Archives of Pulmonology and Respiratory Care
An atypical Klippel-Treanunay-Weber syndrome
Muammer Bilir1, Cuneyt Tetikkurt2* and Halil Yanardag1
2Professor, MD, Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul University, Turkey
Cite this as
Bilir M, Tetikkurt C, Yanardag H (2018) An atypical Klippel-Treanunay-Weber syndrome. Arch Pulmonol Respir Care 4(1): 012-014. DOI: 10.17352/aprc.000033A 44 year old female was referred for hyperbaric oxygen treatment of leg ulcers. The patient had port-wine stain, hand vitiligo, hypertrophy of one extremity, collateral abdominal, and varicose leg veins. Calcium was 12 mg/dL and PTH was 309 ng/L. Hepatomegaly, deep vein thrombosis of the right leg, transudative ascites, hypersplenism, parathyroid adenoma, mesenteric cyst, and papillary thyroid carcinoma were identified. The final clinical diagnosis was Klippel-Treanunay-Weber syndrome.
Several theories such as intrauterine damage to sympathetic ganglia, deep vein abnormalities, mesodermal and ectodermal dysplasia exist for the pathogenesis of this syndrome. This is the first case of Klipel-Treanunay-Weber syndrome with coexisting vitiligo, hypersplenism, non-chirrotic hepatic fibrosis, a mesenteric cyst, a parathyroid adenoma, and a papillary thyroid carcinoma. The vitiligo, mesenteric cyst, parathyroid adenoma, and the papillary thyroid carcinoma are associated with the genetic abnormalities of the syndrome while hypersplenism and hepatic fibrosis are the late complications the disease.
Introduction
KTW [Klippel-Trenaunay-Weber (KTW)] syndrome is a complex congenital disorder that has been defined as the triad of capillary malformation, venous malformation, and limb overgrowth [1-4]. Although in most cases the syndrome is sporadic, an occasional familial occurence has been reported [5,6]. Various genetic defects arise as the eventual causative factors for the KTW syndrome, like the overexpression of the angiogenic factor VG5Q and the de novo supernumerary ring chromosome 18 [7,8]. Most patients with KTW carry postzygotic somatic mutations in the PIK3CA [phosphatidylinositol-4, 5-bisphospate 3-kinase (PIK3CA)], catalytic subunit alpha gene [9]. These mutations activate PKB/AKT [protein kinase B/AKT (PKB/AKT)] and mTOR [mammalian target of rapamycin (mTOR)] that eventuate in cell proliferation and angiogenesis. Such genetic mutations have alson been delineated in epidermal nevi and skeletal anomalies CLOVES [Congenital, Lipomatous, Overgrowth, Vascular Malformations, Epidermal Nevi and Spinal/Skeletal Anomalies and/or Scoliosis syndrome (CLOVES)], FAVA [fibro-adipose vascular anomaly (FAVA)], and MCAP [Megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP)] syndrome [9-11].
We report a case of Klippel-Trenaunay-Weber (KTW) syndrome presenting with different clinical findings from the classical patient profile. The patient has unique clinical manifestations that differ from the classical aspectcts of the syndrome depending on the variable genetic mutations. The term PROS [PIK3CA-related overgrowth spectrum (PROS)] may encompass these distinct overlapping conditions that may better describe such patients.
