Archives of Organ Transplantation
Effects on organ donation of transition from apnoeic-oxygenation to radioisotope brain scanning to diagnose brain death in children
James Tibballs1*, Sainath Raman2 and Peter Francis3
2Senior Medical Officer, Pediatric Intensive Care Unit, Queensland Children’s Hospital, Australia
3Nuclear Medicine Physician, Medical Imaging, Royal Children’s Hospital, Melbourne, Australia
Cite this as
Tibballs J, Raman S, Francis P (2021) Effects on organ donation of transition from apnoeic-oxygenation to radioisotope brain scanning to diagnose brain death in children. Arch Organ Transplant 6(1): 001-007. DOI: 10.17352/2640-7973.000017We studied rates of cadaveric organ donation on transition from apnoeic-oxygenation to radioisotope brain perfusion scanning to diagnose brain death. We studied records of children who were organ donors and/or had Technetium Tc99m Exametazine brain scans over 1989-2018. Donation after Brain Death (DBD) commenced in 1989, Donation after Circulatory Death (DCD) in 2011. Brain scanning was adopted in 2002 and apnoeic-oxygenation abandoned in 2007. In total, 95 of 1930 children (4.9%) donated organs (81 DBD, 14 DCD). During 1989-2001, with brain death diagnosed by clinical tests including apnoeic-oxygenation, 40 of 1059 children (3.8%) donated organs at average 3.1/annum. During 2002-2007 when either apnoeic-oxygenation or scanning was used, 18 of 351 children (5.1%) donated organs at average 3.0/year. During 2008-2018 with scanning in lieu of apnoeic-oxygenation, 23 of 520 children (4.4%) donated organs at average 2.1/annum. The difference in DBD rate between eras was not significant (P=0.52). Of 77 children scanned, 65(84%) were diagnosed brain dead after 1 or 2 scans, and 29 (45%) of these donated organs. Of 12 children with limited brain perfusion (not brain dead), DCD proceeded in 8 (67%). DCD or DBD (death diagnosed by scanning) was performed during 2011-2018 in 35 of 370 children (9.5%) which is significantly more (P<0.0001) than DBD alone (death diagnosed by apnoeic-oxygenation) in 1989-2001 (3.8%). Transition from apnoeic-oxygenation to scanning to diagnose brain death did not change DBD and may have facilitated DCD. Brain death can be diagnosed by radionuclide scanning in lieu of apnoeic-oxygenation.
Introduction
The task of diagnosing brain death for the purposes of organ donation (DBD) and for guiding cessation of futile treatment for severe brain injury has traditionally been by clinical examination of the state of consciousness and by tests of brain-stem function. However, the key test of brain-stem function, the apnoeic oxygenation test, may not always be possible or be confounded by numerous factors. When this occurs, ancillary tests of brain function such as assessment of brain perfusion with lipophilic radioisotopes are recommended by authorities on organ donation such as the Australian and New Zealand Intensive Care Society (ANZICS) [1]. A test of brain perfusion may be a potential routine but more stringent alternative to apnoeic-oxygenation in clinical testing to diagnose brain death. We hypothesised that use of brain scanning in lieu of apnoeic-oxygenation testing would make no difference to the rate of organ donation.
Lipophilic radionuclide scanning has been used since the late 1980’s to diagnose brain death in adults and children [2,3] but the effect of this diagnostic mode on organ donation has not been studied. This study examines an institutional transition from the use of apnoeic-oxygenation testing as part of clinical testing of brain-stem function to the use of Single-Photon Emission Computed Tomography (SPECT) or radiopharmaceutical scan of the brain using CeretecTM - Technetium Tc99m Exametazine [(RR,SS)-4.8-diaza-3,6,6,9-tetramethylundecane-2, 10-dione bisoxime, formerly known as Tc-99m HMPAO (hexamethylpropylene amine oxime)]. CeretecTM is a diffusible lipophilic radiopharmaceutical which is rapidly taken up, retained and metabolised to a hydrophilic form by living brain tissue [4]. It attains a maximum concentration in the brain within one minute of intravenous injection followed by a loss of 15% over the ensuing two minutes. The radioactivity of the retained tracer subsequently decays [5].
