Annals of Pancreatic Disorders and Treatment
Survival benefits and safety of chemotherapy regimens for pancreatic cancer: An umbrella review of meta-analyses of randomized controlled trials
Aditi Kharat1, Chia Jie Tan1, Manit Saeteaw2, Anindit Chhibber1, Joseph Biskupiak1 and Sajesh K Veettil1, and Nathorn Chaiyakunapruk1,3*
2Division of Pharmacy Practice, Ubon Ratchathani University, Ubon Ratchathani, 34190, Thailand
3IDEAS Center, Veterans Affairs Salt Lake City Healthcare System, Salt Lake City, UT, 84148, USA
Cite this as
Kharat A, Tan CJ, Saeteaw M, Chhibber A, Chaiyakunapruk N, et al. (2022) Survival benefits and safety of chemotherapy regimens for pancreatic cancer: An umbrella review of meta-analyses of randomized controlled trials. Ann Pancreat Disord Treatm 4(1): 001-020. DOI: 10.17352/apdt.000008Copyright
© 2022 Kharat A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Several meta-analyses have reported the survival benefits and safety issues of chemotherapy regimens for pancreatic cancer (PC). The aim was to perform an umbrella review to summarize the existing evidence from meta-analyses of randomized controlled trials (RCTs).
Methods: EMBASE, PubMed, Cochrane database of systematic reviews, and Epistemonikos were searched from inception to October 31st, 2021.Methodological quality was assessed using the A Measurement Tool to Assess Systematic Reviews (AMSTAR-2). The quality of evidence was evaluated using GRADE criteria (Grading of Recommendations, Assessment, Development, and Evaluations).
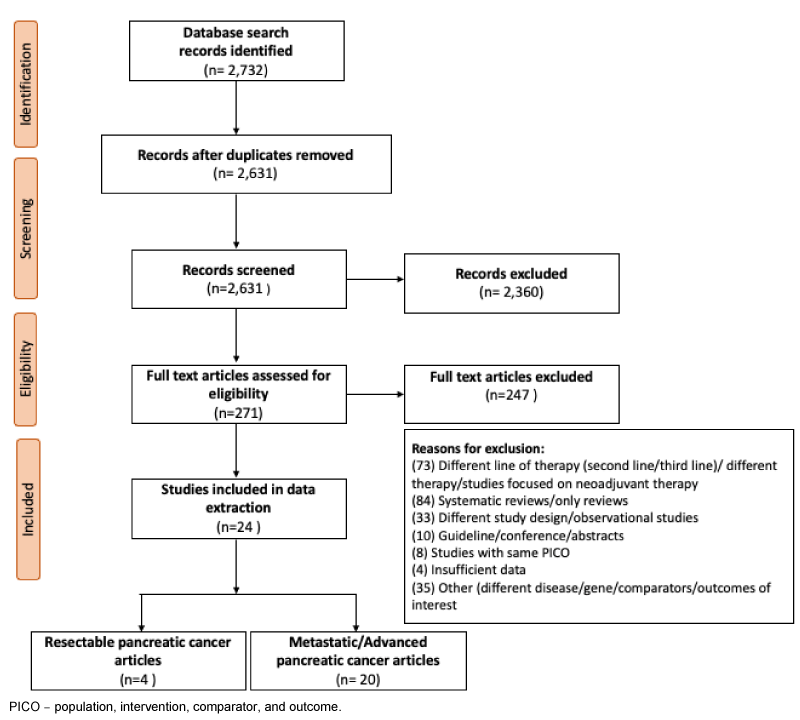
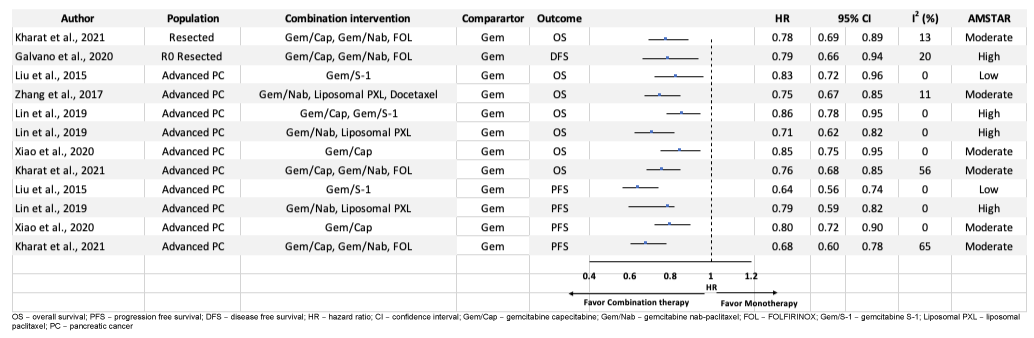
Results: A total of 2,732 records were identified with 24 articles corresponding to 168 meta-analyses in resected/metastatic PC. Two (8.3%) studies were found to be of high methodological quality. Eighty (47.6%) meta-analyses reported survival benefits of using combination chemotherapy, while 88 (52.4%) meta-analyses reported safety outcomes. 78 (46.42%; 36-efficacy, 42-safety outcomes) of the 168 meta-analyses were statistically significant (P ≤0.05). No meta-analyses were found to be of high-quality evidence. Twelve meta-analyses reporting the survival benefits of gemcitabine combinations were graded as moderate quality of evidence. Combination regimen FOLFIRINOX, gemcitabine nab-paclitaxel (gem/nab), and gemcitabine capecitabine (gem/cap) compared to gemcitabine monotherapy were found to improve overall survival (OS) and progression free survival (PFS) for both resected (OS: HR = 0.78 (0.69-0.89); PFS: HR=0.79 (0.66-0.94)) and advanced PC (OS: HR = 0.76 (0.68-0.85); PFS: HR = 0.68 (0.60 -0.78)). One meta-analysis comparing the gemcitabine combination regimens (with Nab/Paclitaxel or Capecitabine) versus monotherapy among metastatic PC patients was upgraded to high quality after a sensitivity analysis excluding small-sized studies (PFS; HR = 0.78 (95% CI, 0.69-0.88)). The remaining meta-analyses were either low or very low quality of evidence.
