EML4-ALK fusion is the most common gene fusion found in LADC [58], yet it is still rare, especially with micropapillary growth pattern [56]. In a study from India on 240 LADC cases, 17 cases harbored ALK rearrangement, of which only one case had micropapillary predominant histology [73]. In a study of samples from 18 patients with LADC who had a primary presentation with metastatic pleural effusion, 10 cases had EGFR mutations, three had KRAS mutations, and one case showed ALK translocation [74]. The detection of ALK translocation predicts enhanced sensitivity to ALK specific tyrosine kinase inhibitors, such as crizotinib, ceritinib, and alectinib. Treatment with these targeted therapies improves outcomes in selected patients [56].
Programmed Death 1 (PD-1) and Programmed Death Ligand 1 (PD-L1): The interaction of PD-1 and PD-L1 confers immunity to cancers cells against T-cell targeted killing when the cancer cells express PD-L1. PD-L1 testing by immunohistochemistry indicates use of monoclonal antibodies against PD-1, e.g. pembrolizumab, in patients with advanced NSCLC. In their study of 476 patients with LADC, Kim et al. [75], showed that PD-L1 mRNA and protein expression in primary LADC is more frequent in tumors with higher grade micropapillary and solid patterns compared to lower grade lepidic predominant patterns. Furthermore, they showed that PD-L1 expression was associated with higher rates of KRAS mutations, but lower rates of EGFR mutations. Conversely, Kwong et al. [76], showed that of 74 LADCs studied, 53% of micropapillary and solid components showed PD-L1 membranous positivity by IHC staining, compared to 35% in the acinar and papillary components and <1% in the lepidic and invasive mucinous components. Interestingly, Takada et al. [77], showed in a study of 417 surgically resected primary LADCs that PD-L1 protein expression, detected by IHC staining, was correlated with higher histologic grades (i.e. micropapillary and solid predominant patterns), wild-type EGFR status, higher T and N stages, and presence of pleural and vascular invasions, as well as male sex and smoking history. Finally, another report by Kim et al. concluded that PD-L1 expression is also correlated with nodal metastasis [58].
Glucose Transporter 1 (GLUT1): Glucose Transporter 1 (GLUT1): Glucose transporter 1 (GLUT1) is a cell surface transporter of glucose normally found in red blood cells and the blood-brain barrier [78]. Aberrant expression of GLUT1 has been documented in several malignant neoplasms, including those of the breast, prostate, thyroid, stomach, and squamous cell carcinomas of the head and neck [79]. The expression of GLUT1 and carbonic anhydrase IX in NSCLC, in conjunction with metabolic tumor volume and total lesion glycolysis measured by positron emission tomography (PET) scanning, was assessed by Koh et al. as a prognostic marker in 269 resected NSCLC. Multivariate analysis showed that GLUT1 expression was an independent risk factor for a lower OS rate in patients with LADC. GLUT1 expression was also associated with micropapillary and solid patterns, higher rate of lymphovascular invasion, and advanced pTNM stage [80]. Glucose transporter 1 (GLUT1) is a cell surface transporter of glucose normally found in red blood cells and the blood-brain barrier [78]. Aberrant expression of GLUT1 has been documented in several malignant neoplasms, including those of the breast, prostate, thyroid, stomach, and squamous cell carcinomas of the head and neck [79]. The expression of GLUT1 and carbonic anhydrase IX in NSCLC, in conjunction with metabolic tumor volume and total lesion glycolysis measured by positron emission tomography (PET) scanning, was assessed by Koh et al. as a prognostic marker in 269 resected NSCLC. Multivariate analysis showed that GLUT1 expression was an independent risk factor for a lower OS rate in patients with LADC. GLUT1 expression was also associated with micropapillary and solid patterns, higher rate of lymphovascular invasion, and advanced pTNM stage [80].
In summary, there is wide variation in the genetic profiles that drive the growth, evolution, and development of LADC. Gene mutations and translocations can be exploited as targets for new medication that offer better outcomes for select patients. Precise molecular testing in LADC is becoming more critical to identify patient subsets that will benefit from targeted therapies. Factors like race, gender, age, and smoking history influence molecular alterations. Despite the advances in molecular testing, around 40% of all LADC cases have an unknown mutation status. Moreover, several identifiable mutations either have no targeted therapies or have targeted therapies that are in still in clinical trials. More studies are needed to explore the full molecular landscape of LADC and identify actionable mutations.