The premise underlying use of radioisotope scanning to diagnose brain death is that complete absence of radioactivity in the whole brain after intravenous administration signifies absence of perfusion and/or uptake of the tracer which represents cessation of whole brain function, as legally required in Australia to diagnose brain death.
The objective of the study was to determine the effects of transition from apnoeic-oxygenation to brain radioisotope scanning on organ donation after brain death (DBD) and circulatory death (DCD).
Materials and methods
This is a retrospective study of cohorts of children who were organ donors and/or had CeretecTM brain perfusion scan during the period January 1989 to December 2018. The study was conducted by reviewing the records of the Imaging Department and of the Paediatric Intensive Care Unit (PICU) of the Royal Children’s Hospital (RCH) and data provided by DonateLife Australia.
Organ Donation after Brain Death (DBD) commenced at RCH in 1989 while organ donation after circulatory death (DCD) commenced in 2011. Brain scanning with Technetium Tc99m Exametazine (CeretecTM) was introduced into clinical practice to diagnose brain death in 2002 after it was found to be a reliable, safe and cost-effective [6,7] and has been used exclusively at RCH to diagnose brain death in lieu of apnoeic-oxygenation since 2008. Apnoeic-oxygenation was abandoned in 2007. In the interim (2002-2007) both techniques were used.
The dose of the radiopharmaceutical was adjusted for weight according to the European Association of Nuclear Medicine paediatric dose guidelines [8]. Scanning was commenced at least 15 minutes after intravenous administration. SPECT images were obtained from a dual head camera with low energy collimation positioned as close as possible to the patient, 128 x 128 matrix, 20 frames/second, 2.81 degrees angular step, 128 steps, zoom 1.46. Images were reconstructed and displayed in three planes using colour scales with 1 pixel thick slices. Brain death was reported if there was no discernible uptake of tracer within brain structures and the brain stem.
Brain death by clinical testing was defined as in the ANZICS Statement 1 which essentially is unresponsive coma, the absence of all brain stem reflexes and the absence of respiratory centre function during induced oxygenated hypercapnia (i.e., apnoea) in the clinical setting in which these findings are irreversible. In addition, there must be definite clinical or neuro-imaging evidence of acute brain pathology consistent with the irreversible loss of neurological function. Brain death may also be determined by imaging that demonstrates the absence of intracranial blood flow.
We compared the number of donors in whom brain death was diagnosed by clinical testing including apnoeic-oxygenation with the number in whom brain death was diagnosed by CeretecTM brain perfusion scanning. In addition, we analysed the role of brain perfusion scanning in organ donation after circulatory death. The differences in the rate of organ donation between eras were analysed for significance with Chi-square test (Vassarstats.net). The project was approved by the RCH Human Research Ethics Committee.
Results
PICU admissions and mortality
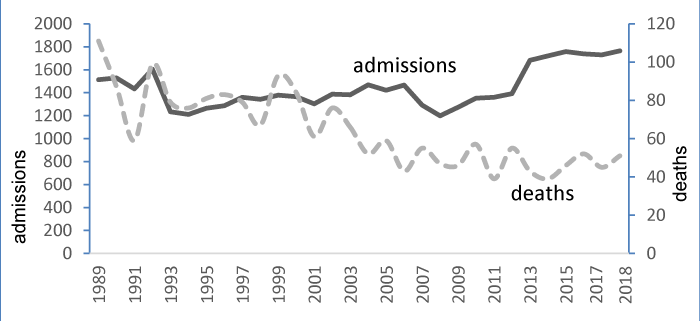
Throughout the period of study, 43,217 patients were admitted to PICU with 1930 deaths (crude mortality 4.5%). Annual admissions to PICU increased steadily (with minor variation) while the annual mortality decreased initially during this period but remained relatively static during the latter part. For example, annual deaths were approximately 100 and 110 respectively in 1989 and 1990 but declined to 40-60 and remained static over the period 2003-2018 (Figure 1), resulting in a decreasing number of potential organ donors Figure 1.