Conclusion: Our review showed that the use of combination chemotherapy regimens demonstrated survival benefits over gemcitabine monotherapy, which were supported by moderate to high-quality evidence. Gemcitabine combined with taxanes particularly showed high benefits for overall survival but only a modest benefit for progression free survival for metastatic PC. SWOG-1505 study compared perioperative FOLFIRINOX vs gem/nab in patients with resectable PC but no differences in survival was found. To date, FOLFIRINOX and gem/nab have been compared in the perioperative setting but no phase III trials have performed direct head-to-head comparisons for FOLFIRINOX against gemcitabine-based combination treatments in the metastatic setting. In future, head-to-head clinical trials comparing safety and efficacy for FOLFIRINOX vs gemcitabine-based combinations regimens (specifically gem/nab and gem/cap) in the metastatic setting are required.
Introduction
Pancreatic cancer (PC) is associated with high morbidity, mortality, and poor prognosis. Although accounting for only 2.6% of the global cancer burden in 2022 with an estimated total of 495,773 new cases, this malignancy was the seventh leading cause of cancer mortality, leading to 466,003 deaths worldwide[1]. At its current trajectory, PC is expected to surpass breast cancer as the third leading cause of cancer death by 2025 despite the relatively small number of cases [2]. While overall cancer survival has increased over the last two decades due to superior therapeutic strategies and earlier detection, improvements in survival for pancreatic ductal adenocarcinoma has been modest [3,4]. For resected PC, there have been significant improvements in survival with adjuvant chemotherapy regimen FOLFIRINOX [4] .
Approximately 80% of PC patients have an unresectable disease. Of these, 50% have metastatic disease and 30% have locally advanced disease [4]. Only about 20% are diagnosed in their early stages when surgical resection is possible. For advanced PC, chemotherapy is the mainstay treatment and for resectable PC, although surgery is the primary treatment modality, survival is prolonged with the use of chemotherapy either in the adjuvant or neoadjuvant setting. In current practice, FOLFIRINOX, gemcitabine-nab paclitaxel (gem/nab), gemcitabine-capecitabine (gem/cap), gemcitabine S-1 are commonly used treatment regimens for PC. Other agents such as irinotecan, and platinum agents like cisplatin and oxaliplatin have also been added to the array of treatments for PC, vastly widening the range of chemotherapy agents available [5].
Several meta-analyses of randomized controlled trials (RCTs) have been published that assessed the safety and the survival benefits of different combination chemotherapy regimens for PC. However, no attempt has been made to summarize the findings from these meta-analyses and assess the quality of evidence. Umbrella reviews make it feasible to summarize the evidence from multiple meta-analyses on the same topic [6,7]. This umbrella review aimed to systematically identify relevant meta-analyses of RCTs, summarize their findings, and assess the quality of evidence to provide an aggregate picture of survival benefits and safety concerns associated with different combination chemotherapy regimens for PC. This study could help determine which chemotherapy regimen is optimal for resectable and metastatic PC in terms of both efficacy and safety.
Methods
This umbrella review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [8]. The protocol of this umbrella review has been registered in OSF Registries (https://osf.io/32dze/).
Search strategy
A systematic literature search was conducted in PubMed, EMBASE, Cochrane database of systematic reviews, and Epistemonikos from inception until October 31st, 2021. Additional articles from reference list of included studies were also searched. Our search strategy included PC type, line of therapy, and different chemotherapy treatments. The search strategy is listed in the supplementary documents (eTable 1).
Eligibility criteria
We included systematically reviewed meta-analysis and network meta-analysis (NMA) of randomized controlled trials (RCTs) that compare survival benefits and/or adverse events of at least two or multiple chemotherapy treatment regimens that are used as first line therapy for both resectable and metastatic PC. No language restrictions were applied. When more than one meta-analysis was available for the same research question, we selected the meta-analysis with the largest data set as previously described elsewhere [7,9,10]. We excluded pooled analyses of a non-systematic selection of observational studies, non-systematic reviews, and indirect/mixed treatment estimates from NMA that did not perform grading. We also excluded meta-analysis that provided insufficient or inadequate data for evidence synthesis.