Case Report
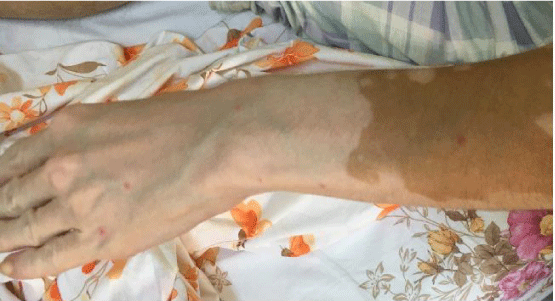
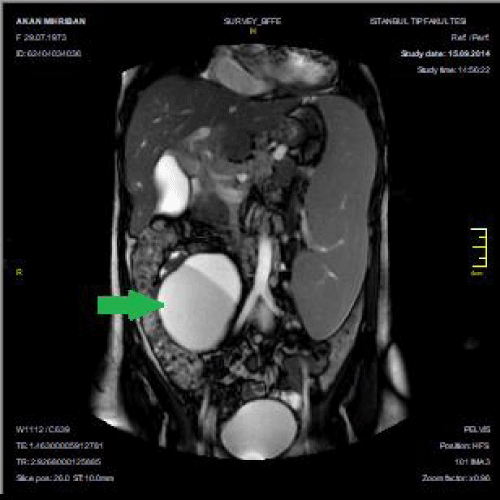
A 44 year old female was admitted for hyperbaric oxygen treatment of leg ulcers. On inspection, port-wine stains (Figure 1), vitiligo of the left hand (Figure 2), varicose leg and abdominal collateral veins were observed. Serum biochemistry, and urine analysis were normal but Ca was 12 mg/dL and PTH was 309 ng/L. Chest x-ray showed bilateral hilar lymphadenopathy. Tuberculine test was negative. ECG revealed a sinus rhytm of 84/minute. Abdominal CT showed vena hepatica thrombosis, ascites, and hepatosplenomegaly. Histopathologic examination of the liver biopsy identified non-chirrotic hepatic fibrosis due to portal hypertension. Heterogenous prothrombine gen mutation was positive. Bone marrow biopsy depicted normal cellullar elements. The low platelet count of 60x103/mm3 was compatible with hypersplenism. The ascites was transudative due to portal hypertension casused by vena hepatica thrombosis. MRI revealed a 10 cm mesenteric cyst (Figure 3). Ultrasonography showed a thyroid and a parathyroid nodule. Histopathology of the resected thyroid gland was compatible with papillary thyroid carcinoma and parathyroid adenoma. Deep venous thrombosis of the right leg was detected by Doppler ultrasound and enoxaparin 6000 U/day was started. Abdominal lymph node biopsy pathology revealed non-necrotizing granulomatous inflammation. Transbronchial lung and lip biopsy were negative. Serum ACE was 20 U/L. The final diagnosis was Klippel-Treanunay-Weber syndrome with coexisting vitiligo of the hand, hypersplenism, non-chirrotic hepatic fibrosis, parathyroid adenoma, and thyroid papillary carcinoma. The patient is currently under follow-up with ongoing enoxaparin treatment.
Discussion
Klippel-Trenaunay-Weber syndrome is a triad of port-wine stains, varicose veins, and bony and soft tissue hypertrophy involving an extremity. Klippel and Trenaunay first described the syndrome in two patients presenting with a port-wine stain and varicosities of an extremity associated with bone and soft tissue of limb [1,2]. Parkes Weber reported a patient with these symptoms and an arteriovenous malformation of an extremity [3,4]. Currently, it is conflicting how to designate these two syndromes as arterivenous malformation can lead to a significant prognostic outcome. Exact cause of Klippel-Trenaunay-Weber syndrome is unknown. The syndrome may be due to intrauterine damage to the sympathetic ganglia or intermediolateral tract leading to dilated microscopic arteriovenous anastomoses [12], to deep vein abnormalities with resultant obstruction of venous flow leading to venous hypertension and the development of varices with limb hypertrophy [13], to a mesodermal defect during fetal development that causes maintenance of microscopic arteriovenous communications [14], or to an underlying mixed mesodermal and ectodermal dysplasia [15].
Most cases are sporadic but may carry a an autosomal dominant pattern of inheritance [16]. A case KTW syndrome with an unaffected twin supports the paradominant genetic pattern [17]. The affiliation amid AGGF1 [Angiogenic factor with G patch and FHA domains 1 (AGGF1)] and KTW appears to be significant while common AGGF1 versions may lead to enhanced risk for this syndrome [18]. Kihiczak has reported that KTW syndrome may result from a pathogenic vascular and tissue overgrowth gene [19]. The syndrome has a complex disease pattern and different genetic mechanisms play a role in the development of this genetic disorder leading to variable clinical manifestations that may create a diagnostic challenge for the clinician. Some of these findings may appear as the natural evolution of the disease while others may come out as complications of the syndrome itself. The classical features of the syndrome were present in our case while vitiligo of the hand, parathyroid adenoma, mesenteric cyst, and the papillary thyroid carcinoma may have arisen due to the distinct genetic defects or mutations. These findings may be coincidental but the distinction is not possible in our patient currently. The other clinical manifestations such as hypersplenism, venous thrombosis, and hepatic fibrosis, on the other hand, may have occured as the expected long-term complications of the disease. Our patient was a sporadic case without a previous family history.
