Annals of Pancreatic Disorders and Treatment
Three decades of survival in Pancreatic Neuroendocrine Tumor with Unresectable Liver Metastases
Maddibande Ramachar Sreevathsa* and Nishnata Koirala
Cite this as
Sreevathsa MR, Koirala N (2018) Three decades of survival in Pancreatic Neuroendocrine Tumor with Unresectable Liver Metastases. Ann Pancreat Disord Treatm 2(1): 002-006. DOI: 10.17352/apdt.000005Pancreatic neuro-endocrine tumors are rare and have a slow growth rate. They have long-term survival even when associated with hepatic metastases, after organ directed surgical treatment. Several prognostic factors have been identified for survival in pancreatic neuro-endocrine tumors with or without liver metastases. We describe a 32-year-old male patient with BMI of 16.4, presenting initially with recurrent, multiple, peptic ulcers and later carcinoid syndrome. A pancreatic neuro-endocrine tumor with synchronous bilobar liver metastases was discovered on laparotomy. The primary tumor was 7.5 centimeter in size, low-grade and well differentiated variety. Chromogranin A level was 81ng/liter (normal 39ng/liter) and Ki67 expression was 3%. The serum 5-Hydroxy indole acetic acid was 3246ng/ml(normal 0-22ng/ml), 24 hour urinary 5-hydroxy indole acetic acid was 1742 mg (normal 0-2.7mg) , serum gastrin level was 1060 pg/ml (normal <100pg/ml) and patient is on long-term omeprazole maintenance therapy. He has survived for 32 years without organ directed surgical treatment. Literature is briefly reviewed.
Abbreviations
PNET: Pancreatic Neuroendocrine Tumor; NET: Neuroendocrine Tumor; BMI: Body Mass Index; D1: First Part of Duodenum; D2: Second Part of Duodenum; GJ: Gastro-Jejunostomy; HPE: Histo-Pathological Examination; HIAA: Hydroxy Indole Acetic Acid; CT: Computerized Tomography; USG: Ultrasonography; PA: Pulmonary Artery; TNM: Tumor Node Metastasis; SEER: Surveillance Epidemiology and End Results; PRRT: Peptide Receptor Radionuclide Therapy
Introduction
Amongst the neuro-endocrine tumors, carcinoids are the most frequently encountered tumor type. Pancreatic carcinoids contribute to 0.55% of all carcinoid tumors [1,2]. Pancreatic neuro-endocrine tumors constitute 1-2% of all pancreatic neoplasms with an incidence of approximately 1 in a lakh population [3-5]. They are heterogeneous by nature and the incidence is rising in the western world. Though majority are functioning, 30-40% of pancreatic neuro-endocrine tumors are non-functional and present with obscure symptoms. They are also characterized by their slow growth and are less aggressive than other neuro-endocrine tumors. More than 80% of pancreatic neuro-endocrine tumors are usually metastatic at the time of presentation. The 5-year survival is reported to be 40-60% with median survival ranging from 38 to 104 months [2,6]. But due to the rarity of these tumors, data regarding survival and it’s prognostic factors come from small volume centers [6]. Long term survival in pancreatic neuro-endocrine tumors with liver metastases without resection is extremely rare.
Materials and Methods
A male aged 32-years with BMI of 16.4 was operated in 1984 in a major hospital for duodenal ulcer perforation. A year later for recurrence of duodenal ulcer which were multiple, patient underwent posterior gastro-jejunostomy in the same hospital. In 1988, he yet again underwent re-laparotomy for adhesions causing abdominal pain when the surgeon noticed micro-nodular cirrhosis of liver, which was not confirmed by biopsy. Later in 1991 patient presented to a Medical College hospital with recurrent haemetemesis, loose stools due to multiple duodenal ulcers in D1, D2, and stomal ulcer, when laparotomy revealed micro-nodular appearance of both lobes of liver and a tan colored 7.5 centimeters tumor in the body of pancreas. The duodenum was found to be deformed and GJ anastomotic area fibrotic. Bilateral truncal vagotomy, antrectomy and resection of GJ with Billroth II reconstruction was done, pancreatic tumor and liver were biopsied.