Title and abstract screenings were done independently by two reviewers (CJ, MS). Full text screening of the potentially eligible articles was also done by the same two reviewers. A third reviewers (AK) was consulted in case of any discrepancies.
Data extraction
Data were extracted at the meta-analysis level. The primary outcome for this review was survival. Types of survival included were overall survival (OS) and progression free survival (PFS) /disease free survival (DFS). Adverse events as defined and graded by Common Terminology Criteria in Adverse Events (CTCAE) were also included as a secondary outcome [11]. Definition for each survival outcome and a list of adverse events included in this review is provided in the supplementary materials (eTable 2).
Methodological quality assessment
The methodological quality of each eligible meta-analysis was assessed using A Measurement Tool to Assess Systematic Reviews (AMSTAR-2) [12], and quality was rated as critically low, low, moderate, or high. Two reviewers (AC, SV) independently performed the data extraction and quality assessments, and a third reviewer (AK) was consulted with any disagreements for resolution.
Data synthesis and sensitivity analyses
For each meta-analysis, we reported the summary estimates, corresponding 95% confidence intervals (CIs), heterogeneity with the I2 statistic, and publication bias according to the original studies. The quality of evidence in a meta-analysis was assessed by using the grading of recommendations, assessments, and evaluations (GRADE) approach [13]. The GRADE approach comprises of five domains 1) risk of bias in the individual studies, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias and the quality of evidence is categorized as very low, low, moderate, and high [14]. GRADEpro version 3.6.1. was used for assessing strength of evidence (McMaster University, 2014).
For each meta-analysis that was initially graded as high or moderate quality of evidence, a series of sensitivity analyses were performed to determine the robustness of the findings as follows: excluding primary studies with a high risk of bias and by excluding small-sized studies (i.e., include 25% of the largest trials and exclude small sized studies regardless of existence of small study effect) [15,16].
Results
Study selection
A total of 2,732 publication were screened and of those 271 (9.92%) full-text articles were assessed for eligibility. From 271 articles, 24 (8.85%) [17-40] articles corresponding to 168 meta-analyses in patients with either resected (n = 34) or metastatic (n = 134) PCs were included in this umbrella review (Figure 1). The reasons for exclusion for the remaining 247 (91.15%) articles are provided in supplementary material (eTable 3).
Study characteristics
Characteristics of the included meta-analyses are summarized in supplementary material (eTable 4, eTable 5). The median number of RCTs per meta-analysis for both survival and adverse event outcomes were 5 [Interquartile range (IQR): survival outcome (3-7); adverse event outcomes (4-9)]. The sample size per meta-analysis was 1043 for survival outcomes (IQR: 468 – 2089) and 1442 for adverse event outcomes (IQR: 916 – 2104). The median duration of follow up time was 41 months (IQR: 34 – 57). The meta-analyses included in this umbrella review were published between 2006 and 2021. Among 168 meta-analyses, 80 (47.61%) and 88 (52.38%) meta-analyses reported survival and adverse event outcomes, respectively.
Quality assessment
Evaluation of methodological quality of included meta-analyses using AMSTAR-2 revealed that only 2 (8%) [36,37] studies were found to be of high methodological quality, 6 (25%) [17,21,24-26] were moderate, 9 (38%) [18,20,30-33,38-40] were low, and the remaining 7 (29%) [19,23,27–29,34,35] were rated as critically low quality.
Findings from survival outcomes
Among 80 meta-analyses, OS was reported in 56 (resected – 5; metastatic – 51) meta-analyses and the remaining 24 (resected – 7; metastatic – 17) reported PFS. A total of 36 meta-analyses (45%) were found to be statistically significant at p ≤0.05 [resected: OS – 3, PFS – 4; metastatic: OS – 16, PFS – 13] (Descriptive characteristics of all statistically significant associations for survival outcomes are included in Table 1). Overall, the findings from these meta-analyses suggested that the combination treatment regimens improved survival outcomes compared to gemcitabine monotherapy. Combination treatments reported a median survival of 16.8 months (IQR: 5 -23) compared to 9.3 months (IQR: 3 – 14) for gemcitabine monotherapy. Forty-four (55%) meta-analyses were found to be non-statistically significant and the descriptive characteristics of survival outcomes without statistical significance at p≤0.05 are presented in eTable 4.
