Archives of Pulmonology and Respiratory Care
Effectiveness of Asthma Inhaler Pharmacotherapy must take into consideration both the device and drug and which is guided by clinically meaningful outcomes
Elissa M McDonald* and Felix SF Ram
Cite this as
McDonald EM, Ram FSF (2017) Effectiveness of Asthma Inhaler Pharmacotherapy must take into consideration both the device and drug and which is guided by clinically meaningful outcomes. Arch Pulmonol Respir Care 3(1): 044-047. DOI: 10.17352/aprc.000023Introduction
The inhalation route remains the mainstay of therapy for asthma and chronic obstructive pulmonary disease (COPD). This confers a number of advantages such as delivery of medication directly to the site of action resulting in faster onset. It also allows smaller doses to be administered and therefore significantly reduces systemic side effects compared with oral therapy. The drug treatment regime for the majority of patients with asthma and COPD is straightforward and is documented in many guidelines [1-3]. However, the choice of which inhaler device to use is less straightforward. Rather than being spoilt for choice, we are frequently confused by the ever-increasing number of devices available. Although inhalation therapy is now the mainstay of asthma treatment, for most patients such treatment is still not optimal. The delivered dose can vary widely between different delivery systems and between patients depending on how well they use a particular device. Many new inhaler devices have become available, and their competing pharmaceutical company promotional claims can confuse both prescribers and patients. This review provides clinical practice recommendations based on evidence to date on the effectiveness of inhaler devices.
Evidence to date
The choice of delivery system should depend on the patient, the drug and the device. The hand-held inhaler device systems available can be broadly divided into two classes: pressurised metered-dose inhalers (pMDIs) used with or without spacer devices and dry powder inhalers (DPIs). Pressurised metered-dose inhalers (pMDIs) are usually the cheapest inhaler device and are often regarded as first choice, but their efficiency is dependent to a large degree on an individual’s ability to use them properly. MDIs require the co-ordination of actuating the delivery of drug with slow inhalation by the patient. Therefore, they are not appropriate for young children, people with arthritis and many elderly patients. Studies have shown that 50% to 80% of adults have difficulty using a pMDI [4-6]. The breath-actuated pMDIs (e.g. Autohaler, Easibreathe) do not require co-ordination of actuation and inhalation.
Inhalers with or without spacer devices
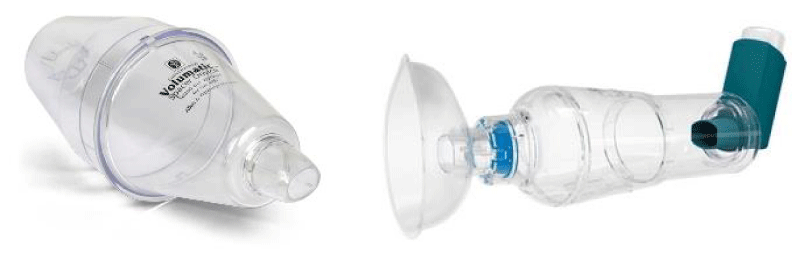
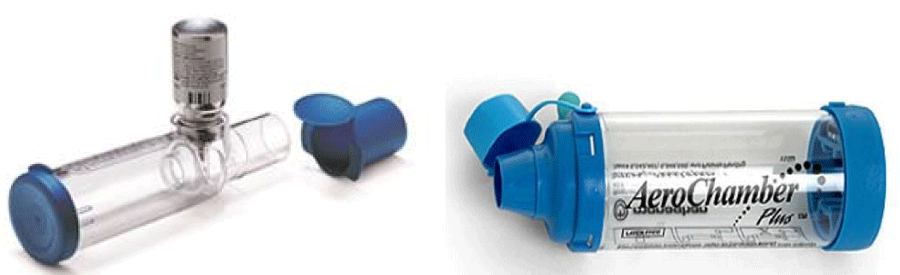
One of the most important advantages of the pMDIs is their use in emergency management of asthma, either alone, or in combination with spacer devices [7-9]. Spacer devices were developed to overcome some of the problems of pMDIs. There are two main types: (1) Valved holding chambers (Figure 1), examples include the Volumatic (GlaxoWellcome, Uxbridge, UK) and the Nebuhaler (AstraZeneca, Kings Langley, UK; (2) Extension devices (Figure 2), examples of which are the Optihaler (Philips Respironics, Parisppany, USA) and Aerochamber (Trudell Medical, Ontario, Canada). The valved holding chambers tend to have large volumes of typically 750 ml and allow the patient to breathe tidally from a reservoir of drug. Extension devices provide a space between the inhaler and the patient, allowing the aerosol to slow and the propellants to evaporate, reducing the size of the drug particles from the pMDIs and trapping large particles in the device. They usually take the form of a tube of similar diameter to the mouthpiece of the pMDI and are of much lower volume (e.g. 125 ml) than the valved holding chambers. The effectiveness of spacer devices (both valved and extension) has not been well documented in terms of pulmonary deposition and side effect profile. Even when using extension devices with pMDIs, coordination is still required for optimal drug delivery but coordination may be less critical with valved holding chambers. In young children valved holding chambers provide the recommended delivery system. The requirement for a degree of coordination still prevents some patients, particularly the elderly from using pMDI and spacer devices of either type.