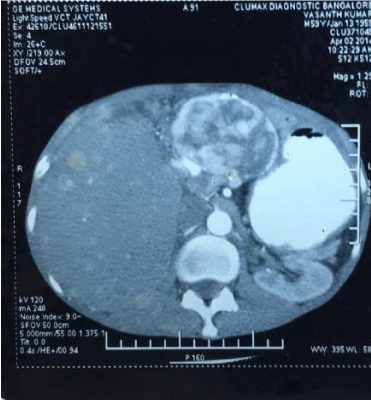
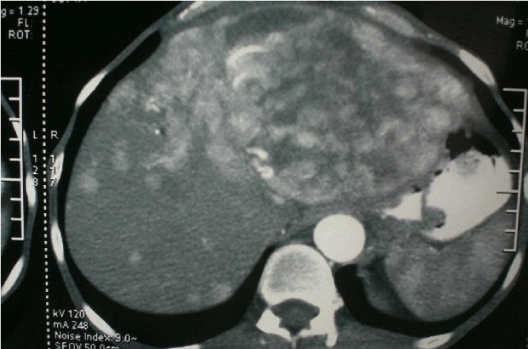
The histopathology findings of pancreas and liver were similar and reported as carcinoid of pancreas with metastases to the liver. The primary in the pancreas was well-differentiated neuroendocrine tumor of low grade variety (6 mitoses per high power field). On immuno-histochemistry the tumor was positive for chromogranin A and synaptophysin. HPE of antrum of stomach revealed chief cell hyperplasia. 24 hr urinary 5-HIAA was 1742 mg (normal 0-2.7mg/ml). The serum 5-HIAA was 3246 ng/ml (normal 0-22 ng/ml) and serum gastrin level was found to be 1060 pg/ml (normal is 0-100 pg/ml). Patient was prescribed Omeprazole 20mg/day and on follow-up in 1992 complained of loose stools with frequency of 4-5/day. He also developed flushing of the face. In 1995, he presented with increased flushing of the face, palpitations, giddiness, abdominal pain, loose stools and respiratory difficulty. There was dermatitis of skin of abdominal wall, malar area and on extensor surface of all four limbs. 2-D Echocardiography revealed tricuspid regurgitation with enlarged right ventricle and right atrium, mild pulmonary artery hypertension with pressure of 45 mm Hg. There was also mild mitral regurgitation. Diagnosis of carcinoid syndrome was made. Patient received 5 cycles of combination chemotherapy with Streptozocin and 5-Fluorouracil. The symptoms of carcinoid syndrome regressed considerably. On abdominal examination there was bilobar hepatomegaly having sharp margins and irregular surface. Patient continued to take maintenance dose of Omeprazole 20 mg/day. In 2014, he presented with abdominal pain, asthenia, ascites, hypo proteinemia and anemia. He had lost 15% of his body weight. The chest x-ray was normal. CT scan abdomen revealed increase in size of metastatic nodules involving entire left lobe and segments V and VIII. The left and middle hepatic veins were not visualized. The liver span was clinically same as in 1995 but the primary tumor in the pancreas had disappeared. Trucut needle biopsy of liver showed, NET low grade, well differentiated variety, positive for chromogranin-A, neuron specific enolase, synaptophysin. Ki 67-expression was 3% and the serum chromogranin-A level was 81ng/liter (normal: 39ng/liter) (Figures 1-4).
2-D Echocardiography also was same as in 1995 but with moderate mitral valve prolapse. His Karnofsky performance score was 65%. He was administered chemotherapy with 4 cycles of Cisplatin-Etoposide after correction of anemia and hypoproteinemia. His general condition became poor after chemotherapy and hence was abandoned. He was maintained on Omeprazole and nutritional support. Gradually he recovered from anemia, asthenia, hypoproteinemia and ascites. He regained weight partially but continued to have flushing of face, dermatitis. USG abdomen in 2017 revealed features of bilobar nodular metastasis in the liver, no pancreatic tumor, no ascites, grade 1 nephropathy with creatinine of 1.8 mg% and patient had Karnofsky performance score of 80%.