GRADE was used to assess strength of evidence and we found that majority of the meta-analyses were graded as either low (N = 28) or very low (N = 22) quality. Twelve meta-analyses focusing on survival outcomes were found to be of moderate quality (Figure 2). We were unable to grade 18 meta-analyses because of lack of either risk of bias data or publication bias data. Among 12 meta-analyses [22,24,26,36,37,40] graded as moderate quality, 2 meta-analyses [22,36] comparing combination therapy (gem/cap, FOLFIRINOX, and gem/nab) with gemcitabine monotherapy demonstrated improved OS (HR=0.78 (0.69-0.89)) and PFS (HR=0.79 (0.66-0.94)) in resected PC patients. Improvements in PFS for resected PC was specific for R0 resection cases whereas OS was applicable to both patients with R0 and R1 resection. Other 6 meta-analyses [22,24,26,37,40] comparing combination therapy with gemcitabine monotherapy demonstrated improved OS in metastatic PC patients (Figure 2). Gem/taxane combinations [nab-paclitaxel and liposomal paclitaxel (HR = 0.71 (0.62-0.82)) and nab-paclitaxel, liposomal paclitaxel, and docetaxel (HR = 0.75 (0.67-0.85))] followed by combination of gem/cap, gem/nab, and FOLFIRINOX (HR = 0.76(0.68-0.85)) were found to have the highest OS improvement. The remaining 4 meta-analyses [22,24,37,40] comparing combination therapy with gemcitabine monotherapy demonstrated improved PFS among metastatic PC patients (Figure 2). Gem/S-1 was found to have the highest PFS with a HR of 0.64 (0.56 – 0.74), followed by gem/cap, gem/nab and FOLFIRINOX (HR = 0.68 (0.60 -0.78)). Gemcitabine combinations with taxanes and capecitabine showed modest improvements in PFS (gem/taxanes: HR = 0.79 (0.59-0.82); gem/cap: HR = 0.80 (0.72-0.90)).
Findings from safety outcomes
Of the 88 meta-analyses reporting adverse event outcomes, 22 were for resected PC and 66 for metastatic PC. The most common adverse events included in meta-analyses were nausea/vomiting (N = 17), neutropenia (N = 16), anemia (N = 13), diarrhea (N = 13), thrombocytopenia (N = 12), fatigue (N = 8), leukopenia (N = 4), neuropathy (N = 1), and combination of different above adverse events (N = 4). Of the 88 meta-analyses, 42 (47.72%) were found to be statistically significant at p≤0.05 (Descriptive characteristics of all statistically significant associations for safety outcomes are included in Table 2). List of statistically significant AEs were as follows: diarrhea (N = 9), neutropenia (N = 8), thrombocytopenia (N = 7), nausea/vomiting (N = 6), fatigue (N = 3), leukopenia (N = 2), anemia (N = 2), neuropathy (N = 1), and combination of different above adverse events (N = 4). Of the 42 meta-analysis that were found to be statistically significant, 32 (76.19%) meta-analysis suggested that combination treatment regimens were associated with increased risk of adverse events among both resected and metastatic PC compared to gemcitabine monotherapy. The remaining 10 (23.81%) meta-analyses suggested that combination regimens did not significantly increase risk of adverse events, specifically neutropenia (metastatic PC – 2; resected PC - 1), thrombocytopenia (metastatic PC – 1; resected PC - 1), followed by anemia (n = 1), leukopenia (n= 1) for resected PC, and nausea/vomiting (n = 2) and diarrhea (n =1) for metastatic PC (Table 2). The remaining 46 (52.27%) meta-analyses were found to be non-statistically significant and the descriptive characteristics of safety outcomes without statistical significance at p≤0.05 are presented in eTable 5.
We found that majority of the meta-analyses were graded as either low (N=36) or very low (N=30) quality. No meta-analysis for adverse events was found to be of moderate or high quality. We could not grade 22 meta-analyses, and this was due to lack of either risk of bias data or publication bias data (eTable 5).
Sensitivity analysis
Findings from sensitivity analyses are presented in supplementary material eTable 6. When RCTs with a high risk of bias were excluded, only one meta-analysis [24] comparing gem/cap to gemcitabine monotherapy for PFS in patients with metastatic PC was downgraded to low quality due to imprecision, while others retained the same rank. A sensitivity analysis excluding small-sized studies showed that one meta-analysis [22] comparing gemcitabine combination therapy (with nab/paclitaxel or capecitabine) versus gemcitabine monotherapy among metastatic PC patients for PFS was upgraded to high quality evidence (HR = 0.78 (95%CI, 0.69-0.88)). Meanwhile, one meta-analysis [26] comparing gem/taxanes (nab-paclitaxel, liposomal paclitaxel, and docetaxel) to gemcitabine monotherapy was downgraded to very low quality because of high imprecision, while others retained the same rank.
Discussions
Chemotherapy is one of the most effective modalities to improve outcomes in PC treatment. Chemotherapeutic agents, such as fluorouracil and gemcitabine, have been used both as monotherapy and in combination with other drugs for PC. With more clinical studies performed, the efficacy and safety of numerous chemotherapy regimens have been gradually improved over the years and are often individualized to patient characteristics. Several meta-analyses have compiled clinical studies to compare clinical outcomes of chemotherapy regimens. However, no attempt has been made to summarize the overall findings from these meta-analyses and assess the quality of evidence. To our understanding, this is the first umbrella review performed to include the most recent evidence on chemotherapy treatments in both resected and metastatic PC exploring efficacy and safety outcomes.
Our findings suggest that there were only 2 of 24 studies of high methodological quality using the AMSTAR-2 tool and 12 of 168 meta-analyses of moderate strength of evidence using GRADE. This shows that majority of the existing evidence is of poor quality, and we need additional studies with high quality to be published. In addition, most meta-analyses were focused on metastatic PC. There were only 34 of 168 meta-analyses that explored outcomes in resected PC. The clinical studies focused on resected PC are limited and we need more investigative studies focusing on safety and efficacy of adjuvant chemotherapy treatments.