Benefits of HFA (CFC-free) inhaler devices
One of the major developments with the pMDIs in the past several years has been the replacement of CFC propellant formulations with hydrofluoroalkane (HFA). The pMDIs contain the drug either dissolved or suspended in one or more propellants, along with a surfactant or dispersal agent (oleic acid), used not only to keep the drug suspended in the propellants but also to lubricate the valve mechanism [10,11]. One of the important differences noted in the HFA propelled inhaler is the “softer” spray. This is due to formulation changes and the required redesign of the valve and actuator in order to provide the same dose delivery as the chlorofluorocarbon (CFC) pMDI [12]. Some clinical trials conducted using bronchodilators [13-16] and corticosteroids [17-19] have shown equivalent response and safety profile of HFA-pMDI to CFC-pMDI. Although the initial design of the HFA-pMDI was to deliver the same drug dose as the old CFC-pMDIs recent large retrospective cohort studies have since shown that the HFA-pMDI containing corticosteroids provides efficacy and safety at roughly half the dose compared to CFC-pMDI [20-22].
Dry powder inhalers
Dry powder inhalers (DPIs) (e.g. Turbuhaler, Diskhaler) create aerosols of dry powder by directing air through a quantity of loose powder. DPIs are either single or multiple dosing and are similar to breath-actuated pMDI devices in that they do not require co-ordination between actuation and inhalation by the patient. However, the dispersion of the powder into respirable particles is dependent on the creation of turbulent flow in the inhaler. This flow is dependent on the patients’ ability to inhale the powder with sufficiently high inspiratory flow rate to effectively dispense the powder. This is important in patients who are not able to generate high enough inspiratory flow rates, especially during acute exacerbations and in severe COPD. Some studies [23-25], have shown that the DPIs have equivalent response to pMDIs while other studies [26-29], have shown greater lung deposition with the DPIs compared to the pMDI. Closer examination of the methodology of studies showing greater deposition with the DPIs highlights inconsistencies in the estimation of percentage lung deposition. These studies failed to account for the dose left behind (40-60% of label claim) in the DPI reservoir in their calculation of deposition. If this was corrected for, lung deposition would be similar to that of the pMDI when used with correct technique [30].
Lung deposition studies and clinical effectiveness
Although it is reasonable to assess the effects of dosage alterations when pulmonary deposition differences are known between inhaler devices, dose adjustments should always be based on clinical outcomes and side effects and not pulmonary deposition alone. The efficacy of any particular inhaler device when used with optimal technique in a clinical trial may not equate to its effectiveness in general use, as such trials usually exclude patients with suboptimal inhaler technique. There is therefore little evidence from clinical trials on which to base inhaler selection in the real world, where patients often use their inhalers incorrectly. The lung deposition of inhaled drug varies according to inhaler device, drug particle size, inhalation technique, and pattern of inspiratory flow. Even with training, not all patients can use their inhalers correctly and maintain inhaler technique; patients may be incapable of handling a particular inhaler, have strong preference for another device or have natural breathing patterns that do not match their prescribed inhaler. Therefore, matching device to the patient may be a better course of action than increasing therapy or training and retraining a patient to use a specific inhaler device, especially if the basis for doing so is based on pharmaceutical company sponsored lung deposition studies. Although inhaler device manufacturers are required by regulatory authorities to provide in vivo drug delivery studies including lung deposition for approval of their drug-device combination which may also include drug bioavailability and adverse effects (especially for a generic drug-device combination), there still remains an incomplete understanding of the relationship between in-vitro and in-vivo lung deposition, drug particle size, aerodynamic diameter, drug mass and clinical effectiveness and side effects. Furthermore, studies conducted in different diseases (asthma versus COPD) and different age groups (children, adults or the elderly) cannot be extrapolated to other or all diseases or ages. It is also important to know how relative efficacy is assessed with each inhaler device. This can be measured in crossover studies, which compare inhalers containing bronchodilators, by measuring lung function (e.g. FEV1). However, comparisons of the efficacy of different devices for delivery of inhaled corticosteroids are not as easy. Studies using corticosteroids usually require longer time periods and crossover designs are rarely suitable due to the requirement for long washout periods. The most frequently encountered problem with studies that compare inhaler devices is that they are designed to be comparative trials with a null hypothesis of bioequivalence (equal efficacy) and are therefore not powered to detect non-equivalence or differences between devices. Failure to detect a difference should not necessarily imply equivalence.