Discussion
Carcinoid tumors are the most frequently encountered neuroendocrine tumors but they rarely arise in the pancreas and some times are accidentally found. In the largest series of carcinoid tumors reported by Modlin and Sandor only 46 of the 8305 cases were pancreatic, accounting for 0.55% of all carcinoids [1,2]. Its incidence varies from 1 to 8.4/lakh population/year [2,7] Pancreatic neuroendocrine tumors (PNET), constituting 1.3% of all pancreatic cancers, are rarely resected for cure as, though they are slow growing nearly 80 % have unresectable liver metastases and some have extra hepatic metastases [3-5]. Even though PNET are slow growing, they do prognostically worse than other neuroendocrine tumors of the gastro-intestinal tract [5]. The World Health Organization has categorized gastroentero-pancreatic neuro-endocrine tumors into two types: well-differentiated neuroendocrine tumors and poorly-differentiated neuroendocrine carcinoma, based on Ki 67 index and number of mitosis in high power fields. Well-differentiated NET are sub-classified into: G1 (mitotic count of < 2 per high power fields and/or Ki67 index < 2%) and G2 (mitotic count of 2 to 20 per high power fields and/or Ki67 index of 3 to 20%). Poorly differentiated neuroendocrine carcinoma are G3 tumors with a mitotic count of > 20 per two high power fields and/or Ki67 index of more than 20% [8].
The treatment of PNET is basically surgical resection as the tumor is slow growing even when metastatic, as other modalities of treatment do not bear good results. But there is dilemma in choosing the correct surgical procedure in the setting of advanced PNET as the results of these surgical procedures are very variable. Sara Ekeblad et al, in their analysis of 324 patients of PNET from a single institution have reported five year and ten year survival to be 64% and 44% respectively with a median survival of 99 months [6]. In patients with neuroendocrine tumors having liver metastases which could be resected for cure, few authors have also reported the five year survival to be greater than 60% and some approaching 80% with mortality of resection less than 5% [9-11]. A review article on treatment of hepatic metastases with neuroendocrine tumors has summarized the 5 years survival to be 80% for R0 and 70% for R1 resection for liver metastases [12,13]. E.L. Glazer et al., earlier had reported similar outcomes in NET patients with hepatic metastases undergoing surgical treatment [10]. A single institution experience of 13 cases of PNET from China reported median survival of 26.6 months in ten out of thirteen patients undergoing radical resection. The other 3 patients succumbed at 26, 32 and 49 months respectively and in three patients with distant metastases none expressed features of carcinoid syndrome [14]. In 172 patients identified from MD Anderson hospital data base (Texas) receiving either RFA or hepatic resection, the median overall survival was 9.6 years (Range: 89 days – 22 years). Krampitz G.W et al, report a 10 year survival of 30% in PNET patients with combined liver and lymph node metastases [15]. Patients with advanced liver metastases which cannot be resected for cure can be subjected to cyto-reductive surgery (also termed debulking) which by reducing the quantum of hormonal secretion in functional tumors spirals down the endocrine symptoms, simultaneously increasing the life span [3]. Cyto-reductive surgery in patients with diffuse bilobar liver metastases due to neuro endocrine tumors is indicated in young patients with good performance status where up to 90% of tumor volume can be resected [13].
Liver transplantation was initially considered an ideal surgical solution for unresectable liver metastases, but now has almost been abandoned. The liver transplantation for neuroendocrine tumor with metastases is considered as an extended indication and hence raises ethical issues. There are strict selection criteria for liver transplantation done with curative intent for unresectable liver metastases in PNET like: a) Age < 55 years, b) Volume of involvement of liver < 50%, c) Low-grade well-differentiated tumors and d) metastatic neuroendocrine tumors where the primary has already been resected for cure a year previously and who now have stable liver metastases with no extra hepatic disease [3]. In selected gastrointestinal NET patients liver transplantation has shown a recurrence rate of < 30% over 5 years [3]. Of 185 liver transplants performed for metastatic neuroendocrine tumors in USA the five year survival was 57.8% [13]. However, it has also been reported that the follow-up of those patients with PNET who underwent liver transplantation is shorter than 6 years but having a high rate of tumor recurrence of almost 60% and hence need a longer surveillance [3]. This raises the question as to which surgical procedure is ideal for liver metastases in patients with PNET.