Brain perfusion scanning
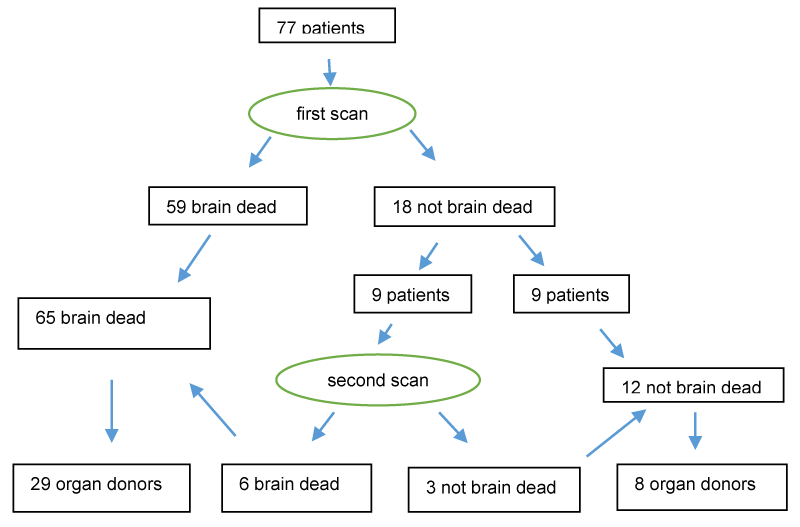
Seventy-seven patients (average age 6.6 years, range 1 week-17 years) had scans over the 17-yr period 2002-2018. Scanning was performed to assist in the assessment of severity of brain injury or to diagnose brain death. The indications to perform brain perfusion scanning were unresponsive coma with unreactive pupils in all patients (but other brainstem reflexes were not recorded routinely) and all patients had had a preceding CT scan or MRI scan which had shown severe brain injury. The aetiology of brain injury is shown in Table 1.
Fifty-nine patients were diagnosed as brain dead on an initial CeretecTM scan, that is, they had no cranial blood flow. Eighteen patients (23%) had some brain perfusion on initial scanning and were diagnosed as not brain dead. Of these latter patients, 9 had a second scan after decay of initial radioactivity at a mean interval of 34.7 hours (range 22.5 – 71.5 hours). Six of these had absent perfusion on the second scan thus constituting 65 of 77 patients scanned (84%) as diagnosed brain dead, and 12 as not brain dead (16%) (Figure 2). In one patient who had had 2 scans, residual radioactivity was absent 71.5 hours after the first scan but was present 17.5 hours after a second scan thus precluding a third scan Figure 2.
Organ donation
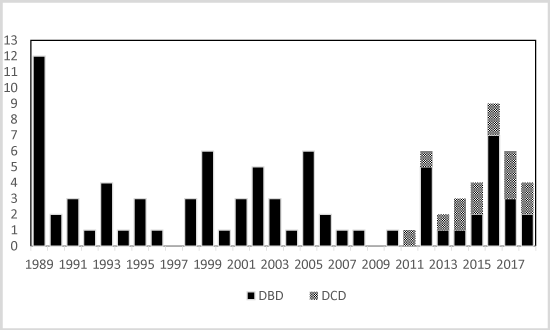
Overall, from 1989 to 2018, organs were donated by 95 of 1930 children (4.9%) after brain death or circulatory death at an average of 3.2/year or at a median percentage of 4.6(interquartile range, IQR, 1.8-5.1). Of these, 81 had been diagnosed with brain death and 14 had sustained circulatory death (Figures 3,4). Brain death was diagnosed in 52 donors by apnoeic-oxygenation and in 29 by brain perfusion scanning.
1. After brain death (DBD)
Organ donation may be considered in eras and according to the mode of diagnosing brain death (Table 2):
Apnoeic-oxygenation: Of 1059 children who died in the period 1989-2001, when brain death was diagnosed by clinical testing including apnoeic-oxygenation, 40 donated organs (3.8%) at an average of 3.1/annum or an annual median percentage of 3.7(IQR, 1.2-5.1).
Apnoeic-oxygenation or brain scanning: From 2002-2007, organs were donated by 18 patients diagnosed brain dead by brain scanning (6 patients) or by apnoeic-oxygenation (12 patients) among 351 deaths (5.1%).
