Archives of Pulmonology and Respiratory Care
Inhaled GM-CSF in a Pulmonary Alveolar Proteinosis Patient Refractory to Plasmapheresis Combined with Multiple Whole Lung Lavages
Francesca Mariani1, Elena Paracchini1, Davide Piloni2, Zamir Kadija1, Elena Salvaterra1, Laura Divizia1, Giuseppe Rodi3, Carmine Tinelli4, Federica Meloni1,2 and Ilaria Campo1*
2Department of Internal Medicine, Pneumology Unit, University of Pavia, Pavia, Italy
3Department of Anesthesiology and Intensive Care, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
4Clinical Epidemiology and Biometric Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Cite this as
Mariani F, Paracchini E, Piloni D, Kadija Z, Salvaterra E, et al. (2017) Inhaled GM-CSF in a Pulmonary Alveolar Proteinosis Patient Refractory to Plasmapheresis Combined with Multiple Whole Lung Lavages. Arch Pulmonol Respir Care 3(1): 016-019. DOI: 10.17352/aprc.000018A autoimmune Pulmonary Alveolar Proteinosis (PAP) patient with persistent disease underwent 3 Whole Lung Lavages (WLLs), 10 plasmapheresis sessions and further 3 WLL, from October 2004 to May 2007. Nevertheless HRTC and pulmonary function test (PFT) showed a persistent residual disease of mild degree. At the beginning of 2010, the patient was admitted to inhaled rGM-CSF (Sargramostim) therapy as compassionate treatment. GM-CSF was administered by Akita 2 nebulizer (Vectura), as follow: 250 mcg/day every other week for 12 weeks, then 250 mcg/day on 2 consecutive days every 2 weeks for 6 months. Follow up visits were scheduled at 3, 10, 18, 30 months and after that once a year. Functional and HRCT data and PaO2 were collected. Since the start of the inhaled GM-CSF therapy, the patient no more required WLL. Furthermore we found a significant increase in DLCO% (p=0.013) and FVC% (p=0.023) while %FEV1 show a positive trend. No substantial differences in blood gas analysis. The pulmonary involvement at HRCT shows a significant decrease of lung infiltrates (p=0.039) in terms of pathological segments. These data underscore the utility of inhaled GM-CSF not only in case of progressing disease but also in case of refractory patients with persistent lung infiltrates, in order to increase the response rate.
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare pulmonary disease characterized by the accumulation of surfactant proteins and lipids within the alveoli, which is caused by a defective macrophage catabolism, due to a disruption in surfactant homeostasis [1].
PAP occurs as autoimmune, congenital and secondary forms. The autoimmune form is the most frequent, accounting for more than 90% of PAP cases and is associated to the presence of anti-GM-CSF (Granulocyte-Macrophage-Colony stimulating factor) autoantibodies directed towards the GM-CSF, which lead to a defective maturation of alveolar macrophages and thus impair their function in surfactant clearance [2]. Moreover, the anti GM-CSF autoantibodies impair the microbicidal activity of neutrophils [3], possibly explaining the basis for recurrent infections in PAP. Congenital forms are caused by mutations in genes coding for GM-CSF receptor [4]. Secondary forms are generally associated to hematological malignancies or exposure to inorganic materials or immunosuppressive drugs toxicity.
Clinical manifestations include progressive exertional dyspnea of insidious onset, or dyspnea and cough. Nail clubbing due to gas exchange impairment and changes in the ventilation/perfusion ratio may be present. However a large fraction of autoimmune PAP patients are asymptomatic (up to 31%) [5].
Disease progression is highly variable, ranging from spontaneous resolution to cardiorespiratory failure.
Chest HRTC findings show areas of patchy ground-glass opacities and interlobular thickening whose combination determines the “crazy paving” pattern which is characteristic but not diagnostic of PAP.
Diagnosis is based on bronchoalveolar lavage (BAL), histology and chest HRTC findings and on the presence of anti-GM-CSF autoantibodies for the autoimmune form.