Touzios J.G et al, have in an analysis of study group of 60 NET patients with liver metastases concluded that aggressive surgery improved the 5-year survival to 72% compared to patients undergoing TACE, who had 50% 5-year survival [16]. Surprisingly some patients with metastatic neuroendocrine tumors had a 5-year survival of 0 to 20% when given only supportive care [17]. In practice patients with heavy load of metastases surgical resection can be carried out in only 10% of patients [17,18]. In poorly differentiated and unresectable liver metastases with PNET, a number of drug therapy options are available .They are : Cisplatinom-- streptozocin based combination chemotherapy ,TACE, selective inhibitor of mammalian target of rapamycin(Everolimus), somatostatin analogue, alpha interferon or a combination of the above [19]. A placebo controlled prospective randomized studies in patients with functional and advanced PNET showed the treatment with sunitinib or m-TOR inhibitor – Everolimus, to be associated with better progression free survival. Hence, these drugs have been recommended as the first-line of treatment for advanced PNET [16,5].
There is again a plethora of data on prognostic variables in PNET. Sara Ekeblad et al, evaluated the various survival factors of 324 patients of pancreatic neuroendocrine tumors seen in a single institution and found a median survival of 99 months. On univariate analysis, they found following to have negative prognostic value: TNM stage IIIb tumors, absence of radical surgery, Ki67>2%, non-functioning tumors, sporadic tumors, chromogranin A level > three times normal upper limit, primary tumor >3cm size, Neuro-endocrine carcinoma (WHO), BMI <20kg/m2 [6]. Krampitz G.W et al, have reported that in PNET patients with combined liver and lymph node metastases, the major determinant of survival was liver metastases and not lymph node metastases [15]. Touzios JG et al, found poor outcomes in patients with more than 50% of the liver occupied by metastases [20]. In a study conducted out of 4 high volume centers inducting 166 patients divided into 3 groups based on treatment arms: a) curative resection – 11% of patients b) palliative resection – 43% of patients c) no resection - 46% of patients, the median overall survival was 97 months, 89 months and 36 months respectively. On multivariate analysis, the factors which were associated with overall survival were bi-lobar metastases, tumor grading and curative resection in the first group, whereas G3 was the only parameter which was associated with a poor survival amongst the patients undergoing any form of surgery [21]. 53 patients with PNET were retrospectively analyzed for long term (> 5 years) survival. Resection of primary tumor, absence of liver metastasis, metachronous liver metastases and aggressive treatment of hepatic metastases were predictors of long term survival [22]. T.R. Halfdanarson analyzed 1483 PNET patients from SEER database (1973 to 2000) and surmised that age, grade, stage, and functional status predicted survival [19]. David Jeremie Birnbaum et al, found in a univariate analysis of 134 patients who were either isolated PNET or locally advanced or metastatic PNET, the following were negative factors of survival: poor differentiation, liver metastases, neuroendocrine carcinoma and resection of adjacent organs along with pancreas. In a multivariate analysis they found that the poor degree of differentiation was an independent factor influencing disease free survival [23]. In summary the predictors of survival seem to be: completeness of resection (R0), grade of tumor (WHO), degree of functional status, quantum of chromogranin expression and extent of metastases.
The subject described here had the following good prognostic factors which might explain his long term survival. The tumor was a low grade, well-differentiated carcinoid with Chromogranin A level of two time’s upper limit of normal and G2(WHO) variety tumor.
There is also controversy about the role of resectional surgery of the primary in PNET with unresectable metastatic liver disease. In a systematic review only 3 cohort studies were found supporting the above philosophy of treatment. It was found that the survival was longer in patients where the primary was resected in the presence of unresectable metastatic liver disease in 2 out-of these 3 cohort studies. Hence, there is insufficient data to advocate resection of primary PNET when the liver metastases is not addressed surgically [24].