Brain scanning: Of 520 children who died in the period 2008-2018, when brain scanning was performed to diagnose brain death, 23(4.4%) donated organs at an average 2.1/year. The annual median percentage DBD rate was 2.6(IQR, 1.9-5.5). There was an increase of 17% in the donor percentage of deaths compared to the previous era (1989-2001) when apnoeic–oxygenation was used to diagnose brain death but was not a significant change (P=0.22).
Overall, there was no significant difference in DBD during the three eras of apnoeic-oxygenation only, apnoeic-oxygenation or brain scanning and brain scanning only (P=0.52).
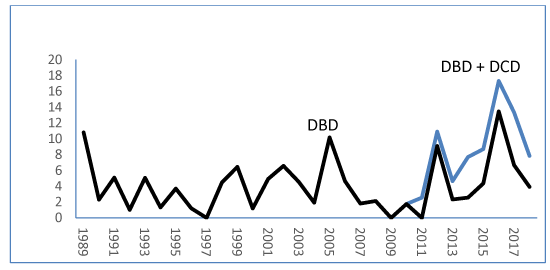
2. After circulatory death (DCD)
A total of 14 children donated organs after circulatory death on withdrawal of life-sustaining treatment in the period 2011-2018. Of these, 8(67%) were diagnosed as not brain-dead with brain scanning showing persistent blood flow after 1 or 2 scans, while 6 donors had not had a brain scan. DCD has constituted 14 of 35 donors (40%) 2011-2018 and 5 of 10 donors in 2017-2018.
3. After brain (DBD) or circulatory death (DCD)
In the period 2011-2018, when brain scanning was used to diagnose brain death, the combined DBD and DCD rate was 35 of 370 deaths (9.5%) which is significantly greater (P<0.0001) than the DBD rate of 40 of 1059 deaths (3.8%) over 1989-2001 when apnoeic-oxygenation testing was used to diagnose brain death, and significantly greater (P<0.0001) than 60 of 1560 deaths (3.8%) over 1989-2010 (data not tabled) when DBD was performed after apnoeic-oxygenation or brain scanning.
Discussion
This study examined the influence of diagnosing brain death by radionuclide scanning in lieu of apnoeic-oxygenation on organ donation. It is not a comparative study of the two methods to diagnose brain death. A notable finding of our study was a decrease in the average annual DBD between the two periods when death was diagnosed by apnoeic-oxygenation (3.1/year) and by scanning (2.1/year), but due to a concomitant decrease in deaths, there was no change in the proportion of donors per deaths (3.8% vs 4.4% respectively). The decrease in absolute annual DBD is consistent with a study of paediatric organ donors across Australia and New Zealand [9] which found a decrease in DBD from an average of 16.8/year (2000-2007) to 13.8/year (2008-2015) in context of a constant percentage of deaths (5.7% vs 5.5%).
Although we regard every dying patient as a potential organ donor, only 4.9% of patients dying in our PICU became organ donors. However, this analysis did not take into account whether dying children were medically suitable or unsuitable for organ donation. Although not directly comparable, only 1.5% of adult patients dying in Australian hospitals were regarded as potential donors and only 0.7% became actual organ donors [10]. Fewer adults than children as percentage of deaths in hospitals may be suitable organ donors because of age-related factors.
Organ donation after brain death during the period of brain scanning may have been affected by numerous unknown factors. Positive factors may have included a greater awareness of organ donation among the public and better education of hospital clinical staff which may have increased organ donation nationally as a percentage of deaths. Such factors probably explain why during the period of brain scanning was used in lieu of apnoeic-oxygenation (2008-2018), organ donors in Australian increased from 12 to 22 donors per million population (ppm) [10].
On the other hand, there may have been negative factors on organ donation. Since some brain perfusion may be present when death is diagnosed by clinical absence of brain-stem reflexes [11-13] the complete absence of brain blood flow is a more stringent condition for diagnosing brain death. It is possible, therefore, that the diagnosis of brain death would be made less frequently by complete absence of perfusion on scanning than by clinical testing, resulting in less brain dead organ donors, but which we did not observe.
