Archives of Pulmonology and Respiratory Care
Influence of the duration of noninvasive ventilation on the cardiorespiratory indicators of preterm infants: A randomized clinical trial
Carmen Silveira ST1, Kamila Leonardi M2*, Ana Paula Melo CF1, José Zaia E2 and Marisa Brunherotti AA2*
2University of Franca – Avenida Dr. Armando de Sáles Oliveira, 201 – Parque Universitario, cidade de Franca, São Paulo, CEP 14404-600 - Brazil
Cite this as
Carmen Silveira ST, Kamila Leonardi M, Ana Paula Melo CF, José Zaia E, Marisa Brunherotti AA (2018) Influence of the duration of noninvasive ventilation on the cardiorespiratory indicators of preterm infants: A randomized clinical trial. Arch Pulmonol Respir Care 4(1): 015-019. DOI: 10.17352/aprc.000034This study aimed to evaluate the cardiorespiratory indicators of preterm infants submitted to two noninvasive ventilator support systems in two periods. It was an controlled randomized clinical trial (RBR-7d9dth). Fifty-two newborns (gestational age of 30.6±2.3 weeks, weight of 1,366±445 g) submitted to continuous positive airway pressure (CPAP) therapy were studied. The infants were randomly allocated to two groups: 31 children received CPAP therapy for 48 hours and 21 children for 72 hours. The respiratory rate, heart rate, oxygen saturation and Silverman-Andersen Score were recorded once a day. Three measurements were obtained in a 15 minutes intervals. The infants were monitored during the predetermined period of noninvasive ventilation and for 24 hours after pressure support withdrawal. The Student t-test was used for comparison of the variables between groups after normality evaluation by the Kolmogorov-Smirnov test and significance was considered when p<0.05. It was found that the birth characteristics were homogenous, without significant differences between groups. The cardiorespiratory indicators did not differ significantly between groups, but better mean values were observed for the 72-hour group. There was a difference in the respiratory rate of 5.0 breaths per minute and the heart rate was 7.8 bpm, while oxygen saturation was similar in the two groups.
Conclusions: Neither the duration of noninvasive ventilation of 48 or 72 hours nor the nasal CPAP and nasal intermittent positive pressure had an influence on the cardiorespiratory indicators of preterm infants. However, the period of 72 hours resulted in lower respiratory and heart rates.
Abbreviations
CPAP: Nasal Continuous Positive Airway Pressure; SAS: Silverman-Anderson Score
Introduction
Nasal continuous positive airway pressure (CPAP) has been valued as a beneficial strategy in neonatal intensive care units. Since nasal CPAP is a noninvasive ventilation method, it reduces the inflammatory response in the lung parenchyma [1], decreases the failure rates of extubation [2] and reintubations [3], minimizes lung injury [4-6] and reduces respiratory problems such as apnea and respiratory acidosis, as well as the use of supplemental oxygen [7]. Despite the known benefits of nasal CPAP in preterm infants, there is a lack of specific knowledge about the parameters adopted [8], modalities used [9] and effects of different interfaces [10,11]. The duration of noninvasive ventilation is also a factor of interest since the literature has shown important variability in the effects of different durations [12,13].
The duration of nasal CPAP in newborns, a lower gestational age and birthweight are directly related to the risk of nasal trauma [14,15]. So far, no studies have addressed the relationship between the duration of noninvasive ventilation and cardiorespiratory response. Part of the scientific studies have focused on the relationship the ventilator support and body position [16-18].
Out hypothesis was that cardiorespiratory indicators in premature newborns had better values when premature infants are submitted to longer periods of noninvasive support and nasal intermittent positive-pressure in better mode.
The purpose of the present study was to evaluate the cardiorespiratory indicators of preterm infants submitted to two noninvasive ventilator support systems in two periods.
Patients And Methods
This was a single-blind, two-arm parallel group, randomized clinical trial conducted at the Infant Intensive Care Center of a public tertiary hospital. The study was approved by the Ethics Committee of the same institution (No. 070/2009) and was registered in the Brazilian Clinical Trials Registry (RBR-7d9dth). The infants were included in the study after the parents or legal guardians had signed the free informed consent form.