Conventionally, combining different chemotherapeutic agents for antineoplastic treatment, especially those with synergistic effects, is expected to enhance the kill rate of cancer cells beyond an additive impact [41]. This could explain the findings of our analysis where combination treatment regimens conferred better survival outcomes in both resected PC and metastatic PC over monotherapy regimens. Recent clinical practice guidelines, including those by the National Comprehensive Cancer Network (NCCN) [5] and the American Society of Clinical Oncology (ASCO) [42,43] recommended combination chemotherapy, such as gemcitabine-based chemotherapy or FOLFIRINOX, as the preferred chemotherapy regimens for resected PC and metastatic PC. For resectable PC, PRODIGE trial comparing FOLFIRINOX to gemcitabine alone found the median overall survival associated with FOLFIRINOX to be 54.4 months, compared to 35 months for gemcitabine (stratified HR for death, 0.64 (95% CI, 0.48-0.86); p = .003), and the median DFS of 21.6 and 12.8 months for FOLFIRINOX and gemcitabine respectively (HR = 0.58 (95% CI, 0.46-0.73); p < 0.001) [44]. In the metastatic setting, FOLFIRINOX compared to gemcitabine alone (OS: 11.1 vs 6.8 months; PFS: 6.4 vs 3.3. months) was associated with a survival advantage ((OS: HR = 0.57 (95% CI, 0.45-0.73); p < 0.001; (PFS: HR = 0.47 (95% CI, 0.37-0.59); p < 0.001)) [45]. SWOG-1505 study compared perioperative FOLFIRINOX vs gem/nab in patients with resectable PC but no differences in survival was found [46]. To date, FOLFIRINOX and gem/nab have been compared in the perioperative setting but no phase III trials have performed direct head-to-head comparisons for FOLFIRINOX against gemcitabine-based combination treatments in the metastatic setting. More trials with FOLFIRINOX are needed to generate further evidence.
Among the gemcitabine-based combinations, results of this study suggest that gemcitabine with nab-paclitaxel represents the most efficacious option, especially among patients with a good performance status (Eastern Cooperative Oncology Group (ECOG) performance score (PS) of 0 to 1). Our findings align with recommendations made by NCCN and ASCO [42,47], that suggest treatment with Gemzar (gemcitabine) and Abraxane (nab-paclitaxel) is more effective than standard single drug therapy for metastatic PC patients [48]. For PC patients with a high ECOG PS, albumin bound paclitaxel combination with gemcitabine has proven to delay cancer progression and improve survival [42,47]. Gemcitabine belongs to a drug class called antimetabolites, which halts growth of cancer cells after being incorporated in the genetic material of the cells. Nab-paclitaxel is a reformulation of an older drug paclitaxel, where the drug particles are bound to albumin, enhancing the delivery to the tumor, hence increasing the kill rate of cancer cells, and reducing off-site adverse effects. From the studies included in our umbrella review, 2 studies [26,37] reporting 4 meta-analyses for gem/nab vs gemcitabine significantly improved survival among patients, but RCTs assessed in these meta-analyses primarily included less severe patients with an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0-1 [49,50]. Nevertheless, clinical trials evaluating chemotherapy regimens in PC usually include patient populations that have good performance status [51]. Among patients with poorer performance status, evidence to support specific treatments is sparse but modification of combination regimens or monotherapy is often recommended [51,52].
When evaluating chemotherapeutic regimens for PC treatment, adverse events are an important issue to consider due to the severity of side effects associated with antineoplastic treatment that can greatly affect the quality of life of patients and have consequences on the treatment, such as dose reduction and treatment discontinuation. While combination chemotherapy may provide higher efficacy due to the synergism of different mechanisms of action, patients may suffer from a wider range and potentially more severe adverse effects. Gemcitabine is one of the backbone chemotherapy agents in PC and myelosuppression chemotherapy induced neutropenia is commonly reported among patient receiving gemcitabine, especially in combination with other chemotherapy. Our study showed a significantly increased risk of adverse events in 34 of 42 meta-analyses of gemcitabine-based combination compared with gemcitabine monotherapy especially in neutropenia and thrombocytopenia. Nevertheless, neutropenia is mostly reversible, and its higher risk associated with gemcitabine combination regimens can be managed with existing treatment guidelines on the monitoring and handling of low blood counts [53-55].
Our umbrella review has several limitations. This umbrella review focused on existing meta-analysis. We found that certain important adverse events such as blood clots, pain, and neurotoxicity were not included in existing meta-analysis, preventing us from making a comprehensive evaluation of safety aspects of chemotherapy treatments. This review did not directly assess the quality or recalculate the effect size along with 95% CI of all primary studies included in each meta-analysis. Instead, we relied on assessments reported by study authors. Because our study was restricted to meta-analysis of RCTs, we could not report real-world effectiveness of these chemotherapy treatments. Differences in baseline characteristics of participants in the individualized clinical studies could influence efficacy and safety outcomes comparison. The focus of our review was to provide an overview of safety and efficacy evidence for various chemotherapy treatments from existing meta-analysis, and therefore, we could not analyze interventions by subgroups such as sex, performance status, resection type, and duration of PC.