Another notable finding of our study was a significant increase in total organ donors when DBD or DCD were undertaken (9.5% of deaths) compared with the previous era when only DBD was undertaken (3.8% of deaths). Since commencement of a programme in 2011, DCD has constituted 14 of 35 donors (40%) and one half of all donors in 2017-2018. The increase in DCD is greater than that observed in Australia and New Zealand in the period 2006-2015 9 when DBD plus DCD amounted to 5.7% of deaths in 2000-2007 but 6.4% in 2008-2015. We speculate that the superior organ procurement in our institution may be in part due to performance of brain scanning which while showing limited blood flow in some patients, precluding a diagnosis of brain death but reinforcing the indication for withdrawing life-sustaining treatment making DCD possible.
Introduction of DCD has been universally successful in increasing organ donors but sometimes at the expense of DBD and with less organs per donor made available than with DBD – an aspect which we did not study. For example, in the United Kingdom, over the period 2003-2012, DCD donors increased from 1.1 to 7.9ppm while DBD donors remained static around 10.5ppm [14]. In the approximate same period (2004-2014) intended DCD donors increased by 292% while intended DBD donors increased only by 11% and while organs procured remained static at 3.30 to 3.26 per DBD donor, the number per DCD donor decreased from 1.54 to 0.99 [15]. In the most recent data from UK [16] organ donation has increased steadily over the last decade (2009-2019) but the increase was disproportionally due to DCD: an increase of 67% in total donation was comprised of an increase in DBD by 54% and in DCD by 90%.
There are numerous reasons why we abandoned a specific aspect of clinical testing, apnoeic-oxygenation, and adopted routine brain radionuclide perfusion scanning to assist in diagnosing brain death. Radionuclide brain perfusion has numerous advantages [17]. We perceive that brain perfusion scanning with lipophilic diffusible radionuclides (Technetium Tc99m Exametazine or Technetium Tc99m-ECD [18]) is more practical, simpler to perform, quicker and more easily accomplished with one test than clinical testing which must be repeated [1]. Scanning is objective, reliable, reproducible, noninvasive, not deleterious to organs and importantly, unlike clinical testing, is not influenced by sedative medication, muscle relaxants, poisons, temperature, metabolic and endocrine derangements, electrolyte disturbances, spinal cord injury and spinal automatism all of which may present in patients with severe brain injuries. Moreover, brain scanning can be applied to all ages, including newborns [19] and abolishes the delay in waiting for medication, which may influence neurological assessment, to be excreted or metabolised [20] and it does not need to be repeated if no perfusion is evident on an initial scan. However, blood pressure must be adequate to enable supply of the radionuclide to the brain unless opposed by intracranial hypertension. Although it is possible to observe an initial angiographic dynamic phase of perfusion in a dead brain [4] in the absence of intracranial hypertension, there will be no uptake of tracer and no radioactivity detected on delayed scanning. Other imaging techniques with computerised tomography or magnetic resonance, scanning with nonlipophilic radiopharmaceuticals and ultrasound techniques are less suitable for assessing brain function in the context of possible brain death because they can only yield initial angiographic data [4,13].
Importantly, images from brain scanning showing complete lack of tracer uptake are convincing evidence that a condition necessary for brain survival, i.e., brain blood flow or brain blood flow with uptake of tracer, is not present. It may be thus convincing not only for medical and nursing personnel that the afflicted child is dead and but also facilitate effective communication with family members [13,21] and is helpful in brain death donor counselling [22]. If limited tracer uptake and hence some blood flow is present, it reinforces clinical assessment and other imaging results which suggest the futility of continuing active treatment. In the absence of a portable camera, performance of scanning carries the risk of transportation out of the PICU, but in a well-developed system poses very little risk. In contrast, clinical testing of brain-stem reflexes is more difficult to explain, to demonstrate and may be less convincing to parents that their child is truly dead when they have a circulation and appear to be breathing when in fact mechanical ventilation is being provided.
