Archives of Pulmonology and Respiratory Care
Effects of Heat and Humidification on Aerosol Delivery during Auto-CPAP non-invasive Ventilation
Marwa Mohsen1, Ahmed A Elberry2, Abeer Salah Eldin3, Raghda RS Hussein1 and Mohamed EA Abdelrahim1,4*
2Clinical Pharmacology Faculty of Medicine, Beni-suef University, Egypt
3Respiratory Department, Faculty of Medicine, Beni-suef University, Beni-suef, Egypt
4Clinical Pharmacy Department, Faculty of Pharmacy, Ahram Canadian University, Giza, Egypt
Cite this as
Mohsen M, Elberry AA, Eldin AS, Hussein RSS, Abdelrahim MEA (2017) Effects of Heat and Humidification on Aerosol Delivery during Auto-CPAP non-invasive Ventilation. Arch Pulmonol Respir Care 3(1): 011-015. DOI: 10.17352/aprc.000017Objective: Although, the use of humidification during non-invasive ventilation (NIV) is an important factor in decreasing nasal airway resistance and assuring patient’s comfort and adherence; many in-vitro studies recommend switching off the humidifier while delivering aerosol to NIV patients.
The aim of the study was to in-vivo determine the effect of humidification on salbutamol delivered via different inhalation devices to chronic obstructive pulmonary disease (COPD) patients using automatic continuous positive airway pressure (Auto-CPAP).
Method: Aerosol delivery to NIV COPD patients by Aerogen Solo vibrating mesh nebuliser (VMN), Jet nebuliser (JET) and a metered dose inhaler (MDI) with AeroChamber MV spacer (AC) were compared with and without humidification. Auto-CPAP was adjusted at non-invasive ventilation mode with the integrated heated humidifier (IHH), as a source of humidity. The heater was set to the default setting 3, equivalent to 50ºC. Urine samples, 30 minutes and 24 hours post inhalation, as an index of the relative pulmonary and systemic bioavailability respectively, were provided by subjects and aliquots were retained for salbutamol analysis using solid phase extraction and high performance liquid chromatography (HPLC).
Results: There was no significant difference in the urinary excreted salbutamol post inhalation between the humidified and dry conditions. However, there was a significant difference between devices. The MDI with AC spacer had the highest percentage of 30 minutes urinary excreted salbutamol and JET had the lowest (p<0.01). VMN the highest percentage of 24 hours urinary excreted salbutamol and JET had the lowest but the difference was not significant.
Conclusion: Significance of switching off humidity during aerosol delivered to ventilated patient was not as previously shown in in-vitro literatures. We recommend delivering aerosol medication to Auto-CPAP NIV patient using humidity without fear of lower delivery.
Introduction
Non-invasive ventilation (NIV) is one of the standard of care for the treatment of exacerbation of chronic obstructive pulmonary disease (COPD), can prevent intubation and reduce morbidity and mortality [1].
Aerosol drug delivery in NIV is affected by several factors, including type of ventilator, mode of ventilation, circuit conditions, type of interface, type of aerosol generator, breathing parameters, drug-related factors, and patient-related factors [2-4].
Humidification of the ventilator circuit is one of the factors that were well studied for optimization of aerosol delivery in NIV patients [5-11]. Optimal humidification of the inhaled gas could improve patient comfort, facilitate use of the mask, and improve bronchodilator effects in those requiring NIV [2]. Also the use of heated humidification attenuated the increase in nasal resistance and drop in tidal volume observed during nasal NIV [12].
Most studies of humidification effects on aerosol delivery were in-vitro and all of them recommend switching off the humidifier while delivering aerosol to NIV patients for better delivery [5-11]. However, the undesirable effect of inhaled dry gas, e.g. the increase in the airway resistance, negates the potential benefits of aerosol bronchodilator therapy [1].
The source of conflict among the previous researches is between the impact of adding humidity to the circuit to prevent airway dryness and resistance [2,13], vs assuming that humidity would decrease the inhaled drug aerosol in the tested lung [5-7,14].