Nearly all the patients demonstrate all three signs of the clinical syndrome: port-wine stain, varicose veins, and bony and soft tissue hypertrophies. In a series of 252 patients at the Mayo Clinic, 63% of patients had all three features and 37% had two of the three features. Port-wine stain was seen in 98% of patients, varicosities or venous malformations in 72%, and limb hypertrophy in 67% of the patients [20]. Brain abnormalities such as hemorrhage, infarction, hemimegalencephaly, venous malformation, arteriovenous malformations, cavernoma, aneurysm, hydrocephalus, choroid plexus abnormalities, atrophy, calcifications, leptomeningeal enhancement, cortical dysplasia, and seizures may occur while cerebral infarctions and brain tumors are encountered rarely [21-23]. Pulmonary emboli may develop secondary to venous limb thrombosis [24]. Consequently, KTW syndrome may present with many different manifestations due to the genetic structure and the anatomic location or the type of vascular malformations as it is the case in our patient.
Our case is unique for the manifestations such as the vitiligo of the hand, parathyroid adenoma, mesenteric cyst, and the papillary thyroid carcinoma that have not been described in the literature previously. On the other hand, vena hepatica thrombosis, hypersplenism, hepatic fibrosis, and deep vein thrombosis are expected long term complications of this syndrome. Clinicians should bear in mind that KTW syndrome may present with an unusual clinical profile that may be either due to the primary genetic profile of the patient or the secondary to the long-term complications of the disease. Complex genetic pattern or genetic mutations may lead to an atypical presentation in patients with the KTW syndrome. The case was sporadic in nature. An atypical presentation due to the variable genetic profile of the syndrome with coexisting disease complications was considered in this patient.
Conclusions
Klippel-Trenaunay-Weber syndrome is a rare and a sporadic genetic disease. The syndrome may present with atypical manifestations that are probably related to the genetic differences or mutations of the disease. The vitiligo of the hand, mesenteric cyst, parathyroid adenoma, and the papillary thyroid carcinoma may resut from chromosomal abnormalities, in utero mesodermal and ectodermal dysplasia. On the other hand, long term complications of the syndrome accompany the clinical profile thereby leading to a diagnostic challenge. Clinicians should bear in mind that the atypical presentation may be related to the genetic features along with the expected complications of this syndrome. Differential diagnosis of Klippel-Trenaunay-Weber syndrome from other capillary malformation-overgrowth syndromes such as isolated capillary malformation, diffuse capillary malformation with overgrowth (DCMO), and other overgrowth syndromes is necessary. The PROS term can encompass different overlapping conditions of this syndrome and thereby may better designate such patients.
Author contributions
Muammer Bilir has contributed to patient evaluation and follow-up.
Cuneyt Tetikkurt has performed case report preparation and written the case report.
Halil Yanardag has contributed to patient data evaluation and references.
- Klippel M, Trenaunay P (1900) Varicose osteo-hypertrophic nevus. Arch Gen Med 3: 641-672.
- Klippel M, Trenaunay P (1900) Varicose osteo-hypertrophic nevus. Journal des Practiciens 14: 65-70.
- Weber FP (1907) Angioma-formation in connection with hypertrophy of limbs and hemi-hypertrophy. Br J Dermatol 19: 231-235. Link: https://tinyurl.com/yabk6ufv
- Weber FP (1918) Haemangiectatic hypertrophy of limbs; congenital phlebarteriectasis and so called congenital varicose veins. Br J Child Dis 15: 13-17.