Eighty preterm infants with a birthweight ranging from 540 to 2,450 g and a gestational age of 24 to 36 weeks, who received noninvasive ventilation support (CPAP) through a silicon nasal prong (Hudson® RCI Infant Nasal Prong CPAP cannula system, Teleflex, Inc., USA), were eligible. Children with heart diseases, congenital malformations, hydrocephalus and surgical indications, and newborns who did not complete the selected period of nasal CPAP were excluded.
Study protocol
Noninvasive ventilation was administered with a mechanical breathing system consisting of an expiratory valve for the control of positive expiratory pressure and a mixer for the oxygen fraction. For nasal CPAP, a continuous flow of 7 to 8 l/min, a positive expiratory pressure of 5 cmH2O, and an oxygen fraction of 21 to 40% were used. The following parameters were adopted for CPAP with nasal intermittent positive pressure: continuous flow (7 to 8 l/min), positive expiratory pressure (5 cmH2O), inspiratory pressure (15 cmH2O), controlled respiratory rate (14 breaths per minute), time of inspiration (0.6 s), and oxygen fraction (21 to 40%).
The children were allocated randomly to the groups using sealed envelopes containing the identification of the method (Nasal CPAP and Nasal intermittent positive-pressure) stratified into two times (48 and 72 hours).
The following cardiorespiratory variables were collected: respiratory rate determined by observing the number of chest movements over one minute timed with a chronometer, heart rate and oxygen saturation evaluated noninvasively with a Dixtal monitor (DX2010), and respiratory distress using the Silverman-Anderson Score (SAS), which ranges from 0 (no respiratory distress) to 10 (maximum respiratory distress). The parameters analyzed were: grunting, nasal flaring, intercostal retractions, xiphoid retractions, and synchrony of chest and abdominal movements.
The variables were collected once a day at the same time. Three measurements were obtained at intervals of 15 minutes. The infants were monitored during the predetermined period of ventilatory support (48 and 72 hours) and 24 hours after pressure support withdrawal.
This was a non-probability sample since the group had homogenous birth characteristics. The birth characteristics are reported descriptively as the mean, standard deviation, and relative frequency. The Student t-test was used for comparison of the variables between groups after normality evaluation by the Kolmogorov-Smirnov test and WilcoxonMann-Whitney test for non-parametric variables. All analyses were performed using the GraphPad InStat software (version 3.06 for Windows) and significance was considered when p<0.05.
Results
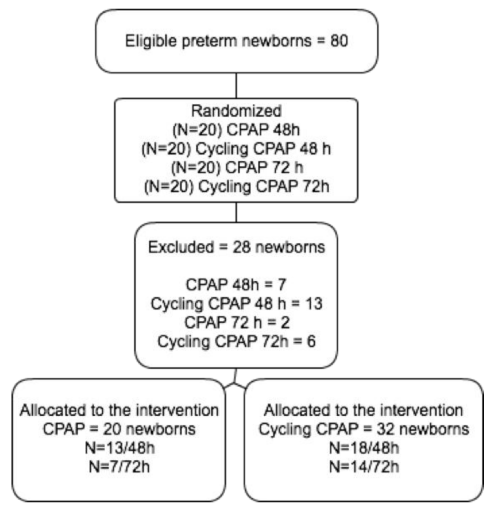
During the study, 28 newborns were excluded because they did not complete the preestablished period of noninvasive ventilator support. The final sample consisted of 52 preterm infants that received noninvasive ventilatory support [Figure 1].
The two groups submitted to 48 and 72 hours of noninvasive ventilation were homogenous in terms of their birth characteristics, with no significant difference in the variables analyzed (Weight, gestational age, male, antenatal corticoid, previous mechanical ventilation and surfactant), p>0.5. The only variable that differed significantly between groups, but without a direct influence on the outcome, was the frequency of cesarean delivery, which was 21.3% higher in the 72-hour group (p=0.027).