The phenomena of long term survival in the index patient can be attributed to spontaneous regression/slow progression of pancreatic tumor mass and probably the liver metastases. The explanation for this phenomena of spontaneous regression/slow growth in PNET can be obtained in an observational study on the growth pattern of pancreatic endocrine tumors in patients with Von Hippel Lindau disease. 20% of 87 PNET patients with anatomic, functional and advanced imaging analysis done over 4 years of follow up showed decrease in their size. This imaging analysis showed a non-linear growth pattern, which includes periods of no growth and apparent decrease in size in this group [25]. There are only three case reports of intra-abdominal neuroendocrine tumors undergoing spontaneous regression in the world literature. These were anecdotal reports of a large gastric carcinoid, peritoneal spread of pelvic carcinoid and a bile duct neuroendocrine tumor. They regressed either partially or disappeared completely. The inciting agent in the first case was open biopsy of tumor, in the second case pregnancy and in the third case endoscopic forceps biopsy of tumor [26-28].
The supposed mechanisms contributing to spontaneous regression of cancers have been hypothesized by several authors and include: a) Immunological response in the host which has been best appreciated in renal cell carcinoma, b) Pro-apoptotic pathway activation, c) Release of cytokines and other related factors. The pro-apoptotic pathway activation is also supposedly driven by cytokines like TNF-α. Yet another hypothesis is the anti-proliferative reaction of tumor suppressor gene leading to vascular endothelial growth factor receptor blockage resulting in reversal of neo-vascularity of the cancer reducing its growth [27].
- Buchanan KD, Johnston CF, OHare MM, Ardill SE, Shaw C, et al, (1986) Neuroendocrine tumors. A European view. AM J Med 81:14-22. Link: https://tinyurl.com/yadj7alr
- Modlin IM, Sandor A (1997) An analysis of 8305 cases of carcinoid tumors. Cancer 15: 813-829. Link: https://tinyurl.com/y7aqly37
- Hori T, Takaori K, Uemoto S (2014) “Pancreatic Neuroendocrine Tumor Accompanied with Multiple Liver Metastases.” World Journal of Hepatology 6: 596–600. Link: https://tinyurl.com/ycheodgb
- Kimura W, Tezuka K, Hirai I (2011) Surgical management of pancreatic neuroendocrine tumors. Surgery 41: 1332-1343-15. Link: https://tinyurl.com/y9tpdxla
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, et al. (2011) Everolimus for advanced pancreatic neuroendocrine tumors. New England Journal of Medicine 364: 514–523. Link: https://tinyurl.com/yaqbkf3m
- Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B (2008) Prognostic factors and survival in 324 patients with pancreatic endocrine tumours treated at a single institution. Clinical cancer research 14: 7798-7803. Link: https://tinyurl.com/yc4pjslu
- Berge T, Linell F (1976) Carcinoid tumors, Frequency in a defined population during a 12 year period. Acta Pathol Microbiol Scand [a] 844: 322-330. Link: https://tinyurl.com/ya72tbjt
- De Lellis R, Lloyd R, Heitz P, Eng C (2004) editors. World Health Organization classification of tumors: pathology and genetics of tumors of endocrine organs. 1st ed. Lyon: IARC Pres 177–182. Link: https://tinyurl.com/y9etw5d5
- Schurr PG, Strate T, Rese K, Kaifi JT, Reichelt U, et al. (2007) Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Annals of surgery 245: 273-281. Link: https://tinyurl.com/y9fx29fp
- Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, et al. (2010) “Long term survival after surgical management of neuroendocrine hepatic metastasis”. HPB 12: 427-433. Link: https://tinyurl.com/ybs42e88
- Frilling A, Li J, Malamutmann E, Schmid KW, Bockisch A, et al. (2009) Treatment of liver metastases from neuroendocrine tumors in relation to the extent of hepatic disease. The British journal of surgery 96: 175-184. Link: https://tinyurl.com/ybw4n56q