Conclusion
Due to the ever increasing number of inhaler devices and the competing claims made by pharmaceutical companies, it is often difficult for prescribers to choose the best device for their patients. The most extensive systematic review with meta-analysis conducted to date [31], involving over 100 randomised controlled trials and 1000’s of patients concluded that in patients with stable asthma the standard pMDIs is as effective as any other hand-held inhaler device and that the therefore the cheapest available device that the patient is able to use should always be considered. The review also called for pharmaceutical companies include clinical outcome data (as opposed to in vitro data) when submitting medicine applications for approval by regulatory authorities, particularly in support of any dosing schedules greater than 1:1 when compared with the standard pMDI. Results of this large systematic review clearly suggests that all inhaler devices are suitable delivery systems for many patients although there are known limitations for some patients (e.g. young, physically impaired and the elderly). Therefore, healthcare professionals advising patients should be encouraged to use the cheapest drug delivery device that the patient is able to use while at the same time considering optimal efficacy, inspiratory flow rates, side effects, patient preference and compliance as important factors.
- British Guideline on the management of asthma: Scottish Intercollegiate Guidelines Network (SIGN). September 2016. ISBN 978 1 909103 29 0.
- Amir Qaseem, Timothy JW, Steven EW, Nicola AH, Gerard C, et al. (2011) Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 155: 179-191. Link: https://goo.gl/3GLvuo
- Siafakas NM, Vermeire P, Pride NB, J. Gibson, P. Howard, et al. (1995) Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J 8:1398-420. Link: https://goo.gl/pQXDDo
- Crompton GK (1990) The adult patient’s difficulties with inhalers. Lung 658-662. Link: https://goo.gl/ydXeBj
- Larsen JS, Hahn M, Ekholm B, Wick KA (1994) Evaluation of conventional press-and-breathe metered-dose inhaler technique in 501 patients. J Asthma 31: 193-199. Link: https://goo.gl/JoPzbF
- Van Beerendonk I, Mesters I, Mudde AN, Tan TD (1998) Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma 35: 273-279. Link: https://goo.gl/FbSJAv
- Horner SD (2000) A low dose of albuterol by metered-dose inhaler with a spacer was as effective as higher doses by metered-dose inhaler or low doses by nebulizer in children with mild acute asthma. West J Med; 172: 247. Link: https://goo.gl/WdZEq0
- Mandelberg A, Tsehori S, Houri S, Gilad E, Morag B, et al. (2000) Is nebulized aerosol treatment necessary in the pediatric emergency department? Chest 117: 1309-1313. Link: https://goo.gl/KDWWcK
- Robertson CF, Norden MA, Fitzgerald DA, Connor FL, Van Asperen PP, et al. (1998) Treatment of acute asthma: salbutamol via jet nebuliser vs spacer and metered dose inhaler. J Paediatr Child Health 34: 142-146. Link: https://goo.gl/NwfqOp
- Berg E (1995) In vitro properties of pressurized metered dose inhalers with and without spacer devices. J Aerosol Med 8: S3-11. Link: https://goo.gl/Y5rPD2
- Swift DL (1993) Dose distribution of pharmaceutical aerosols. Aerosol Sci Technol 18: 272-278. Link: https://goo.gl/8OPeHk
- June D (1995) A new generation of inhaler technology. Brit J Clin Prac 79:18-21.