The gold standard of treatment is the whole lung lavage (WLL), even if this procedure is not yet standardized [6,7] and is associated with a very variable response rate [8,9]. WLL physically removes the accumulated surfactant, however in some patients it is ineffective, despite repeated WLL treatments.
Based on recent studies, new therapeutic strategies are available for patients who are poor responder to WLL, including plasmapheresis [10], recombinant (r) GM-CSF administration or Rituximab [11].
Case Report
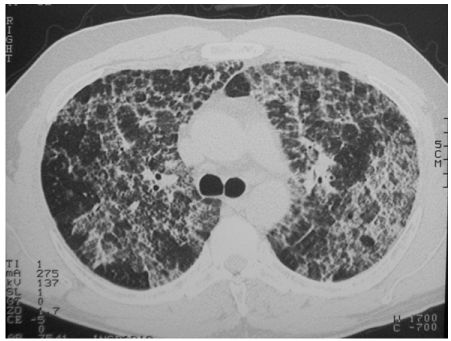
In 2004 a 40-year-old man was diagnosed for autoimmune PAP according to lung biopsy, HRTC and elevated neutralizing GM-CSF autoantibody serum level. Because of progressive respiratory failure and bilaterally spread crazy paving HRTC pattern (Figure 1) he was admitted to WLL. The procedure was performed under general anesthesia in Intensive Care Unit and provided the infusion of 30 L warm normal saline for each lung combined to manual chest percussion, in order to remove the proteinaceous material. The procedure produced a limited and short clinical benefit, thus the patients needed a second and a third WLL in February and in August 2005, respectively. Given the poor response to WLL and the need for frequent procedures, according to previous reports [10], the patient was submitted to serial plasmapheresis sessions [12]. Between February and March 2006, a total of 10 sessions were performed. Each plasmapheresis involved a reduced volume (1.5L) of plasma exchange in order to minimize the potential complications, such as infections and prothrombotic state.
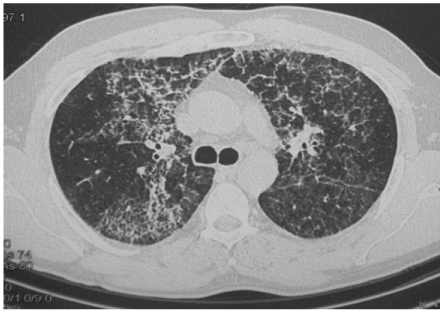
This treatment did not improve significantly neither the clinical condition nor the pulmonary function or chest HRCT findings (Figure 2). GM-CSF autoantibodies serum level decreased from 250 mcg/ml to 130 mcg/ml during treatment.
After plasmapheresis, three additional WLLs were required (March 2006, July 2006 and May 2007) and the autoantibodies furtherly stepped down to a level 56 mcg/ml which is still higher than the critical threshold, while HRTC and pulmonary function test (PFT) showed a persistent residual disease of mild degree.
The patient showed a clinical and functional stability until January 2010 when the patient experienced a worsening of exertional dyspnoea and PaO2 (from 81 mmHg on July 2009 to 64.9 mmHg), despite a substancial stability in lung function. He was evaluated by CT scan which demonstrated sustained lung infiltrates, associated to a crazy paving pattern.
After approval by the Ethics Committee of Fondazione IRCCS Policlinico San Matteo Pavia, the patient was admitted to a compassionate treatment with inhaled rGM-CSF (Sargramostim, Leukine®, Genzyme). Inhaled rGM-CSF was administered according to 2 phases, by using Akita 2 (Vectura), an apparatus which combines a vibrating mesh nebulizer with a computerized compressor controlling both inhalation flow rate and inhaled volume [13]. A 12 weeks acute phase (daily single dose of 250 mcg, for 5 days per week) was followed by a 6 months chronic phase (250 mcg/day on 2 consecutive days every 2 weeks), which started one month after the end of the acute phase. Follow up visits were scheduled at 3, 10, 18, 30 months and then once a year.