According to some investigators the absence of cerebral blood flow is the mainstay of neurological determination of death [23] while brain perfusion scanning with a lipophilic radionuclide has become the “gold-test” [6,24,25]. Nonetheless, a diagnosis of brain death made by this technique does not always result in organ donation. In this study, 29 of 65 (45%) children diagnosed with brain death by scanning became organ donors compared with 69% of cases in a study of adults [25]. We were not able to analyse the rate of consent given by parents to organ donation from their child when brain death was diagnosed by clinical testing. We do not know if absent brain perfusion determined by scanning is more or is less convincing than clinical testing for parents that their child is dead. This aspect requires research.
If reassessment of brain perfusion is required with a second scan, it is necessary to wait sufficient time until residual radioactivity in the brain from the first scan has decayed. That interval is largely determined by the half-life of technetium Tc99m which is 6 hours. After 24 hours, the fraction of remaining radioactivity is less than 0.063 [5]. In 9 of the patients in this cohort who presented for a second scan, no residual radioactivity from the first scan was detected after a mean of 35 hours whereas radioactivity in one patient was present after 17.5 hours. Thus, a delay of at least 24 hours after a first scan is probably required before a repeat scan is performed to avoid a spurious result attributable to residual radioactivity, but we cannot specify a minimum necessary delay based on this data. If performed to diagnose brain death, and to avoid delay required for a second scan, it is recommended that scanning be performed only when all the clinical conditions to diagnose brain death (except testing with apnoeic-oxygenation) are met. If performed too early a finding of cerebral perfusion may delay rather than expedite the diagnosis of brain death [26]. Although the presence of bilateral unreactive pupils is the best predictor of non-survival [27], the absence of the pupillary reflex does not necessarily signify brain death [28]. In this study, some brain perfusion on brain scanning was observed in approximately one quarter of patients who had no pupillary reflexes and were subsequently defined as not brain dead.
In contrast to radionuclide scanning, the key test of brain-stem function, indeed the sine qua non, the apnoeic-oxygenation test, has disadvantages. Even without confounding factors it can be argued to be unscientific and unreliable [29,30], principally because in infants and children [31-35], (otherwise fulfilling the criteria for brain death), the onset of spontaneous ventilation has been observed at blood levels of partial pressures of carbon dioxide well above those at which brain death may be diagnosed (60mmHg in Australia, Canada, USA; 50mmHg in Britain) and moreover hypercarbia itself may depress brain function, which the ANZICS Statement concedes [1]. Thus the test may provide a false positive result, that is of diagnosing death when it is not present. Importantly, the test itself may be theoretically harmful – it may cause brain death since the test requires creation of hypercapnia which, in the functioning brain, is known to cause cerebral vasodilation and intracranial hypertension and consequently may cause brain death if not already established. The test has thus been criticised as a self-fulfilling one: that of potentially causing the condition, brain death, which it purports to diagnose [29,30,36]. Moreover, the clinical testing, including apnoeic-oxygenation testing must be performed twice, at intervals, and by different examiners even if the first test is diagnostic of brain death and may thus prolong the process and jeopardise the viability of organs. Lastly, although the apnoeic-oxygenation test is widely used, it is not applied uniformly, is difficult to perform safely with frequent reports of hypotension and hypoxaemia [29,30]. On the other hand, the test has the advantage of permitting family members to observe in their afflicted child the lack of respiration [37], a universally recognised cardinal sign of death.
In our current practice, possibly unique, when a child shows signs consistent with brain death such as unresponsiveness, unreactive pupils or loss of other brain stem reflexes (apnoeic-oxygenation is not tested), and who would be a suitable organ donor, a brain perfusion scan is performed. However, if any reflex is present scanning is deferred to avoid foregoing the possibility of DBD. Organ procurement under the condition of brain death is then considered if perfusion is absent while organ procurement after circulatory death is considered if any perfusion remains but when further treatment would be futile.
Conclusion
In our institution, the routine adoption of radionuclide brain scanning to diagnose brain death has not adversely affected organ donation after brain death, which has remained constant. We suggest that lipophilic radionuclide brain scanning could be adopted as a routine test in lieu of apnoeic-oxygenation when other clinical examination including unresponsive coma, dilated unreactive pupils and radiological imaging are suggestive but not confirmatory of the diagnosis of brain death, rather than be regarded as a confirmatory test or ancillary test used to circumvent factors confounding clinical testing.