- Elias D, Lasser P, Ducreux M, Duvillard P, Ouellet JF, et al. (2003) Liver resection (and associated extra hepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery 133: 375-382. Link: https://tinyurl.com/ybp7u4ak
- Harring Tr, Nguyen NT, Goss JA, O’Mahoney CA (2011) Review article. Treatment of liver metastasis in patients with neuroendocrine tumors: A comprehensive review. International Journal of Hepatology. Article ID 154541. Link: https://tinyurl.com/y9ucuggq
- Feng-Hualiu, Wang C, Ya-Ling Xing, Jiang-Hua Wu, Tang Y (2015) Clinical characteristics and prognosis of primary pancreatic carcinoid tumors: A report of 13 cases from a single institution. Oncology letters 9: 780-784. Link: https://tinyurl.com/ybpnconl
- Krampitz GW, Norton JA, Poultsides GA, Visser BC, Sun L, et al. (2012) Lymph nodes and survival in pancreatic neuroendocrine tumors. Archives of Surgery 147: 820-827. Link: https://tinyurl.com/yamr7t2a
- Gu P, Wu J, Newman E, Muggia F (2012) Treatment of liver metastases in patients with neuroendocrine tumors of gastro esophageal and pancreatic origin. International journal of hepatology. Link: https://tinyurl.com/yadxkm37
- Frilling A., Sotiropoulos GC, Li J, Kornasiewicz O, Plöckinger U (2010) Multimodal management of neuroendocrine liver metastases. HPB 12: 361-379. Link: https://tinyurl.com/y9gh4ghs
- Madoff DC, Gupta S, Ahrar K, Murthy R, Yao JC (2006) Update on the management of neuroendocrine hepatic metastases. Journal of Vascular and Interventional Radiology 17: 1235-1250. Link: https://tinyurl.com/y9q76wwr
- Halfdanarson TR, Rabe KG, Rubin J, Petersen GM (2008) Pancreatic neuroendocrine Tumors (PNETS): incidence, prognosis and recent trend towards improved survival. Annals of oncology 19: 1727-1733. Link: https://tinyurl.com/y6vft5ba
- Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, et al. (2005) Neuroendocrine hepatic metastases: does aggressive management improve survival? Annals of surgery 241: 776-785. Link:
- Partelli S, Inama M, Rinke A, Begum N, Valente R, et al. (2015) Long-term outcomes of surgical management of pancreatic neuroendocrine tumors with synchronous liver metastases. Neuroendocrinology 102: 68-76. Link: https://tinyurl.com/yd6nfzez
- Quyen DC, Hill HC, Douglassr HO, Driscoll D (2002) Predictive factors associated with long term survival in patients with neuroendocrine tumors of the pancreas. Annals of Surgical Oncology 9: 855-862. Link: https://tinyurl.com/y7dt9k5o
- Birnbaum DJ, Turrini O, Vigano L, Russolillo N, Autret A, et al. (2015) Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Annals of surgical oncology 22: 1000-1007. Link: https://tinyurl.com/yb2mc9e5
- Capurso G, Bettini R, Rinzivillo M, Boninsegna L, Delle Fave G, et al. (2011) Role of resection of the primary pancreatic neuroendocrine tumor only in patients with unresectable metastatic liver disease: a systematic review. Neuroendocrinology 93: 223-229. Link: https://tinyurl.com/y9d3zoua
- Weisbrod AB, Kitano M, Thomas F (2014) Assessment of Tumor Growth in Pancreatic Neuroendocrine Tumors in von Hippel Lindau Syndrome. J Am Coll Surg 218: 163–169. Link: https://tinyurl.com/ycvw3hok
- Sawant PD, Nanivadekar SA, Shroff CP, Srinivas A, Dewoolkar VV (1989) “Spontaneous regression of large gastric carcinoid” Ind.J.Gastroenterol 8: 289-290. Link: https://tinyurl.com/ybpfquyv
- SewPaul A, Bargiela D, James A, Johnson SJ, French JJ (2014) Spontaneous regression of carcinoid tumour following pregnancy. Case reports in Endocrinology Article ID 481823. Link: https://tinyurl.com/y9rfymxy
- Sano I, Kuwatani M, Sugiura R, Kato S, Kawakubo K, et al. (2017) Hepatobiliary and Pancreatic: A rare case of a well-differentiated neuroendocrine tumor in the bile duct with spontaneous regression diagnosed by EUS-FNA. Journal of gastroenterology and hepatology 32: 11. Link: https://tinyurl.com/ya9az8h3
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