Radiology/imaging: Currently, it is well-accepted that the micropapillary component of tumors represents a distinct entity associated with higher tumor aggressiveness. In the past, it has been shown that even the most modern multimodality PET or including computed tomography (CT) imaging technology may fail to adequately visualize this important component, with relevant prognostic implications. Thus, histopathology remained the single, most crucial diagnostic tool in the surgical specimen or in preoperative biopsies or cytology [83]. Few recent studies have shown progress to address this diagnostic deficit. For example, Yoshida et al. recently demonstrated that adenocarcinoma with a micropapillary component was significantly more frequently detected with high-resolution computed tomography (HRCT) in solid nodules (17.8%, 16/90) than in either ground-glass nodules (1.5%, 1/67) or part-solid nodules (5.3%, 4/76) [45].
In a separate study, Lee et al. evaluated in 723 tumors the prognostic value of the newest classification system recommended by the IASLC/ATS/ERS together with the value of imaging biomarkers, including PET and CT. The predominant histological subtype and pattern subgroups were quantified, and the tumor-shadow disappearance ratio (TDR) on CT and maximum standardized uptake value (SUVmax) on PET were assessed. Over a period of 3.8 years of follow up, only 3.2% of cases had micropapillary predominant features. These results showed that in patients with stage I LADC, histologic subtypes and radiologic features, including TDR-4, TDR-2, and SUVmax were found to be significant predictors of DFS and OS. Particularly micropapillary, along with solid morphologies, in LADC had the lowest TDRs and the highest SUVs, both of which are associated with worse prognosis. The TDRs on CT and SUVmax on PET, along with new histologic classification schemes, appear to be promising parameters for the prognostic stratification of patients with LADCs, allowing for the triage of patients who necessitate further staging workup and adjuvant therapy [84].
Moon et al. performed a retrospective review of 350 patients that underwent curative LADC tumor resection. A total of 87% of the tumors were PN0, and 12.9% were PN1/2. Their data showed that in cases diagnosed as clinical N0 by chest CT and PET scanning, the possibility of occult lymph node metastasis increases with SUVmax greater than 5. Additionally, in those same cases, the pleural, lymphatic, and vascular invasion, as well as a micropapillary component, were more frequently observed [85].
Finally, a single study correlating mutational status of tumors and findings on imaging was reported by Wang et al. They retrospectively reviewed 153 patients underwent who surgery to treat LADC. After identifying the histological subtype, they detected EGFR mutations and retrospectively analyzed the characteristics assessed by CT in the tumor compared to a mutation-negative cohort. There findings demonstrated that EGFR mutations correlated with micropapillary morphology subtype and with air bronchograms on CT imaging. In addition, EGFR mutations were independently associated with other CT-identified characteristics, including ground-glass opacity/tumor ratio [86].
Conclusion
Our review of the literature published about micropapillary LADCs provides a current and accurate update of the field, including review articles, retrospective reviews, and studies with large patient cohorts. We included all articles identified by our search criteria dated from more than 10 years ago to evaluate how this entity was discovered and how it was accepted by researchers. In our opinion, the work of Amin et al. was crucial to assert that micropapillary carcinoma was an entity likely to be found in LADCs, and that its presence did not necessarily persist in metastatic tumors, though it is a possibility in primary tumors [6]. Early researchers found that lymph node involvement, lymphovascular/ pleural invasion, DFS, and OS were significantly worse in patients with micropapillary morphology in their tumors, and that those patients had a poor overall prognosis. To date, even more studies have continued to confirm these findings.