In general, comparison during and in the first 24 hours after pressure support withdrawal showed no significant difference in the respiratory rate between the 48- and 72-hour groups or between modalities. However, mean respiratory rates tended to be lower in the 72hour group. Separate analysis showed a significant difference in the respiratory rate between the 48-hour and the 72-hour cycling CPAP groups in the first 24 hours after pressure support withdrawal (p=0.029), with a difference of 5.2 breaths per minute in the 72-hour group. A significant difference during and after noninvasive ventilation was only observed when the 48hour CPAP group was compared to the 72-hour cycling CPAP group. The difference between mean respiratory rates was about 6 breaths per minute, with a lower rate in the 72-hour cycling CPAP group [Table 1].
The maximum boletim Silverman Anderson did not exceed 1.5 and was similar in the groups monitored. A significant difference was only observed for the 72-hour CPAP group when the cycling and conventional groups were compared after weaning (p=0.024), but this difference had no clinical repercussions.
The heart rate showed a similar behavior when the periods of 48 and 72 hours and the nasal CPAP modalities (with and without cycling) were compared. The mean heart rates were within the expected range for age and did not result in undesired clinical repercussions [Table 2].
Oxygen saturation did not differ significantly between infants submitted for 48 and 72 hours to nasal CPAP and nasal intermittent positive pressure for 48 and 72 hours. There was also no difference when the period of noninvasive ventilation and the first 24 hours after weaning were compared. A significant difference was only observed between the groups submitted to CPAP for 48 hours with and without intermittent positive pressure (p=0.001). However, the mean oxygen saturation values were similar in the groups and within the normal range. All groups exhibited desirable mean oxygen saturation levels [Table 3].
Discussion
Noninvasive ventilation of newborns with respiratory distress before extubation is a recognized and valued practice in intensive care units. However, there are no reports in the scientific literature investigating the influence of the duration of noninvasive ventilation on neonatal cardiorespiratory indicators. Evidence indicates wide variation ranging from hours to weeks in the duration of nasal CPAP among newborns [12,13].
The relationship between a longer duration of the CPAP device in the nostrils and nasal trauma has been recognized [19]. The occurrence of nasal bleeding has been shown to be directly proportional to the duration of nasal CPAP therapy in preterm infants, with the observation of a higher risk after 48 hours of therapy [13].
In the present study investigating the duration and modality of noninvasive ventilation, we found no important difference in the mean values of the cardiorespiratory variables studied. Thus, the infants showed a similar behavior during the two periods and in the two modalities.
Although the cardiorespiratory variables were within the desired range for age in the present study, infants submitted to noninvasive ventilation for 72 hours tended to have lower mean respiratory rates, with a difference of up to 5.9 breaths per minute during noninvasive ventilation. The same trend was observed in the first 24 hours after weaning from the pressure support, with a difference of up to 5.2 breaths per minute. There was also a trend towards lower respiratory rates in the 72-hour intermittent positive pressure CPAP group. The sample studied exhibited mean SAS scores less than 1.5 and the lowest mean score was observed in the 72-hour intermittent positive pressure CPAP group (0.6), demonstrating that the infants were not experiencing respiratory distress. This result can be explained by the influence of positive expiratory pressure through noninvasive ventilation. However, the infants exhibited a low Boletim Silverman Andersen even after pressure support withdrawal, irrespective of the duration of noninvasive ventilation.
Respiratory rate responses of preterm infants have been demonstrated under different clinical conditions. Studies have shown lower mean respiratory rates in newborns in the prone position before and after feeding [20], during tube feeding [21], in oxygen-dependent infants [22], and during quiet sleep [23]. In the present study, despite the lack of significant differences between groups, lower respiratory rates were observed during the period of 72 hours of noninvasive ventilation and in the intermittent positive pressure modality.
The literature indicates that the heart rate also responds to neonatal conditions. Lower mean heart rates of newborns have been demonstrated in the supine position, during active sleep [24], during spontaneous breathing [25] and during ventilator support [17]. However, the infants studied here exhibited mean heart rates expected for age. The noninvasive ventilation modality and the duration of pressure support did not influence the heart rate of the groups. The threshold of 170 bpm was observed in only one infant during noninvasive ventilation and in five infants after withdrawal of the pressure support. The latter may have been due to support withdrawal and not to the modality and/or duration of ventilation.