So, the aim of this study was to evaluate the effect of humidification on the relative pulmonary and systemic bioavailability of salbutamol delivery to Auto-CPAP non-invasively ventilated COPD patients via different inhalation methods.
Method
The study was designed to identify the effect of humidity on aerosol drug delivery during automatic continuous positive airway pressure (Auto-CPAP) non-invasive ventilation to ventilated COPD patients. As recommended previously by many studies [15,16], the inhalation devices were placed closed to the patient in the ventilator circuit as shown in Figure 1.
The Auto-CPAP (3B Medical, USA), used for ventilation, was adjusted at non invasive ventilation mode with built in integrated heated humidifier (IHH) as a source of humidity. The heater was set to the default setting 3, equivalent to 50ºC.
The study included 36 patients divided into three groups (12 (6 females) subjects each) with a previous diagnosis of chronic obstructive pulmonary disease (COPD) that had been admitted to the chest department of Beni-suef University Hospital & Chest Hospital, Beni-suef, Egypt and required Auto-CPAP support ventilation. A local hospital research ethics committee approval number: FMBSU REC FWA#: FWA00015574 was obtained for the patients in the study.
Patients in the study received ipratropium bromide (Atrovent inhalation solution containing nominal dose of 25µg.mL-1, Boehringer Ingelheim, Egypt) in place of their normal salbutamol dose before and between dosing as the high performance liquid chromatography (HPLC) analysis method differentiates between these two drugs. Each group of patients had been provided with one inhalation device along the duration of study. Group 1, 2 and 3 used Aerogen Solo vibrating mesh nebuliser nebuliser (VMN, Aerogen Limited, Galway, Ireland), Jet nebuliser (JET, Philips Respironics, UK) attached to a compressor (PortaNeb, Philips Respironics, UK) and metered dose inhaler (MDI, Ventoline, GlaxoSmithKline, Egypt) with AeroChamber MV (AC, Trudell Medical International, Canada) spacer (MDI+AC), respectively.
Patients were randomized to humidification condition studied (with or without heated humidification) on days 1 and 3. Each patient participated in the study for 4 days received two salbutamol study doses on interchangeable days with 24 hour washout period in between to allow the body to excrete the total past dose in order to prevent doses accumulation. The two doses were given to patients on days 1 and 3 with either the IHH switched on, to provide humidified air to the patient, or IHH switched off, to provide dry air using the same device.
A 5000 µg (in1 mL) salbutamol respiratory solution (Farcolin respirator solution, 5000 µg.mL-1; Pharco Pharmaceuticals, Egypt) was nebulised using VMN, and JET. The compressor provides an air flow of 6 L.min-1 into the nebuliser to aerosolize the liquid.
Sixteen MDI doses, containing 100 µg salbutamol per each puff, were delivered using AeroChamber MV spacer (AC). Before MDI insertion in the spacer, MDI was well shaken then a single actuation of salbutamol MDI was fired into the air as priming. The MDI canister was inserted in its place in the AC spacer. Actuations of the sixteen puffs were synchronized with the start of patients’ inspiration for better aerosol delivery [16,17].
The choice of inhaled salbutamol dosage for different devices was in accordance with the previous literatures [17-21].
Subjects were asked to provide a urine sample 30 minute post each salbutamol study dose (USAL0.5) as a measure of the relative bioavailability of salbutamol to the lungs [22] and to collect all their urine into a container over the next 24 hours (USAL24) as a measure of the relative systemic bioavailability of salbutamol. The volume of the 30 minute and cumulative urine samples were measured and aliquots were retained for salbutamol analysis by using solid phase extraction with Oasis MCX cartridge (Waters Corporation, USA) and HPLC assay.
HPLC with UV detection was used to identify amounts of salbutamol base in urine samples. The method used HPLC (Agilent 1260 Infinity, Germany) instrument equipped with Agilent 1260 Infinity preparative pump (G1361A), Agilent 1260 Infinity Diode array detector VL (G131SD), Agilent 1260 Infinity thermostated column compartment (G1316A) and Agilent 1260 Infinity preparative Auto-sampler (G2260A). Separation and quantitation was performed on ZORBAX Eclipse Plus C18 column (250 × 4.6 mm i.d, 5μm particle size (USA).