- Dockhorn R, Vanden Burgt JA, Ekholm BP, Donnell D, Cullen MT (1995) Clinical equivalence of a novel non-chlorofluorocarbon-containing salbutamol sulfate metered-dose inhaler and a conventional chlorofluorocarbon inhaler in patients with asthma. J Allergy Clin Immunol 96: 50-56. Link: https://goo.gl/0OQMl2
- Kleerup EC, Tashkin DP, Cline AC, Ekholm BP (1996) Cumulative dose-response study of non-CFC propellant HFA 134a salbutamol sulfate metered-dose inhaler in patients with asthma. Chest 109: 702-707. Link: https://goo.gl/ud4Cbe
- Ramsdell JW, Colice GL, Ekholm BP, Klinger NM (1998) Cumulative dose response study comparing HFA-134a albuterol sulfate and conventional CFC albuterol in patients with asthma. Ann Allergy Asthma Immunol 81: 593-599. Link: https://goo.gl/7OAuYI
- Taggart SC, Custovic A, Richards DH, Woodcock A (1995) GR106642X: a new, non-ozone depleting propellant for inhalers. BMJ 310: 1639-1640. Link: https://goo.gl/bE9wtx
- Jenkins M (1995) Clinical evaluation of CFC-free metered dose inhalers. J Aerosol Med 8: S41-47. Link: https://goo.gl/Ybnpej
- Milanowski J, Qualtrough J, Perrin V (1999) Inhaled beclomethasone (BDP) with non-CFC propellant (HFA-134a) is equivalent to BDP-CFC for the treatment of asthma. Respir Med 93: 245-251. Link: https://goo.gl/RIwZiZ
- Dahl R, Ringdal N, Ward S, Stampone P, Donnell D (1997) Equivalence of asthma control with new CFC-free formulation HFA-134a beclomethasone dipropionate and CFC-beclomethasone dipropionate. BJCP 5:11-15. Link: https://goo.gl/twYeYd
- Barnes N, Price D, Colice G, A Chisholm, P Dorinsky et al. (2011) Asthma control with extrafine-particle hydrofluoroalkane-beclometasone vs. large-particle chlorofluorocarbon-beclometasone: a real-world observational study. Clin Exp Allergy. 41: 1521-1532. Link: https://goo.gl/3dNZzS
- Paggiaro P, Nicolini G, Papi A (2008) Extrafine beclomethasone dipropionate/formoterol hydrofluoroalkane-propelled inhaler in asthma. Expert Rev Respir Med. 2: 161-166. Link: https://goo.gl/TW10ed
- Price D, Thomas M, Haughney J, Richard A. Lewis, Anne Burden et al. (2013) Real-life comparison of beclometasone dipropionate as an extrafine- or larger-particle formulation for asthma. Respir Med. 107: 987-1000. Link: https://goo.gl/yYv2cs
- Bondesson E, Friberg K, Soliman S, Löfdahl CG (1998) Safety and efficacy of a high cumulative dose of salbutamol inhaled via turbuhaler or via a pressurized metered-dose inhaler in patients with asthma. Respir Med 92: 325-330. Link: https://goo.gl/bq7E5J
- Bronsky E, Bucholtz GA, Busse WW, Chervinsky P, Condemi J, et. al. (1987) Comparison of inhaled albuterol powder and aerosol in asthma. J Allergy Clin Immunol 79 : 741-7. Link: https://goo.gl/XIUiIX
- Haahtela T, Vidgren M, Nyberg A, Korhonen P, Laurikainen K, et al. (1994) A novel multiple dose powder inhaler. Salbutamol powder and aerosol give equal bronchodilatation with equal doses. Ann Allergy 72: 178-182. Link: https://goo.gl/bf6qBN
- Borgström L, Derom E, Ståhl E, R Pauwels (1996) The inhalation device influences lung deposition and bronchodilating effect of terbutaline. Am J Respir Crit Care Med 153:1636-1640. Link: https://goo.gl/6mIzdo
- Carlsson LG, Arwestron E, Friberg K (1995) Half the dose of salbutamol required by turbuhaler compared with diskhaler for the same bronchodilating effect. Eur Respir J 19: 201.
- Ekström T, Andersson AC, Skedinger M, Lindbladh C, Ståhl E (1995) Dose potency relationship of terbutaline inhaled via Turbuhaler or via a pressurized metered dose inhaler. Ann Allergy Asthma Immunol 74: 328-332. Link: https://goo.gl/RmIf8H
- Tonnesen F, Laursen LC, Evald T, Elisabeth Ståhl, Thomas B. Ibsen (1994) Bronchodilating effect of terbutaline powder in acute severe bronchial obstruction. Chest 105: 697-700. Link: https://goo.gl/OinT6K
- Newman SP, Weisz AW, Talaee N, Clarke SW (1991) Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler technique. Thorax; 46: 712-716. Link: https://goo.gl/Ifhxjk
- Ram FS (2003) Clinical efficacy of inhaler devices containing beta2-agonist bronchodilators in the treatment of asthma: Cochrane systematic review and meta-analysis of more than 100 randomized, controlled trials. Am J Respir Med. 2: 349-365. Link: https://goo.gl/DkcSan
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