Oxygen saturation did also not differ between the groups submitted to noninvasive ventilation for 48 and 72 hours. The oxygen mixer was part of the nasal CPAP system and the inspiratory fraction used ranged from 21 to 40%. This inhaled fraction of oxygen may have directly influenced the oxygen saturation values obtained, since there was no effect of the duration of noninvasive ventilatory support. Another important factor is the positive expiratory pressure provided by the nasal CPAP prong, which increases the alveolar area and consequently the functional residual capacity, optimizing gas exchange and increasing saturation [13]. It was therefore expected that oxygen saturation was influenced by these factors. However, the infants continued to exhibit desirable values after weaning from noninvasive ventilation and no significant difference was observed between groups.
However, some limitations of the study should be mentioned. The sample size of each separate group was small and the results therefore only apply to this profile of preterm infants. Furthermore, the infants received pressure support for 48 and 72 hours, a period during which the respiratory system achieves greater balance of ventilatory mechanics. Future studies should therefore include a group submitted to noninvasive ventilation for 24 hours.
Conclusion
In conclusion, the results suggest no advantages of noninvasive ventilation for 48 and 72 hours or of the intermittent positive pressure and conventional modality of nasal CPAP in preterm infants. However, the period of 72 hours resulted in lower respiratory and heart rates.
We thank the group of professionals of the Pediatric Intensive Care Unit of the Santa Casa de Franca.
Authors’contributions
Carmen Silveira, participated in the development of the protocol, contributed to the writing of the manuscript, had primary responsibility for protocol development, patient screening, entollment, outcome assessment and writing the manuscript Kamila Leonardi and Ana Paula Melo, participated in the development of the protocol, had primary responsiblity for protocol development, patient screening, enrollment, outcome assessment José Zaia, analytical framework for the study Marisa Brunherotti, supervised the design and execution of the study, performed the final data analyses and contributed to the writing of the manuscript.
All authors read and approved the final manuscript
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study wsa approved by Ethics Committee of the University of Franca (number 070/2009, Feb 2010) and was registered in the Brazilian Clinical Trials Registry (RBR-7d9dth).
- Carvalho CG, Silveira RC, Procianoy RS (2013) Ventilator-induced lung injury in preterm infants. Rev Bras Ter Intensiva 25: 319-326. Link: https://goo.gl/hWdZqk
- Gupta P, Kuperstock JE, Hashmi S, Arnolde V, Gossett JM, et al. (2013) Efficacy and predictors of success of noninvasive ventilation for prevention of extubation failure in critically ill children with heart disease. Pediatr Cardiol 34: 964-977. Link: https://goo.gl/BvP2nK
- Mayordomo-Colunga J, Medina A, Rey C, Concha A, Menéndez S, et al. (2010) Noninvasive ventilation after extubation in paediatric patients: a preliminary study. BMC Pediatr 5: 10-29. Link: https://goo.gl/B9vZYB
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. (2010) Early CPAP versus surfactant in extremely preterms infants. N Engl J Med 362: 1970-1979. Link: https://goo.gl/iJfHLv
- Jatana KR, Oplatek A, Stein M, Philips G, Kang DR, et al. (2010) Effects of nasal continuous positive airway pressure and cannula use in the neonatal intensive care unit setting. Arch Otolaryngol Head Neck Surg 136: 287-291. Link: https://goo.gl/s7fKL7
- Winter JP, Vries MA, Zimmermann LJ (2010) Clinical practice: noninvasive respiratory support in newborns. Eur J Pediatr 169: 777-782. Link: https://goo.gl/4hdbdT
- Davis PG, Henderson-Smart DJ (2010) Nasal continuous positive airway pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews. In: The Cochrane Library. 10: CD000143. Link: https://goo.gl/JeFngY