Statistical analysis
One-way ANOVA with the application of least significant difference (LSD) correction was used to evaluate the effect of humidity and inhalation device changes on the relative bioavailability of salbutamol to patients.
Results
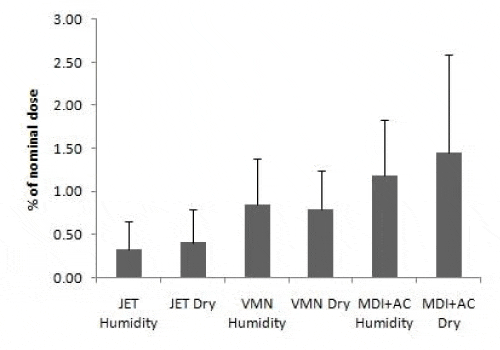
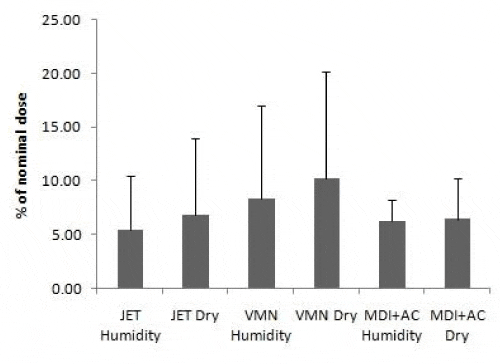
Tables 1, 2 and Figure 2 and 3 provide a summary of the mean (SD) urinary excretion of salbutamol 30 minute and 24 hours post inhalation of the study dose expressed as µg and percentage of nominal dose.
There was no significant effect of heated humidification on the urinary excreted amounts of salbutamol post inhalation with higher but not significant results with dry delivery.
There was a significantly higher urinary excretion of salbutamol 30 minutes post dose via VMN versus JET in both conditions (p<0.05). There was no significant difference between the means amount of salbutamol excreted 24 hours via VMN vs JET with higher but non-significant results by VMN.
MDI with AC spacer showed significantly higher percentage of nominal dose of salbutamol excreted in 30 minutes post dose (USAL0.5%) compared to JET (p<0.01) in both conditions. The MDI with AC spacer had a significantly higher USAL0.5% (p=0.014) compared to VMN at the dry condition only.
There was no significant difference in amounts salbutamol urinary excreted 24 hours post dosing (USAL24%) among the three inhalation devices with relatively higher percent by VMN.
Discussion
It has been shown that the coefficient of variation for urinary salbutamol was approximately double that for plasma salbutamol which means that the urine had double precision the plasma for the assay for salbutamol detection [23]. Also, serum or plasma drug concentrations are low because inhaled doses are small and the volume of distribution is large [24]. Many β-agonists are polar and basic therefore, have a high renal clearance, which is unaffected by pH. So, could be easily detected in urine. Urinary excreted salbutamol 24 hours post inhalation (USAL24) is greater than the amount excreted 30 minutes post dosing (USAL0.5). Hindle and Chrystyn explained the cause for this indication by documenting that these values represent the relative amount absorbed through combined pulmonary and oral route, and through the lung alone, respectively [25]. Similarly, it has been reported that gastrointestinal absorption contributes only to 0.3% of the overall systemic absorption from the inhaled dose 30 minutes after inhalation [26]. Hindle and Chrystyn also reported that the amount of salbutamol excreted in urine during the first 30 minutes after inhalation can be used as an index of the amount of salbutamol deposited in the lungs and the amount of salbutamol excreted in the urine in the first 24 hours as an index of the systemic absorption of salbutamol following inhalation [22]. Therefore we used this method in our study as it is simple non-invasive in-vivo method.
Similar to many previous studies, VMN provided higher USAL0.5, hence a higher pulmonary bioavailability, than JET [9,15,16, 27-29]. That means higher efficiency of the VMN in aerosol drug delivery. Moreover, MDI with spacer significantly increased the relative pulmonary bioavailability (USAL0.5%) compared to JET and VMN [14,16] and that could be because we synchronized the MDI dose delivery and the patient inhalation which increased the lung deposition [16,30].