We thank Duncan Veysey of the RCH Imaging Department for assistance with data concerning CeretecTM scanning; Mark McDonald of the Organ and Tissue Authority and Jenny Thompson of RCH PICU data collection team for data concerning organ donation, and Drs Robert Henning and Michael Clifford for critiques of the manuscript.
- ANZICS Statement on Death and Organ Donation. Link: https://bit.ly/3pXF6Bs
- Schober O, Galaske R, Heyer R (1987) Determination of brain death with 123I-IMP and 99mTc-HM-PAO. Neurosurg Rev 10: 19-22. Link: https://bit.ly/2O01gG3
- Galaske RG, Schober O (1988) Determination of brain death in children : 99mTc-HM-PAO and 123(I)-amphetamine scintigraphy as a new, noninvasive method. Wien Klin Wochenschr 100: 555-561. Link: http://bit.ly/3bK2mhg
- Weckesser M, Schober O (1999) Brain death revisited: utility confirmed for nuclear medicine. Eur J Nucl Med 26: 1387-1391. Link: http://bit.ly/3aRtTye
- GE Healthcare. Ceretec™ Kit for the Preparation of Technetium Tc99m Exametazime Injection. Link: https://bit.ly/3dOks4k
- Wieler H, Marohl K, Kaiser KP, Klawki P, Frossler H (1993) Tc-99m HMPAO cerebral scintigraphy. A reliable noninvasive method for determination of brain death. Clin Nuc Med 18: 104-109. Link: http://bit.ly/3uu0cLz
- Kurtek RW, Lai KK, Tauxe WN, Eidelman BH, Fung JJ (2000) Tc-99m hexamethylpropylene amine oxime scintigraphy in the diagnosis of brain death and its implications for the harvesting of organs used for transplantation. Clin Nuc Med 25: 7-10. Link: http://bit.ly/2MvhNl4
- Kapuca ÖL, Nobili F, Varrone A, Booij J, Vander Borght T, et al. (2009) EANM procedure guidelines for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging 36: 2093-2102. Link: http://bit.ly/3uz9sOw
- Corkery-Lavender T, Millar J, Cavazzoni E, Gelbart B (2017) Patterns of organ donation in children in Australia and New Zealand. Crit Care Resusc 19: 296-302. Link: http://bit.ly/2MpkLHA
- Australian and New Zealand Organ Donor Registry. 2019 Annual Report. Link: http://bit.ly/37OJpcp
- Wijdicks EFM (2010) The case against confirmatory tests for determining brain death in adults. Neurology 75: 77-83. Link: http://bit.ly/3kpNKrm
- Wijdicks EFM (2013) Pitfalls and slip-ups in brain death determination. Neurol Res 35: 169-173. Link: http://bit.ly/3uBymg6
- Zuckier LS (2016) Radionuclide evaluation of brain death in the post-McMath era. J Nuc Med 57: 1560-1568. Link: http://bit.ly/3suGRI9
- Johnson RJ, Bradbury LL, Martin K, Neuberger J (2014) UK Transplant Registry. Organ donation and transplantation in the UK – the last decade: a report from the UK national transplant registry. Transplantation 97: S1-S27. Link: http://bit.ly/2NE5GCA
- Hodgson R, Young AL, Attia MA, Lodge JPA (2017) Impact of a national controlled donation after circulatory death (DCD) program on organ donation in the United Kingdom: A 10-year study. Am J Transplant 17: 3172-3182. Link: http://bit.ly/3kqoSQv
- National Health Service. Organ donation and transplantation. Activity report 2019/2019.