The initial findings about the importance of the micropapillary component on prognosis were so significant that the IASLC/ATS/ERS revised the classification of LADC by proposing new morphological criteria in order to provide a uniform diagnostic terminology for multidisciplinary patient management. This terminology was described in the most recent version of the WHO recommendations in 2014. Additional details about this subtype of morphology have emerged; however the majority are for prognostic purposes. Interestingly, we did not discover any studies that have investigated the morphologic criteria used to objectively diagnose micropapillary adenocarcinoma. However, in our experience, we have seen that there are differences in the criteria used among pathologists, even within the same institution. In our opinion, we assert that more than four cells per cluster, multiple clusters in single alveolar/lacunar space (very slender, 3-4 cell thickness), and inverted nuclear polarity is a clinically useful diagnostic triad. However, there is an unmet need to publish consensus among experts in the field and to objectively evaluate the criteria that allow high levels of concordance among pathologists.
Molecular studies have been exponentially increasing, and they present promising results regarding potential targeted therapeutic alternatives for patients. At this time, increasing the accuracy of prognosis and the OS rates remains a huge challenge in the field. Advanced radiological and imaging techniques can be helpful to determine prognostic relevance and assess potential therapeutic modalities. Nonetheless, morphology is and will likely continue to be one of the most important methods to rely on when diagnosing and predicting lung carcinoma.
- American Cancer Society (2018) Cancer Facts & Figures 2018. Atlanta: American Cancer Society. Link: https://goo.gl/GJo4QC
- Waller DA (2018) Surgical management of lung cancer with multiple lesions: implication of the new recommendations of the 8th edition of the TNM classification for lung cancer. J Thorac Dis 10: S2686-S2691. Link: https://goo.gl/bxHjRb
- Zugazagoitia J, Enguita AB, Nuñez JA, Iglesias L, Ponce S (2014) The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: current concepts and future prospects. J Thorac Dis 6: S526-536. Link: https://goo.gl/UUbe38
- Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, et al. (2011) Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 24: 653-664. Link: https://goo.gl/6uJk5Q
- Travis WD (2014) The 2015 WHO classification of lung tumors. Pathologe. 35 Suppl 2: 188. Link: https://goo.gl/qQ62oF
- Amin MB, Tamboli P, Merchant SH, Ordóñez NG, Ro J, et al. (2002) Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 26: 358-364. Link: https://goo.gl/CUvgVV
- Miyoshi T, Satoh Y, Okumura S, Nakagawa K, Shirakusa T, et al. (2003) Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 27: 101-109. Link: https://goo.gl/ftjMs3
- Tsutsumida H, Nomoto M, Goto M, Kitajima S, Kubota I, et al. (2007) A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 20: 638-647. Link: https://goo.gl/CFftrK
- Kamiya K, Hayashi Y, Douguchi J, Hashiguchi A, Yamada T, et al. (2008) Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 21: 992-1001. Link: https://goo.gl/Mv5jft
- Roh MS, Lee JI, Choi PJ, Hong YS (2004) Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology 45: 580-586. Link: https://goo.gl/NKBkmu
- Kawakami T, Nabeshima K, Makimoto Y, Hamasaki M, Iwasaki A, et al. (2007) Micropapillary pattern and grade of stromal invasion in pT1 adenocarcinoma of the lung: usefulness as prognostic factors. Mod Pathol 20: 514-521. Link: https://goo.gl/jvU34G
- Makimoto Y, Nabeshima K, Iwasaki H, Miyoshi T, Enatsu S, et al. (2005) Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi's type C tumours). Histopathology 46: 677-684. Link: https://goo.gl/PjdZuj