The non-significant difference between the USAL 24% could be due to the use of each device with different group of patients which represent an additional variable that would affect the result. The same three devices when compared within the same patients resulted in a significantly higher USAL24% by the VMN [29,30].
The main finding in our study was the absence of significance difference in the amounts of salbutamol between the dry and humidified settings. This finding indicates that aerosol delivery while humidifier switched off is of no significance effect on the pulmonary and systemic bioavailability of inhaled salbutamol. Meanwhile, the impact of adding humidity to aerosol delivery in NIV mainly comes from its ability to decrease airway resistance [1]. Previous in-vitro studies recommended switching off the humidifier for better delivery [8-11]. However, our results showed that the humidity had much less effect on aerosol delivery during Auto-CPAP NIV and those in-vitro studies over estimate the effect of humidity on aerosol delivery. So, we suggest delivering aerosol medication with humidifier on to avoid the risk of dry air since the difference was not significant. In the meantime, the ventilator setting and the presence of the aerosol delivery methods close to the patient in our study compared to the other studies could decrease the humidification effect on aerosol delivery and that could also be a reason for such a variation. Hence further in-vivo studies are recommended with different aerosol delivery position in NIV circuit and different ventilator setting.
Conclusion
MDI with AeroChamber MV spacer and vibrating mesh nebuliser deliver more aerosol to Auto-CPAP NIV patient lung than jet nebuliser.
No significant difference was found when humidifier was switched on or off during aerosol delivery; therefore, we recommend keeping the humidifier on while delivering aerosol to Auto-CPAP NIV patient to avoid the adverse effect of dry gas.
Further in-vivo studies are recommended to determine the humidification effect on aerosol delivery in different ventilator setting.
- Branson RD, Gentile MA (2010) Is humidification always necessary during noninvasive ventilation in the hospital? Respiratory Care 55: p209-216. Link: https://goo.gl/WU0VFB
- Dhand R (2012) Aerosol therapy in patients receiving noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv 25: p63-78. Link: https://goo.gl/bYjIIp
- Dhand R (2004) Basic techniques for aerosol delivery during mechanical ventilation. Respir Care 49: p611-22. Link: https://goo.gl/lvfOth
- Dolovich MB, Dhand R (2011) Aerosol drug delivery: developments in device design and clinical use. The Lancet 377: p1032-1045. Link: https://goo.gl/7JvEAM
- Thille AW, Bertholon JF, Becquemin MH, Roy M, Lyazidi A, et al. (2011) Aerosol delivery and humidification with the Boussignac continuous positive airway pressure device. Respiratory Care 56: p1526-1532. Link: https://goo.gl/kQQEvi
- Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC (2003) Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluation. American journal of respiratory and critical care medicine 168: p1205-1209. Link: https://goo.gl/Agsjwg
- Dhand R, Mercier E (2007) Effective inhaled drug administration to mechanically ventilated patients. Expert opinion on drug delivery 4: p47-61. Link: https://goo.gl/YtL2KH
- Lange CF, Finlay (2000) WHOvercoming the adverse effect of humidity in aerosol delivery via pressurized metered-dose inhalers during mechanical ventilation. American journal of respiratory and critical care medicine 161: p1614-1618. Link: https://goo.gl/0q7MmG
- Ari A, Areabi H, Fink J (2010) Evaluation of position of aerosol device in two different ventilator circuits during mechanical ventilation. Respir Care 55: p837-844.
- Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, et al. (2010) Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care 55: p845-851. Link: https://goo.gl/JH5gIE
- Ari A, Sheard M, Alquaimi MM, Alhamad B, Fink JB (2016) Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity. Respir Care 61: p.600-6. Link: https://goo.gl/kZ1n5T
- Tuggey JM, Delmastro M, Elliott MW (2007) The effect of mouth leak and humidification during nasal non-invasive ventilation. Respiratory medicine 101: p1874-1879. Link: https://goo.gl/cklG6n
- Nava S, Navalesi P, Gregoretti C (2009) Interfaces and humidification for noninvasive mechanical ventilation. Respiratory Care 54: p71-84. Link: https://goo.gl/tt5qaV
- Piccuito CM, Hess DR (2005) Albuterol delivery via tracheostomy tube. Respiratory Care 50: p1071-1076. Link: https://goo.gl/FFjky7
- Abdelrahim ME, Plant P, Chrystyn H (2010) In-vitro characterisation of the nebulised dose during non-invasive ventilation. J Pharm Pharmacol 62: p966-972. Link: https://goo.gl/VTasuC
- Hassan A, Rabea H, Hussein RRS, Eldin RS, Abdelrahman MM, et al. (2016) In-vitro Characterization of the Aerosolized Dose During Non-Invasive Automatic Continuous Positive Airway Pressure Ventilation. Pulmonary Therapy 2: p115-126. Link: https://goo.gl/TerT6G
- Hussein RRS, Ali AMA, Salem HF, Abdelrahman MM, Said ASA, et al. (2015) In vitro/in vivo correlation and modeling of emitted dose and lung deposition of inhaled salbutamol from metered dose inhalers with different types of spacers in noninvasively ventilated patients. Pharm Dev Technol p1-10. Link: https://goo.gl/rcg5C1
- Berlinski A, Willis JR (2013) Albuterol delivery by 4 different nebulizers placed in 4 different positions in a pediatric ventilator in vitro model. Respir Care 58: p1124-1133. Link: https://goo.gl/Dkav8c
- Pallin M, Naughton MT (2014) Noninvasive ventilation in acute asthma. J Crit Care 29: p586-93. Link: https://goo.gl/lyeqJS
- Ari A, Harwood RJ, Sheard MM, Fink JB (2015) Pressurized Metered-Dose Inhalers Versus Nebulizers in the Treatment of Mechanically Ventilated Subjects With Artificial Airways: An In Vitro Study. Respir Care 60: p1570-4. Link: https://goo.gl/fHg4uP
- Rabea H, Ali AMA, Eldin RS, Abdelrahman MM, Said ASA, et al. (2017) Modelling of in-vitro and in-vivo performance of aerosol emitted from different vibrating mesh nebulisers in non-invasive ventilation circuit. Eur J Pharm Sci 97: p182-191. Link: https://goo.gl/OjX5hq
- Hindle M, Chrystyn H (1992) Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol 34: p311-315. Link: https://goo.gl/p8P21g
- Clark D, McPhate G, Clark G, Lipworth BJ (1996) Lung bioavailability of generic and innovator salbutamol metered dose inhalers. Thorax 51: p325-326. Link: https://goo.gl/jGUK6U
- Chrystyn H (1994) Standards for bioequivalence of inhaled products. Clin Pharmacokinet 26: p1-6. Link: https://goo.gl/U9gVdw
- Hindle M, Chrystyn H, Newton DA (1994) Relative bioavailability of salbutamol to the lung following inhalation using metered dose inhalation methods and spacer devices. Thorax 49: p549-53. Link: https://goo.gl/w7uShi
- Anhoj J, Bisgaard H, Lipworth BJ (1999) Effect of electrostatic charge in plastic spacers on the lung delivery of HFA-salbutamol in children. Br J Clin Pharmacol 47: p333-336. Link: https://goo.gl/brfA2b
- Wilkins RL, Stoller JK, Scanlan CL (2004) Egan's fundamentals of respiratory care. Recording for the Blind & Dyslexic.
- ElHansy MHE, Boules ME, Farid H, Chrystyn H, El-Maraghi SK, et al. (2016) In vitro aerodynamic characteristics of aerosol delivered from different inhalation methods in mechanical ventilation. Pharmaceutical Development and Technology 1-6. Link: https://goo.gl/9HHRSL
- Abdelrahim ME, Assi KH, Chrystyn H (2011) Relative bioavailability of terbutaline to the lung following inhalation, using urinary excretion. Br J Clin Pharmacol 71: p608-610. Link: https://goo.gl/Ss3Tdj
- Hassan A, Eldin RS, Abdelrahman MM, Abdelrahim ME (2017) In-vitro / In-vivo comparison of inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during non-invasive ventilation. Experimental Lung Research. Link: https://goo.gl/AEvcgD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