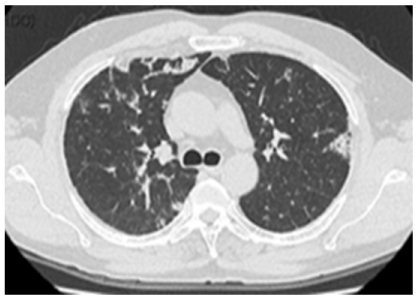
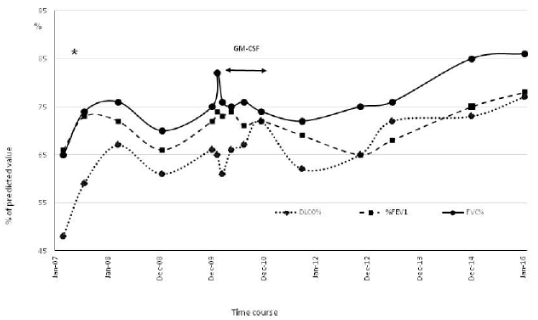
At the end of treatment, patient was evaluated by PFT and chest HRCT (Figure 3). We found a significant increase in DLCO% (p=0.013) and FVC% (p=0.023) while %FEV1 showed a positive trend (Figure 4). No substantial differences in blood gas analysis were detected. The pulmonary involvement evaluated by the CT-assisted lung profusion score [14], showed a significant decrease of lung infiltrates (p=0.039) in terms of pathological segments. Of note, since the start of the inhaled rGM-CSF therapy, the patient did not deteriorate further and no more required WLL. No drug-related adverse effects were reported.
Discussion
This case report describes a relapsing disease not controlled by the standard WLL treatment, even if serially repeated. Although WLL still represents the gold standard for the treatment of PAP, the discovery of neutralizing anti-GM-CSF autoantibodies in the serum of autoimmune PAP patients led to plasmapheresis as a potential treatment option [10]. In this way, an improvement of the surfactant clearance could be gain by restoring the normal surfactant catabolism in alveolar macrophage, through the decrease of anti-GM-CSF autoantibodies serum level.
Nevertheless, even after multiple plasmapheresis, we did not obtain an improvement in clinical conditions nor a reduced need for WLL.
Previous studies have challenged the efficacy of rGM-CSF supplementation in PAP patients, consequently subcutaneous and inhaled administration were considered. Although WLL is still considered the gold standard for treatment, the discovery of alveolar macrophage involvement and anti GM-CSF neutralizing antibodies led to the use of GM-CSF as a potential therapeutic approach for PAP.
In particular, two papers reported a positive but transient clinical response to subcutaneous administration, however the overall response rate was less than 50% of the patient treated. In the first one, 14 autoimmune PAP patients received subcutaneously administered GM-CSF in escalating doses (5-20 mcg/kg/day) over a 3-month-period [15]. In the second study, 21 autoimmune PAP patients were treated with escalating subcutaneous doses of 5-18 mcg/kg/day for 6-12-months [16].
On the other hands, the overall response rate to inhaled rGM-CSF was about 68%, as reported in Wylam [17] and Tazawa [18], studies, with no drug-related adverse effects reported. The first was a retrospective study including 12 autoimmune PAP patients, treated with a rGM-CSF dosage of 250 mg/day every other week (one patient required dose escalation at 500 mg/day and different treatment length). In the second one, 35 autoimmune PAP patients were treated according to an induction dose (250 mcg on days 1-8 of 14, x 6 cycles) followed by maintenance dose (125 mcg on days 1-4 of 14, x 6 cycles).
Since it is conceivable that in autoimmune PAP the alveolar space is the main site of rGM-CSF signal disruption, with the impairment of surfactant catabolism, then it is reasonable to propose rGM-CSF supplementation by inhalatory route.
Starting from these data and considering the unsuccessful results obtained with the therapeutic approaches tried before, we decided to admit our patient to inhaled rGM-CSF therapy. From the start of the inhalatory treatment, our patient no more required WLL. Furthermore we found a significant improvement in pulmonary function in terms of FVC% and DLCO%, while a positive trend of %FEV1 was identified. Consistent with pulmonary function test data, the lung infiltration gradually decreased during the inhaled rGM-CSF treatment.