- Hoshi R, Tsuzuku M, Horai T, Ishikawa Y, Satoh Y (2004) Micropapillary clusters in early-stage lung adenocarcinomas: a distinct cytologic sign of significantly poor prognosis. Cancer 102: 81-86. Link: https://goo.gl/WtHavy
- Duncan LD, Jacob S, Atkinson S (2007) Fine needle aspiration cytologic findings of micropapillary carcinoma in the lung: a case report. Acta Cytol 51: 605-609. Link: https://goo.gl/9C99bP
- Kuroda N, Hamaguchi N, Takeuchi E, Ohara M, Hirouchi T, et al. (2006) Lung adenocarcinoma with a micropapillary pattern: a clinicopathological study of 25 cases. APMIS 114: 381-385. Link: https://goo.gl/uWiEXr
- Sánchez-Mora N, Presmanes MC, Monroy V, Moreno N, Lara-Martínez JM, et al. (2008) Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol 39: 324-330. Link: https://goo.gl/mTq74e
- Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, et al. (2008) Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 32: 810-827. Link: https://goo.gl/1jatqb
- Ninomiya H, Hiramatsu M, Inamura K, Nomura K, Okui M, et al. (2009) Correlation between morphology and EGFR mutations in lung adenocarcinomas Significance of the micropapillary pattern and the hobnail cell type. Lung Cancer 63: 235-240. Link: https://goo.gl/vBWHTp
- Miyoshi T, Shirakusa T, Ishikawa Y, Iwasaki A, Shiraishi T, et al. (2005) Possible mechanism of metastasis in lung adenocarcinomas with a micropapillary pattern. Pathol Int 55: 419-424. Link: https://goo.gl/fbtcFk
- Cao Y, Zhu LZ, Jiang MJ, Yuan Y (2015) Clinical impacts of a micropapillary pattern in lung adenocarcinoma: a review. Onco Targets Ther 9: 149-158. Link: https://goo.gl/yxZTVK
- Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, et al. (2010) A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 34: 1155–1162. Link: https://goo.gl/u3Wkat
- Motono N, Matsui T, Machida Y, Usuda K, Uramoto H (2017) Prognostic significance of histologic subtype in pStage I lung adenocarcinoma. Med Oncol 34: 100. Link: https://goo.gl/urix9V
- Zhao ZR, Xi SY, Li W, Situ DR, Chen KM, et al. (2015) Prognostic impact of pattern-based grading system by the new IASLC/ATS/ERS classification in Asian patients with stage I lung adenocarcinoma. Lung Cancer 90: 604-609. Link: https://goo.gl/q8Mifb
- Zombori T, Furák J, Nyári T, Cserni G, Tiszlavicz L (2018) Evaluation of grading systems in stage I lung adenocarcinomas: a retrospective cohort study. J Clin Pathol 71: 135-140. Link: https://goo.gl/kfw6wH
- Yanagawa N, Shiono S, Abiko M, Katahira M, Osakabe M, et al. (2016) The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma. J Thorac Oncol 11: 1976-1983. Link: https://goo.gl/MYACWA
- Zhao Y, Wang R, Shen X, Pan Y, Cheng C, et al. (2016) Minor Components of Micropapillary and Solid Subtypes in Lung Adenocarcinoma are Predictors of Lymph Node Metastasis and Poor Prognosis. Ann Surg Oncol 23: 2099-2105. Link: https://goo.gl/2wFknA
- Yoshiya T, Mimae T, Tsutani Y, Tsubokawa N, Sasada S, et al. (2016) Prognostic Role of Subtype Classification in Small-Sized Pathologic N0 Invasive Lung Adenocarcinoma. Ann Thorac Surg 102: 1668-1673. Link: https://goo.gl/TV2Sye
- Wang Y, Zheng D, Zheng J, Huang Q, Han B, et al. (2018) Predictors of recurrence and survival of pathological T1N0M0 invasive adenocarcinoma following lobectomy. J Cancer Res Clin Oncol 144: 1015-1023. Link: https://goo.gl/DHW2TG
- Zhao X, Zhang Y, Qian K, Zhao L, Wang W, et al. (2017) Prognostic significance of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of stage I lung adenocarcinoma: A retrospective study based on analysis of 110 Chinese patients. Thorac Cancer 8: 565-571. Link: https://goo.gl/HmDNBs
- Yuan Y, Ma G, Zhang Y, Chen H (2018) Presence of micropapillary and solid patterns are associated with nodal upstaging and unfavorable prognosis among patient with cT1N0M0 lung adenocarcinoma: a large-scale analysis. J Cancer Res Clin Oncol 144: 743-749. Link: https://goo.gl/njM1BH
- Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, et al. (2015) Non Predominant lepidic pattern correlates with better outcome in invasive lung adenocarcinoma. Lung Cancer 90: 568-574. Link: https://goo.gl/99c1pB
- Tsubokawa N, Mimae T, Sasada S, Yoshiya T, Mimura T, et al. (2016) Negative prognostic influence of micropapillary pattern in stage IA lung adenocarcinoma. Eur J Cardiothorac Surg 49: 293-299. Link: https://goo.gl/DXT7pD
- Dai C, Xie H, Kadeer X, Su H, Xie D, et al. (2017) Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 41: 1212-1220. Link: https://goo.gl/sNxkem
- Yeh YC, Kadota K, Nitadori J, Sima CS, Rizk NP, et al. (2016) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification predicts occult lymph node metastasis in clinically mediastinal node-negative lung adenocarcinoma. Eur J Cardiothorac Surg 49: e9-e15. Link: https://goo.gl/x3gLiY
- Hung JJ, Jeng WJ, Wu YC, Chou TY, Hsu WH. (2016) Factors predicting organ-specific distant metastasis in patients with completely resected lung adenocarcinoma. Oncotarget 7: 58261-58273. Link: https://goo.gl/qJMCVw
- Luo J, Huang Q, Wang R, Han B, Zhang J, et al. (2016) Prognostic and predictive value of the novel classification of lung adenocarcinoma in patients with stage IB. J Cancer Res Clin Oncol 142: 2031-2040. Link: https://goo.gl/krp8Cp
- Morimoto J, Nakajima T, Suzuki H, Nagato K, Iwata T, et al. (2016) Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 152: 64-72. Link: https://goo.gl/1S8ddc
- Masai K, Sakurai H, Sukeda A, Suzuki S, Asakura K, et al. (2017) Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 12: 1788-1797. Link: https://goo.gl/zYLi1N
- Kubouchi Y, Yurugi Y, Wakahara M, Sakabe T, Haruki T, et al. (2018) Podoplanin expression in cancer-associated fibroblasts predicts unfavorable prognosis in patients with pathological stage IA lung adenocarcinoma. Histopathology 72: 490-499. Link: https://goo.gl/mxeXcH
- Leeman JE, Rimner A, Montecalvo J, Hsu M, Zhang Z, et al. (2017) Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 97: 138-145. Link: https://goo.gl/n1SDCJ
- Lee G, Choi ER, Lee HY, Jeong JY, Ahn JH, et al. (2016) Pathologic heterogeneity of lung adenocarcinomas: A novel pathologic index predicts survival. Oncotarget 7: 70353-70363. Link: https://goo.gl/Q61sJp
- Yu Y, Ding Z, Jian H, Shen L, Zhu L, et al. (2016) Prognostic value of MMP9 activity level in resected stage I B lung adenocarcinoma. Cancer Med 5: 2323-2331. Link: https://goo.gl/bHooNn
- Kamata T, Yoshida A, Shiraishi K, Furuta K, Kosuge T, et al. (2016) Mucinous micropapillary pattern in lung adenocarcinomas: a unique histology with genetic correlates. Histopathology 68: 356-366. Link: https://goo.gl/y5GHfs
- Zhao W, Wang H, Xie J, Tian B (2018) A Clinicopathological Study of Small Lung Adenocarcinoma 1 cm or Less in Size: Emphasis on Histological Subtypes Associated With Lymph Node Metastasis and Recurrence. Int J Surg Pathol 26: 4-11. Link: https://goo.gl/UNK4JM
- Yoshida Y, Nitadori JI, Shinozaki-Ushiku A, Sato J, Miyaji T, et al. (2017) Micropapillary histological subtype in lung adenocarcinoma of 2 cm or less: impact on recurrence and clinical predictors. Gen Thorac Cardiovasc Surg 65: 273-279. Link: https://goo.gl/i6o9za
- Hung JJ, Yeh YC, Wu YC, Chou TY, Hsu WH (2017) Prognostic Factors in Completely Resected Node-Negative Lung Adenocarcinoma of 3 cm or Smaller. J Thorac Oncol 12: 1824-1833. Link: https://goo.gl/jTJj8f
- Hung JJ, Yeh YC, Jeng WJ, Wu YC, Chou TY, et al. (2016) Factors predicting occult lymph node metastasis in completely resected lung adenocarcinoma of 3 cm or smaller. Eur J Cardiothorac Surg 50: 329-336. Link: https://goo.gl/YqXdCC
- Yu Y, Jian H, Shen L, Zhu L, Lu S (2016) Lymph node involvement influenced by lung adenocarcinoma subtypes in tumor size ≤3 cm disease: A study of 2268 cases. Eur J Surg Oncol 42: 1714-1719. Link: https://goo.gl/ACZaXU