- Conrad GR, Sinha P (2003) Scintigraphy as a confirmatory test of brain death. Semin Nuc Med 33: 312-323. Link: http://bit.ly/3aVzEen
- Bertagna F, Barozzi O, Puta E, Lucchini S, Paghera B, et al. (2009) Residual brain viability, evaluated by 99mTc-ECD SPECT, in patients with suspected brain death and with confounding clinical factors. Nucl Med Commun 30: 815-821. Link: http://bit.ly/3suHmSx
- Okuyaz C, Gucuyener K, Karabacak NI, Aydin K, Serdarroglu A, et al. (2004) Tc-99m-HMPAO SPECT in the diagnosis of brain death in children. Ped Internat 46: 711-714. Link: http://bit.ly/3bJOYcW
- Lopez-Navidad A, Caballero F, Domingo P, Marruecos L, Estorch M, et al. (2000) Early diagnosis of brain death in patients treated with central nervous system depressant drugs. Transplantation 70: 131-135. Link: http://bit.ly/2NZvRnk
- Nakagawa TA, Ashwal S, Mathur M, Mysore M (2011) Clinical report – guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Pediatrics 128: e720-e740. Link: http://bit.ly/3aRx7Sh
- Palaniswamy V, Sadhasivam S, Selvakumaran C, Jayabal P, Ananth SR (2016) Single-photon emission computed tomography imaging for brain death donor counseling. Indian J Crit Care Med 20: 477-479. Link: http://bit.ly/3qYaUHN
- Heran MK, Heran NS, Shemie SD (2008) A review of ancillary tests in evaluating brain death. Can J Neurolog Sci 35: 409-419. Link: http://bit.ly/3pVooCX
- Munari M, Zucchetta P, Carollo C, Gallo F, De Nardin M, et al. (2005) Confirmatory tests in the diagnosis of brain death: a comparison between SPECT and contrast angiography. Crit Care Med 33: 2068-2073. Link: http://bit.ly/2PdFLCl
- Banzo J, Razola P, Araiz JJ, Larraga J, Tardín L, et al. (2012) Cerebral perfusion scintigraphy study as confirmation test of brain death in the process or organ donation for transplant. Rev Esp Med Nucl Imagen Mol 31: 278-285. Link: http://bit.ly/3aT4moo
- Larar GN, Nagel JS (1992) Technetium-99m-HMPAO cerebral perfusion scintigraphy: considerations for timely brain death declaration. J Nuc Med 33: 2209-2211. Link: http://bit.ly/3dMgVDR
- Scholefeld BR, Gao F, Duncan HP, Tasker RC, Parslow RC, et al. (2015) Observational study of children admitted to United Kingdom and Republic of Ireland Paediatric Intensive Care Units after out-of-hospital cardiac arrest. Resuscitation 97: 122-128. Link: http://bit.ly/2ZRGvit
- Carter BG, Butt W, Taylor A (2007) Bilaterally absent pupillary responses: not always a bad sign. Anaesth Intensive Care 35: 984-987. Link: http://bit.ly/3dK2H6p
- Tibballs J (2010) A critique of the apneic oxygenation test for the diagnosis of “brain death”. Pediatr Crit Care Med 11: 475-478. Link: http://bit.ly/3kpOoVO
- Joffe AR, Anton NR, Duff JP (2010) The apnea test: rationale, confounders, and criticism. J Child Neurol 25: 1435-1443. Link: http://bit.ly/2ZRqZmL
- Brilli RJ, Bigos D (1995) Altered apnea threshold in a child with suspected brain death. J Child Neurol 10: 245-246. Link: http://bit.ly/3st6Z67
- Levin SD, Whyte RK (1988) Brain death sans frontières. N Eng J Med 318: 852-853. Link: http://bit.ly/3sun5fM
- Vardis R, Pollack MM (1998) Increased apnea threshold in a pediatric patient with suspected brain death. Crit Care Med 26: 1917-1919. Link: http://bit.ly/3uxU6JP
- Okamoto K, Sugimoto T (1995) Return of spontaneous respiration in an infant who fulfilled current criteria to determine brain death. Pediatrics 96: 518-520. Link: http://bit.ly/3dK2XCp
- Joffe AR, Kolski H, Duff J, de Caen AR (2009) A 10-month-old infant with reversible findings of brain death. Pediatr Neurol 41: 378-382. Link: http://bit.ly/2ZRtjdx
- Joffe AR (2020) The apnea test: Requiring consent for a test that is a self-fulfilling prophecy, not fit for purpose, and always confounded? Amer J Bioethics 20: 42-44. Link: http://bit.ly/3ssr9gH
- Tibballs J (1990) Demonstrating brain death. Med J Aust 153: 233. Link: http://bit.ly/3bHOB2Q
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