Conclusion
The use of combination treatment regimens demonstrated survival benefits over gemcitabine monotherapy, but it was also associated with higher adverse events. Gem/taxanes showed high survival benefits for OS but only a modest benefit for PFS for metastatic PC. Combination of FOLFIRINOX, gem/nab, and gem/cap compared to gemcitabine monotherapy was particularly beneficial for both OS and PFS among resected PC patients. In future, head-to-head clinical trials comparing safety and efficacy for FOLFIRINOX vs gemcitabine-based combinations regimens (specifically gem/nab, gem/cap, and gem/cisplatin) are required.
Statement of ethics
This paper is exempt from ethical committee approval because only previously published data from peer reviewed publications was used. These data do not contain any information that could identify subjects.
Author contributions
All authors have equal contribution towards conceptualizing and designing the study. Aditi Kharat, Chia Jie Tan, Manit Saeteaw, and Anindit Chhibber were involved in screening the articles, collecting, and analyzing data. Sajesh Veettil, Joseph Biskupiak, and Nathorn Chaiyakunapruk supervised the project and critically reviewed the study. All authors have read and approved the final article.
Data availability: Data from previously published meta-analyses was used for this study. All data generated or analyzed during this study are included in this article. Further enquiries can be directed to Dr. Chaiyakunapruk.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016 Sep-Oct;55(9-10):1158-1160. doi: 10.1080/0284186X.2016.1197419. Epub 2016 Aug 23. PMID: 27551890.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. doi: 10.3322/caac.21708. Epub 2022 Jan 12. PMID: 35020204.
- Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021 Sep 7;326(9):851-862. doi: 10.1001/jama.2021.13027. Erratum in: JAMA. 2021 Nov 23;326(20):2081. PMID: 34547082.
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Apr 1;19(4):439-457. doi: 10.6004/jnccn.2021.0017. PMID: 33845462.
- Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, Giovannucci EL, Varady KA, Chaiyakunapruk N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw Open. 2021 Dec 1;4(12):e2139558. doi: 10.1001/jamanetworkopen.2021.39558. PMID: 34919135; PMCID: PMC8683964.
- Veettil SK, Wong TY, Loo YS, Playdon MC, Lai NM, Giovannucci EL, Chaiyakunapruk N. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw Open. 2021 Feb 1;4(2):e2037341. doi: 10.1001/jamanetworkopen.2020.37341. PMID: 33591366; PMCID: PMC7887658.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021 Mar 29;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. PMID: 33780438; PMCID: PMC8007028.
- Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, Stubbs B, Firth J, Fornaro M, Tsartsalis D, Carvalho AF, Vieta E, McGuire P, Young AH, Shin JI, Correll CU, Evangelou E. Association of Antidepressant Use With Adverse Health Outcomes: A Systematic Umbrella Review. JAMA Psychiatry. 2019 Dec 1;76(12):1241-1255. doi: 10.1001/jamapsychiatry.2019.2859. Erratum in: JAMA Psychiatry. 2021 May 1;78(5):569. PMID: 31577342; PMCID: PMC6777224.
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014 Apr 1;348:g2035. doi: 10.1136/bmj.g2035. PMID: 24690624; PMCID: PMC3972415.
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. 2022.
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. doi: 10.1136/bmj.j4008. PMID: 28935701; PMCID: PMC5833365.
- Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth. 2019 Nov;123(5):554-559. doi: 10.1016/j.bja.2019.08.015. Epub 2019 Sep 24. PMID: 31558313.
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Syst Rev. 2013 Sep 23;2:81. doi: 10.1186/2046-4053-2-81. PMID: 24059250; PMCID: PMC3849859.
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA. 2014 Aug 13;312(6):623-30. doi: 10.1001/jama.2014.8166. PMID: 25117131.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. doi: 10.1136/bmj.l4898. PMID: 31462531.
- Chan K, Shah K, Lien K, Coyle D, Lam H, Ko YJ. A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PLoS One. 2014 Oct 6;9(10):e108749. doi: 10.1371/journal.pone.0108749. PMID: 25286060; PMCID: PMC4186762.
- Parmar A, Chaves-Porras J, Saluja R, Perry K, Rahmadian AP, Santos SD, Ko YJ, Berry S, Doherty M, Chan KKW. Adjuvant treatment for resected pancreatic adenocarcinoma: A systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2020 Jan;145:102817. doi: 10.1016/j.critrevonc.2019.102817. Epub 2019 Oct 23. PMID: 31955005.
- Tu C, Zheng F, Wang JY, Li YY, Qian KQ. An Updated Meta-analysis and System Review:is Gemcitabine+Fluoropyrimidine in Combination a Better Therapy Versus Gemcitabine Alone for Advanced and Unresectable Pancreatic Cancer? Asian Pac J Cancer Prev. 2015;16(14):5681-6. doi: 10.7314/apjcp.2015.16.14.5681. PMID: 26320435.
- Gresham GK, Wells GA, Gill S, Cameron C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta-analysis. BMC Cancer. 2014 Jun 27;14:471. doi: 10.1186/1471-2407-14-471. PMID: 24972449; PMCID: PMC4097092.
- Jin J, Teng C, Li T. Combination therapy versus gemcitabine monotherapy in the treatment of elderly pancreatic cancer: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018 Mar 7;12:475-480. doi: 10.2147/DDDT.S156766. PMID: 29563772; PMCID: PMC5846317.