Our experience demonstrates for the first time how the administration of rGM-CSF by inhalatory route is more efficacious than standard and unconventional therapy in refractory patients, leading to a significant and persistent improvement which lasted years after the end of the treatment.
In conclusion, our results underscore the utility of inhaled rGM-CSF in case of relapsing and refractory disease, in order to increase the response rate and to avoid the recurrence of WLL.
Author’s contribution
FMa, EP and ZK managed the patient during the follow up; ES and LD collected data; GR performed the whole lung lavages; CT analyzed data; IC coordinate the study and wrote the paper; DP wrote the paper; FMe revised the draft.
- Trapnell BC, Whitsett JA, Nakata K (2003) Pulmonary alveolar proteinosis. N Engl J Med 349: 2527-2539. Link: https://goo.gl/o3mkkC
- Sakagami T, Uchida K, Suzuki T, Carey CB, Wood RE, et al. (2009) Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med 361: 2679-2681. Link: https://goo.gl/H017zF
- Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, et al. (2007) GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 356: 567-579. Link: https://goo.gl/sDLbDB
- Suzuki T, Sakagami T, Young LR, Carey BC, Wood RE, et al. (2010) Hereditary Pulmonary Alveolar Proteinosis: Pathogenesis, Presentation, Diagnosis, and Therapy. Am J Respir Crit Care Med 182: 1292-1304. Link: https://goo.gl/QLCjzc
- Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, et al. (2008) Characteristics of a Large Cohort of Autoimmune Pulmonary Alveolar Proteinosis Patients in Japan. Am J Respir Crit Care Med 177: 752-762. Link: https://goo.gl/lvmI8v
- Campo I, Luisetti M, Griese M, Trapnell BC, Bonella F, et al. (2016) Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet J Rare Dis 11: 115-124. Link: https://goo.gl/IN1noq
- Campo I, Luisetti M, Griese M, Trapnell BC, Bonella F, et al. (2016) A Global Survey on Whole Lung Lavage in Pulmonary Alveolar Proteinosis. Chest 150: 251-253. Link: https://goo.gl/8tM9Vf
- Seymour JF, Presneill JJ (2002) Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 166: 215-235. Link: https://goo.gl/Aca0Gc
- Beccaria M, Luisetti M, Rodi G, Corsico A, Zoia MC, et al. (2004) Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 23: 526-531. Link: https://goo.gl/yAOtcD
- Bonfield TL, Kavuru MS, Thomassen MJ (2002) Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin Immunol 105: 342-350. Link: https://goo.gl/yjvVoj
- Kavuru MS, Malur A, Marshall I, Barna BP, Meziane M, et al. (2011) An open-label trial of rituximab therapy in pulmonary alveolar proteinosis. Eur Respir J 38: 1361-1367. Link: https://goo.gl/rSmWk7
- Luisetti M, Rodi G, Perotti C, et al. (2009) Plasmapheresis for treatment of pulmonary alveolar proteinosis. Eur Respir J 33: 1220-1222. Link: https://goo.gl/xACDqB
- Luisetti M, Kroneberg P, Suzuki T, Kadija Z, Muellinger B, et al. (2011) Physical properties, lung deposition modeling, and bioactivity of recombinant GM-CSF aerosolised with a highly efficient nebulizer. Pulm Pharmacol Ther 24: 123-127. Link: https://goo.gl/FkWVfG
- Akira M, Inoue Y, Yamamoto S, Sakatani M (2000) Non-specific interstitial pneumonia: findings on sequential CT scans of nine patients. Thorax 55: 854-859. Link: https://goo.gl/nFbF2o
- Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, et al. (2001) Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med 163: 524-531. Link: https://goo.gl/SRYJ2p
- Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, et al. (2006) An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest 130: 227-237. Link: https://goo.gl/kKIsaj
- Wylam ME, Ten R, Prakash UB, Nadrous HF, Clawson ML, et al. (2006) Aerosol granulocyte-macrophage colony-stimulating factor for pulmonary alveolar proteinosis. Eur Respir J 27: 585-593. Link: https://goo.gl/QDmsz2
- Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, et al. (2010) Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med 181: 1345-1354. Link: https://goo.gl/yeKAw1
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