- Matsuoka Y, Yurugi Y, Takagi Y, Wakahara M, Kubouchi Y, et al. (2016) Prognostic Significance of Solid and Micropapillary Components in Invasive Lung Adenocarcinomas Measuring ≤3 cm. Anticancer Res 36: 4923-4930. Link: https://goo.gl/tpNfgt
- Albert A (1974) Selective toxicity. 5th ed. London: Chapman and Hall; 1973. Link: https://goo.gl/zFjJVG
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92-98. Link: https://goo.gl/QzhctM
- Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, et al. (2004) Jun Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22: 2184-2191. Link: https://goo.gl/De5241
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med 361: 947–957. Link: https://goo.gl/KGPtet - Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, et al. (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363: 1693–1703. Link: https://goo.gl/FwiapD
- Guo Z, Yi F, Yin W, Zhang Y, Li Q, et al. (2017) Clinical value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma. Thorac Cancer 8: 159-169. Link: https://goo.gl/1fKHku
- Warth A, Penzel R, Lindenmaier H, Brandt R, Stenzinger A, et al. (2014) EGFR, KRAS, BRAF and ALK gene alterations in lung adenocarcinomas: patient outcome, interplay with morphology and immunophenotype. Eur Respir J 43: 872-883. Link: https://goo.gl/fwij7n
- Pyo JS, Kim JH (2018) Clinicopathological Significance of Micropapillary Pattern in Lung Adenocarcinoma. Pathol Oncol Res 24: 547-555. Link: https://goo.gl/9wYYJa
- Kim J, Jang SJ, Choi CM, Ro JY (2016) Correlation of Histologic Subtypes and Molecular Alterations in Pulmonary Adenocarcinoma: Therapeutic and Prognostic Implications. Adv Anat Pathol 23: 330-338. Link: https://goo.gl/RWhXj5
- Casali C, Rossi G, Marchioni A, Sartori G, Maselli F, et al. (2010) A Single Institution-Based Retrospective Study of Surgically Treated Bronchioloalveolar Adenocarcinoma of the Lung: Clinicopathologic Analysis, Molecular Features, and Possible Pitfalls in Routine Practice. J Thorac Oncol 5: 830-836. Link: https://goo.gl/R2GMSY
- De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA (2009) Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol 131: 694-700. Link: https://goo.gl/8vBaiy
- Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, et al. (2012) Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 18: 4910-4918. Link: https://goo.gl/t5TAzB
- Herbst RS (2004) Review of epidermal growth factor receptor biology. International Journal of Radiation Oncology • Biology • Physics 59: S21 - S26. Link: https://goo.gl/CMJEEa
- Matsumura M, Okudela K, Kojima Y, Umeda S, Tateishi Y, et al. (2016) A Histopathological Feature of EGFR-Mutated Lung Adenocarcinomas with Highly Malignant Potential - An Implication of Micropapillary Element. PLoS One 11: e0166795. Link: https://goo.gl/3nnGxT
- Shim HS, Lee DH, Park EJ, Kim SH (2011) Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 135: 1329-1334. Link: https://goo.gl/1SNg8V
- Suda K, Murakami I, Yu H, Ellison K, Shimoji M, et al. (2016) Heterogeneity of EGFR Aberrations and Correlation with Histological Structures: Analyses of Therapy-Naive Isogenic Lung Cancer Lesions with EGFR Mutation. J Thorac Oncol 11: 1711-1717. Link: https://goo.gl/B8q1Wp
- Wang H, Zhang W, Wang K, Li X (2018) Jun Correlation between EML4-ALK, EGFR and clinicopathological features based on IASLC/ATS/ERS classification of lung adenocarcinoma. Medicine (Baltimore) 97: e11116. Link: https://goo.gl/Hp63nw
- Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, et al. (2008) Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 32: 810-827. Link: https://goo.gl/xatGSb
- Koh YW, Chun SM, Park YS, Song JS, Lee GK, et al. (2016) Association between the CpG island methylator phenotype and its prognostic significance in primary pulmonary adenocarcinoma. Tumour Biol 37: 10675-10684. Link: https://goo.gl/7CsALB