- Craven N, Wright AL (1995) Familial Klippel-Trenaunay syndrome: a case report. Clin Exp Dermatol 20:76-79. Link: https://tinyurl.com/yaz8lj69
- Steijlen PM, van Steensel MA (1999) Paradominant inheritance, a hypothesis explaining occasional familial occurrence of sporadic syndromes. Am J Med Genet 85: 359-360. Link: https://tinyurl.com/yacwffta
- Tian XL, Kadaba R, You SA, Liu M, Timur AA, et al. (2004) Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature 427: 640-645. Link: https://tinyurl.com/y8z6mq92
- Timur AA, Sadgephour A, Graf M, Schwartz S, Libby ED, et al. (2004) Identification and molecular characterization of a de novo supernumerary ring chromosome 18 in a patient with Klippel-Trenaunay syndrome. Ann Hum Genet 68: 353-361. Link: https://tinyurl.com/yd4wk4qo
- Luks VL, Kamitaki N, Vivero MP, Uller W, Rab R, et al. (2015) Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr 166: 1048-1054. Link: https://tinyurl.com/y9nw3k6u
- Alomari AI, Spencer SA, Arnold RW, Chaudry G, Kasser JR, et al. (2014) Fibro-adipose vascular anomaly: clinical-radiologic-pathologic features of a newly delineated disorder of the extremity. J Pediatr Orthop 34: 109. Link: https://tinyurl.com/ybrughd9
- Yeung KS, Ip JJ, Chow CP, Kuong EY, Tam PK, et al. (2017) Somatic PIK3CA mutations in seven patients with PIK3CA-related overgrowth spectrum. Am J Med Genet A 173: 978-984. Link: https://tinyurl.com/ydz5dgtt
- Bliznak J, Staple TW (1974) Radiology of angiodysplasias of the limb. Radiology 110: 35-44. Link: https://tinyurl.com/ybbwdgw8
- Servelle M (1985) Klippel and Trenaunay's syndrome. 768 operated cases. Ann Surg 201: 365-373. Link: https://tinyurl.com/ya26qzhc
- Baskerville PA, Ackroyd JS, Browse NL (1985) The etiology of the Klippel-Trenaunay syndrome. Ann Surg 202: 624-627. Link: https://tinyurl.com/ybz572oo
- McGrory BJ, Amadio PC (1993) Klippel-Trenaunay syndrome: orthopaedic considerations. Orthop Rev 22: 41-50. Link: https://tinyurl.com/yb2pmqqb
- Ceballos-Quintal JM, Pinto-Escalante D, Castillo-Zapata I (1996) A new case of Klippel-Trenaunay-Weber (KTW) syndrome: evidence of autosomal dominant inheritance. Am J Med Genet 63: 426-427. Link: https://tinyurl.com/yb8ffhcd
- Hofer T, Frank J, Itin PH (2005) Klippel-Trenaunay syndrome in a monozygotic male twin: supportive evidence for the concept of paradominant inheritance. Eur J Dermatol 15: 341-343. Link: https://tinyurl.com/ycdwyzgv
- Hu Y, Li L, Seidelmann SB, Timur AA, Shen PH, et al. (2008) Identification of association of common AGGF1 variants with susceptibility for Klippel-Trenaunay syndrome using the structure association program. Ann Hum Genet 72: 636-643. Link: https://tinyurl.com/yd5wl7dx
- Kihiczak GG, Meine JG, Schwartz RA, Janniger CK (2006) Klippel-Trenaunay syndrome: a multisystem disorder possibly resulting from a pathogenic gene for vascular and tissue overgrowth. Int J Dermatol 45: 883-890. Link: https://tinyurl.com/ybp5vvz2
- Jacob AG, Driscoll DJ, Shaughnessy WJ, Stanson AW, Clay RP, et al. (1998) Klippel-Trénaunay syndrome: spectrum and management. Mayo Clin Proc 73: 28-36. Link: https://tinyurl.com/ybvwkv8m
- Funayama E, Sasaki S, Oyama A, Furukawa H, Hayashi T, et al. (2011) How do the type and location of a vascular malformation influence growth in Klippel-Trénaunay syndrome ? Plast Reconstr Surg 127: 340-346. Link: https://tinyurl.com/y778j7du
- Renard D, Larue A, Taieb G, Jeanjean L, Labauge P (2012) Recurrent cerebral infarction in Klippel-Trenaunay-Weber syndrome. Clin Neurol Neurosurg 114: 1019-1020. Link: https://tinyurl.com/yb9tkhv6
- Yılmaz T, Cikla U, Kirst A, Baskaya MK (2015) Glioblastoma multiforme in Klippel-Trenaunay-Weber syndrome: a case report. J Med Case Rep 9: 83. Link: https://tinyurl.com/y6tw93by
- Upadhyay H, Sherani K, Vakil A, Babury M (2016) A case of recurrent massive pulmonary embolism in Klippel-Trenaunay-Weber syndrome treated with thrombolytics. Respir Med Case Rep 17: 68-70. Link: https://tinyurl.com/yb9r6axs
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