- Kharat A, Brendle M, Chhibber A, Chaiyakunapruk N, Biskupiak J. Comparative Safety and Efficacy of Therapeutic Options in Resectable and Advanced/Metastatic Pancreatic Cancer: A Systematic Review and Indirect Comparison. Oncol Res Treat. 2021;44(9):476-484. doi: 10.1159/000517409. Epub 2021 Jul 27. PMID: 34315166.
- Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012 Sep 21;18(35):4944-58. doi: 10.3748/wjg.v18.i35.4944. PMID: 23002368; PMCID: PMC3447278.
- Xiao BY, Wang BC, Lin GH, Li PC. Efficacy and safety of gemcitabine plus capecitabine in the treatment of advanced or metastatic pancreatic cancer: a systematic review and meta-analysis. Ann Palliat Med. 2020 Jul;9(4):1631-1642. doi: 10.21037/apm-20-45. Epub 2020 Jun 22. PMID: 32576005.
- Li Q, Yan H, Liu W, Zhen H, Yang Y, Cao B. Efficacy and safety of gemcitabine-fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomized controlled trials. PLoS One. 2014 Aug 5;9(8):e104346. doi: 10.1371/journal.pone.0104346. PMID: 25093849; PMCID: PMC4122434.
- Zhang XW, Ma YX, Sun Y, Cao YB, Li Q, Xu CA. Gemcitabine in Combination with a Second Cytotoxic Agent in the First-Line Treatment of Locally Advanced or Metastatic Pancreatic Cancer: a Systematic Review and Meta-Analysis. Target Oncol. 2017 Jun;12(3):309-321. doi: 10.1007/s11523-017-0486-5. PMID: 28353074.
- Cao C, Kuang M, Xu W, Zhang X, Chen J, Tang C. Gemcitabine plus S-1: a hopeful frontline treatment for Asian patients with unresectable advanced pancreatic cancer. Jpn J Clin Oncol. 2015 Dec;45(12):1122-30. doi: 10.1093/jjco/hyv141. Epub 2015 Oct 30. PMID: 26518328.
- Jin SF, Fan ZK, Pan L, Jin LM. Gemcitabine-based combination therapy compared with gemcitabine alone for advanced pancreatic cancer: a meta-analysis of nine randomized controlled trials. Hepatobiliary Pancreat Dis Int. 2017 Jun;16(3):236-244. doi: 10.1016/s1499-3872(17)60022-5. PMID: 28603091.
- Bria E, Milella M, Gelibter A, Cuppone F, Pino MS, Ruggeri EM, Carlini P, Nisticò C, Terzoli E, Cognetti F, Giannarelli D. Gemcitabine-based combinations for inoperable pancreatic cancer: have we made real progress? A meta-analysis of 20 phase 3 trials. Cancer. 2007 Aug 1;110(3):525-33. doi: 10.1002/cncr.22809. PMID: 17577216.
- Xie DR, Yang Q, Chen DL, Jiang ZM, Bi ZF, Ma W, Zhang YD. Gemcitabine-based cytotoxic doublets chemotherapy for advanced pancreatic cancer: updated subgroup meta-analyses of overall survival. Jpn J Clin Oncol. 2010 May;40(5):432-41. doi: 10.1093/jjco/hyp198. Epub 2010 Feb 10. PMID: 20147334.
- Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007 Jun 20;25(18):2607-15. doi: 10.1200/JCO.2006.09.2551. PMID: 17577041.
- Huang D, Fang J, Luo G. Meta-analysis of gemcitabine and cisplatin combination chemotherapy versus gemcitabine alone for pancreatic cancer. J Cancer Res Ther. 2016 Oct;12(Supplement):104-108. doi: 10.4103/0973-1482.191616. PMID: 27721265.
- Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008 Mar 28;8:82. doi: 10.1186/1471-2407-8-82. PMID: 18373843; PMCID: PMC2292732.
- Xie DR, Liang HL, Wang Y, Guo SS, Yang Q. Meta-analysis on inoperable pancreatic cancer: a comparison between gemcitabine-based combination therapy and gemcitabine alone. World J Gastroenterol. 2006 Nov 21;12(43):6973-81. doi: 10.3748/wjg.v12.i43.6973. PMID: 17109519; PMCID: PMC4087341.
- Chen H, He R, Shi X, Zhou M, Zhao C, Zhang H, Qin R. Meta-analysis on resected pancreatic cancer: a comparison between adjuvant treatments and gemcitabine alone. BMC Cancer. 2018 Oct 23;18(1):1034. doi: 10.1186/s12885-018-4948-7. PMID: 30352573; PMCID: PMC6199735.
- Galvano A, Castiglia M, Rizzo S, Silvestris N, Brunetti O, Vaccaro G, Gristina V, Barraco N, Bono M, Guercio G, Graceffa G, Fulfaro F, Gori S, Bazan V, Russo A. Moving the Target on the Optimal Adjuvant Strategy for Resected Pancreatic Cancers: A Systematic Review with Meta-Analysis. Cancers (Basel). 2020 Feb 26;12(3):534. doi: 10.3390/cancers12030534. PMID: 32110977; PMCID: PMC7139837.