- Iqbal N, Iqbal N (2014) Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int 852748. Link: https://goo.gl/DG5Veh
- Pillai RN, Behera M, Berry LD, Rossi MR, Kris MG, et al. (2017) HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 123: 4099-4105. Link: https://goo.gl/9Pg6TG
- Kamata T, Yoshida A, Shiraishi K, Furuta K, Kosuge T, et al. (2016) Mucinous micropapillary pattern in lung adenocarcinomas: a unique histology with genetic correlates. Histopathology 68: 356-366. Link: https://goo.gl/k9wcws
- Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, et al. (2013) Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31: 1997-2003. Link: https://goo.gl/xQ7mqN
- Bal A, Singh N, Agarwal P, Das A, Behera D. (2016) ALK gene rearranged lung adenocarcinomas: molecular genetics and morphology in cohort of patients from North India. APMIS 124: 832-838. Link: https://goo.gl/x1VdC9
- Rodriguez EF, Shabihkhani M, Carter J, Maleki Z (2017) Molecular Alterations in Patients with Pulmonary Adenocarcinoma Presenting with Malignant Pleural Effusion at the First Diagnosis. Acta Cytol 61: 214-222. Link: https://goo.gl/NwsBPi
- Kim H, Kwon HJ, Park SY, Park Y, Park E, et al. (2018) Clinicopathological analysis and prognostic significance of programmed cell death-ligand 1 protein and mRNA expression in non-small cell lung cancer. PLoS One 13: e0198634. Link: https://goo.gl/cWUi14
- Ng Kee Kwong F, Laggner U, McKinney O, Croud J, Rice A, et al. (2018) Expression of PD-L1 correlates with pleomorphic morphology and histological patterns of non-small-cell lung carcinomas. Histopathology 72: 1024-1032. Link: https://goo.gl/tqws5Y
- Takada K, Okamoto T, Shoji F, Shimokawa M, Akamine T, et al. (2016) Clinical Significance of PD-L1 Protein Expression in Surgically Resected Primary Lung Adenocarcinoma. J Thorac Oncol 11: 1879-1890. Link: https://goo.gl/D3zoHT
- Olson AL, Pessin JE. (1996) Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr 16: 235-256. Link: https://goo.gl/PHHWu2
- Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, et al. (2011) GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo) 66: 965-972. Link: https://goo.gl/yqWpGU
- Koh YW, Lee SJ, Park SY (2017) Differential expression and prognostic significance of GLUT1 according to histologic type of non-small-cell lung cancer and its association with volume-dependent parameters. Lung Cancer 104: 31-37. Link: https://goo.gl/AzHWfM
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, et al. (2002) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37: 375-536. Link: https://goo.gl/ukSXyB
- Vandooren J, Van den Steen PE, Opdenakker G (2013) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol 48: 222-272. Link: https://goo.gl/qckSnK
- Prior JO, Stupp R, Christodoulou M, Letovanec I (2010) Micropapillary pattern in lung adenocarcinoma: aspect on 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging. Interact Cardiovasc Thorac Surg 10: 144-145. Link: https://goo.gl/kXHFht
- Lee HY, Lee SW, Lee KS, Jeong JY, Choi JY, et al. (2015) Role of CT and PET Imaging in Predicting Tumor Recurrence and Survival in Patients with Lung Adenocarcinoma: A Comparison with the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma. J Thorac Oncol 10: 1785-1794. Link: https://goo.gl/8hrwv1
- Moon Y, Kim KS, Lee KY, Sung SW, Kim YK, et al. (2016) Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcisnoma. Ann Thorac Surg 101: 1928-1935. Link: https://goo.gl/4Rr5ZB
- Wang D, Yan N, Yang X, Ge Y, Xu D, et al. (2018) Correlation between epidermal growth factor receptor mutation and histologic subtypes or characteristics of computed tomography findings in patients with resected pulmonary adenocarcinoma. J Cancer Res Ther 14: 240-244. Link: https://goo.gl/nqqAi1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