- Medeiros SK, Carvalho WB, Soriano CF (2012) Practices of use of nasal intermittent positive pressure ventilation (NIPPV) in neonatology in northeastern Brazil. J Pediatr (Rio J) 88: 48-53. Link: https://goo.gl/X9rcVc
- Ricotti A, Salvo V, Zimmermann LJ, Gavilanes AW, Barberi I, et al. (2013) N-SIPPV versus bi-level N-CPAP for early treatment of respiratory distress syndrome in preterm infants. J Matern Fetal Neonatal Med 26: 1346-1351. Link: https://goo.gl/ns7BKM
- Newnam K, McGrath JM, Salyer J, Estes T, Jallo N, et al. (2015) A comparative effectiveness study of continuous positive airway pressure-related Skin breakdown when using different nasal interfaces in the extremely low birth weight neonate. Appl Nurs Res 28: 36-41. Link: https://goo.gl/iJmd8q
- Chandrasekaran A, Thukral A, Jeeva Sankar M, Agarwal R, Paul VK,et al. (2017) Nasal masks or binasal prongs for delivering continuous positive airway pressure in preterm neonates a randomised trial. Eur J Pediatr 176: 379-386. Link: https://goo.gl/VgsRaF
- Kamper J, Ringsted C (1990) Early treatment of idiopathic respiratory distress syndrome using binasal continuous positive airway pressure. Acta Paediatr Scand 79: 581-586. Link: https://goo.gl/Xab8xA
- Rego MAC, Martinez FE (2000) Clinical and laboratorial repercussions of nasal CPAP in preterm newborns. J Pediatr 76: 339-348. Link: https://goo.gl/fg46Pm
- Ficher C, Bertelle V, Hohlfeld J, Forcada-Guex M, Stadelmann-Diaw C, et al. (2010) Nasal trauma due to continuous positive airway pressure in neonates. Arch Dis Child Fetal Neonatal 95: 447-451. Link: https://goo.gl/1xPg4B
- Maruccia M, Fanelli B, Ruggieri M, Onesti MG (2014) Necrosis of the columella associated with nasal continuous positive airway pressure in a preterm infant. International Wound Journal 11: 335-336. Link: https://goo.gl/Jzu3YW
- Pickerd N, Williams EM, Watkin, WJ, Kotecha S (2014) Tidal breathing in preterm infants receiving and weaning from continuous positive airway pressure. Journal of Pediatrics 164: 1058-1063. Link: https://goo.gl/FQGXUt
- Hough J, Trojman A, Schiber A (2016) Effect of time and body position on ventilation in premature infants. Pediatric Research 80: 499-504. Link: https://goo.gl/jGexCG
- Ghorbani F, Asadollahi M, Valizadeh S (2013) Comparison the effect of sleep positioning on cardiorespiratory rate in noninvasive ventilated premature infants. Nurs Midwifery Stud 2: 182-187. Link: https://goo.gl/w839XV
- Yong SC, Chen SJ, Boo NY (2005) Incidence of nasal trauma associate with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birthweight infants: a randomized control study. Arch Dis Child Fetal Neonat 90: 480-483. Link: https://goo.gl/KZsHwE
- Mizuno K, Itabashi K, Okuyama K (1995) Effect of body position on the blood gases and ventilation volume of infants with chronic lung disease before and after feeding. Am J Perinatol 12: 275–277. Link: https://goo.gl/9gZBfT
- Padua G, Martinez EZ, Brunherotti MAA (2009) Cardiorespiratory effects of body position in preterm newborns submitted to an increase in gastric volume. Arq Gastroenterol 46: 321–327. Link: https://goo.gl/DVFvuY
- Leipälä JA, Bhat GF, Rafferty SH, Greenough A (2003) Effect of posture on respiratory function and drive in preterm infants prior to discharge. Pediatr Pulmonol 36: 295-300. Link: https://goo.gl/EZbjcY
- Elder DE, Campbell AJ, Larsen PD, Galletly D (2011) Respiratory variability in preterm and term infants: Effect of sleep state, position and age. Respiratory Physiology & Neurobiology 75: 234-238. Link: https://goo.gl/vJNq5Q
- Ammari A, Schulze KF, Ohira-Kist K (2009) Effects of body position on thermal, cardiorespiratory and metabolic activity in low birth weight infants. Early Hum Dev 85: 497-501. Link: https://goo.gl/3x1E9n
- Heimann K, Vaeben P, Peschgens T, Stanzel S (2010) Impact skin to skin care, prone and supine positioning on cardiorespiratory parameters and thermoregulation in premature infants. Neonatology 97: 311-317. Link: https://goo.gl/75qEKQ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