- Lin KI, Yang JL, Lin YC, Chou CY, Chen JH, Hung CC. Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer. Cancers (Basel). 2019 Nov 7;11(11):1746. doi: 10.3390/cancers11111746. PMID: 31703359; PMCID: PMC6895788.
- Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Polychemotherapy or gemcitabine in advanced pancreatic cancer: a meta-analysis. Dig Liver Dis. 2014 May;46(5):452-9. doi: 10.1016/j.dld.2014.01.001. Epub 2014 Feb 22. PMID: 24565950.
- Ciliberto D, Botta C, Correale P, Rossi M, Caraglia M, Tassone P, Tagliaferri P. Role of gemcitabine-based combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomised trials. Eur J Cancer. 2013 Feb;49(3):593-603. doi: 10.1016/j.ejca.2012.08.019. Epub 2012 Sep 16. PMID: 22989511.
- Liu Y, Huang QK, Hong WD, Wu JM, Sun XC. The addition of S-1 to gemcitabine-based chemotherapy improves survival with increased toxicity for patients with advanced pancreatic cancer: combined meta-analysis of efficacy and safety profile. Clin Res Hepatol Gastroenterol. 2015 Apr;39(2):254-60. doi: 10.1016/j.clinre.2014.08.012. Epub 2014 Oct 7. PMID: 25304193.
- Humphrey RW, Brockway-Lunardi LM, Bonk DT, Dohoney KM, Doroshow JH, Meech SJ, Ratain MJ, Topalian SL, Pardoll DM. Opportunities and challenges in the development of experimental drug combinations for cancer. J Natl Cancer Inst. 2011 Aug 17;103(16):1222-6. doi: 10.1093/jnci/djr246. Epub 2011 Jul 15. PMID: 21765011; PMCID: PMC4415086.
- Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek C, Mumber M, Schulick R, Zeh HJ, Katz MHG. Potentially Curable Pancreatic Adenocarcinoma: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019 Aug 10;37(23):2082-2088. doi: 10.1200/JCO.19.00946. Epub 2019 Jun 10. PMID: 31180816.
- Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T, Krishnamurthi S, Moravek C, O'Reilly EM, Philip PA, Pant S, Shah MA, Sahai V, Uronis HE, Zaidi N, Laheru D. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020 Aug 5:JCO2001364. doi: 10.1200/JCO.20.01364. Epub ahead of print. PMID: 32755482.
- Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018 Dec 20;379(25):2395-2406. doi: 10.1056/NEJMoa1809775. PMID: 30575490.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011 May 12;364(19):1817-25. doi: 10.1056/NEJMoa1011923. PMID: 21561347.
- Ahmad SA, Duong M, Sohal DPS, Gandhi NS, Beg MS, Wang-Gillam A, Wade JL 3rd, Chiorean EG, Guthrie KA, Lowy AM, Philip PA, Hochster HS. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg. 2020 Sep 1;272(3):481-486. doi: 10.1097/SLA.0000000000004155. PMID: 32740235; PMCID: PMC7856053.
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Apr 1;19(4):439-457. doi: 10.6004/jnccn.2021.0017. PMID: 33845462.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013 Oct 31;369(18):1691-703. doi: 10.1056/NEJMoa1304369. Epub 2013 Oct 16. PMID: 24131140; PMCID: PMC4631139.
- Zhang XW, Ma YX, Sun Y, Cao YB, Li Q, Xu CA. Gemcitabine in Combination with a Second Cytotoxic Agent in the First-Line Treatment of Locally Advanced or Metastatic Pancreatic Cancer: a Systematic Review and Meta-Analysis. Target Oncol. 2017 Jun;12(3):309-321. doi: 10.1007/s11523-017-0486-5. PMID: 28353074.
- [Lin KI, Yang JL, Lin YC, Chou CY, Chen JH, Hung CC. Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer. Cancers (Basel). 2019 Nov 7;11(11):1746. doi: 10.3390/cancers11111746. PMID: 31703359; PMCID: PMC6895788.
- Schulz D, Algül H. Chemotherapy of pancreatic cancer in patients with poor performance. Chin Clin Oncol. 2019 Oct;8(S1):S22. doi: 10.21037/cco.2019.08.01. Epub 2019 Aug 20. PMID: 31500430.
- Boeck S, Hinke A, Wilkowski R, Heinemann V. Importance of performance status for treatment outcome in advanced pancreatic cancer. World J Gastroenterol. 2007 Jan 14;13(2):224-7. doi: 10.3748/wjg.v13.i2.224. PMID: 17226900; PMCID: PMC4065949.
- Kuznar W. Updated NCCN Guideline Strongly Recommends Germline Testing for All Patients with Pancreatic Cancer. 2022.
- Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L. Myeloid Growth Factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017 Dec;15(12):1520-1541. doi: 10.6004/jnccn.2017.0175. PMID: 29223990.
- Singh N, Singh Lubana S, Dabrowski L. Isolated Chronic and Transient Neutropenia. Cureus. 2019 Sep 10;11(9):e5616. doi: 10.7759/cureus.5616. PMID: 31720132; PMCID: PMC6823038.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
