Archives of Organ Transplantation
Defining a Steroid Withdrawal Protocol in a newly established Kidney Transplantation Unit
Obadiah Moyo1, Ajay Kumar Sharma2 and Ahmed Halawa3*
2Royal Liverpool University Hospital, Liverpool, UK
3Sheffield Teaching Hospitals, Sheffield Kidney Institute, University of Sheffield, Sheffield, UK
Cite this as
Moyo O, Ajay Kumar S, Halawa A (2017) Defining a Steroid Withdrawal Protocol in a newly established Kidney Transplantation Unit. Arch Organ Transplant 2(1): 030-040. DOI: 10.17352/2640-7973.000009As a key step in setting up the immunosuppression protocols for our kidney transplantation unit, still in its infancy, consideration of the choice of the steroid withdrawal strategy is important. We conducted a review of literature to ascertain a safe steroid withdrawal protocol that would be able to achieve a high allograft survival and function rate, low acute allograft rejection (AR) rate and advantageous in reducing a wide range of adverse effects associated with corticosteroids such as cardiovascular risks, growth retardation in pediatric patients, osteoporosis and other steroid-related complications.
Based on this review, steroid withdrawal was associated with high rates of AR in comparison to steroids maintenance. Specifically, late steroid withdrawal was related to poor outcomes in comparison to total steroid avoidance or very early withdrawals. High immunological risk patients with delayed graft function, prolonged cold ischemia time, donation after cardiac death, black race and those with a history of glomerulonephritis are not recommended for steroid avoidance.
Due to the unclear reduction of adverse effects and co-morbidities through steroid withdrawal or avoidance and inconclusive results and outcomes surrounding this subject which still needs further refinement, the decision taken by our unit was to retain, taper and maintain a very low dosage of corticosteroids on a long term basis with combined use of lymphocyte depleting induction agents coupled with calcineurin inhibitors and anti-proliferative agents for maintenance. Transplant recipients with low immunological risk are ideal candidates for early steroid withdrawal.
Introduction
Chitungwiza Central Hospital, a 500 bedded tertiary teaching hospital located in the city of Harare in the Southern African country of Zimbabwe, is at an advanced stage of establishing a kidney transplantation unit with the guidance of International Society of Nephrology. It is anticipated that the unit will initially perform at least five (5) kidney transplant operations per month when it becomes fully functional. The recommended immunosuppression protocol includes induction by rabbit anti-thymocyte globin (rATG) and maintenance on prednisolone, tacrolimus (TAC) and mycophenolate mofetil (MMF), and does not consider steroid withdrawal. This review is aimed to critically review our unit’s recommended policy of maintaining steroids as part of immunosuppression versus steroid withdrawal strategies implemented by other kidney transplant units. The review considers the advantages and disadvantages of various steroid withdrawal or avoidance protocols using published evidence.
Corticosteroids have been in use in the prevention of AR since the early sixties. Irrespective of their capability in preventing acute rejection, steroids are known to be associated with adverse effects including weight gain, osteoporosis, cataracts, glaucoma, skin atrophy, diabetes mellitus, hyperlipidaemia, avascular necrosis, myopathy and increased infection risk [1,2,3]. In order to reduce the adverse effects, clinicians tended to reduce the dosages of steroids in immunosuppression. Steroid-free immunosuppression was possible since the introduction of calcineurin inhibitors (CNI) such as cyclosporine A (CsA) [3].
Immunosuppression protocols include induction, maintenance regimen and rescue regimen. Induction immunosuppression might include lymphocyte depletion therapy or through supplementary immunosuppressive agents like interleukin-2 (IL-2) receptor antibody. Induction has been shown to reduce AR. The maintenance immunosuppression includes a calcineurin inhibitor such as CsA or TAC, anti-proliferative agent such as MMF or azathioprine (AZA) and a corticosteroid [1]. Steroids promote the activity of uridine diphosphate in mycophenolic acid metabolism. This activity is, however, reversed when steroids are withdrawn thus leading to an accumulation of mycophenolic acid due to its poor clearance resulting in the untoward effects on the recipient’s immunosuppression due to steroid discontinuation [4,5]. Corticosteroids have been considered to inhibit T cell signals, which are responsible for apoptosis. As they reduce the cytokine release, corticosteroids possibly amplify cytokine receptors expression on T Cells. Therefore, a later withdrawal after an initial treatment with steroids may cause a T Cell activation rebound, resulting in AR due to the release of cytokine as a result of upregulated cytokine receptors [6]. By this review we aim to establish the most suitable steroid withdrawal protocol for renal transplant recipients.
Review of Literature
Definitions
Reducing exposure to steroids can be accomplished through steroid avoidance where the steroids are withdrawn very early within the first few days up to 14 days following transplantation or through steroid withdrawal wherein the steroids are discontinued at a particular time well after the transplantation. Steroid withdrawal at 3-6 months after transplantation is categorized as early withdrawal while withdrawal after 6 months and above after transplantation is considered as late withdrawal [1,7].
Early steroid withdrawal
Reports emanating from the CsA era confirm a high AR rates in renal transplant patients on CsA and AZA when early steroid withdrawal was applied [4,8,9]. A random Canadian placebo controlled trial of 523 renal transplant patients recommended for a 5 year follow up to have an in depth assessment of steroid-free protocols. That study showed a reduction in graft survival rates to 73% in patients on CsA therapy who had stopped steroid intake at 3 months after transplantation as compared to a higher figure of 85% survival in study cases who did not discontinue prednisolone intake [4,10]. Hricik et al., in 1993 demonstrated in a meta analysis that avoidance of steroids at transplantation or withdrawing post transplantation intensified AR risk, however with no adverse effect on graft survival [11].
In a double-blinded European multicentre random study of 500 kidney transplant recipients, corticosteroids were administered to one group at half the dose for 3 months from the transplantation date and then stopped while standard doses were maintained in a control group. CsA and MMF were administered to both groups and after the 6 and 12 months the group on half dosage had a relatively higher percentage of AR proven by biopsy (23% and 25%, respectively) than the control group (14% and 15%, respectively). At 12 months, the graft loss rate in the half dose group was 5% in comparison to 4% in the control group, please see table 1 [9]. A similar study in the USA in contrast experienced a massive increase in AR rate in the half dose group. The AR mostly affected African American recipients in the study [12].
Hricik et al., also noted risk of AR in recipients of black race and early withdrawal as risks associated [13]. In a follow up uncontrolled study by Hricik et al., prednisolone was withdrawn at 3 months post transplantation from those African-American patients who had been commenced on sirolimus, tacrolimus, and corticosteroid as the initial treatment and had not experienced any AR. In this study there was AR in 7% of patients that raised concerns. It was concluded that there is need to be cautious about withdrawal of steroids in African-American patients irrespective of them being on strong immunosuppression [14].
Vanrenterghem et al., set up a multicenter Random Clinical Trial (RCT) of 556 low risk renal transplant recipients who were maintained on TAC and MMF. In a group of patients steroids were withdrawn at 3 months. On review of the cases at 6 months, an increased AR rate and reduced cholesterol and low density lipoprotein (LDL) were noted in the steroid withdrawal group commencing from the period 3 up to 6 months post transplantation, refer to table 1 [7,15].
Pascual et al. in 2011, showed that steroid avoidance or early withdrawal within the first 14 days after transplant was safe in recipients who are on an anti-interleukin T receptor antibodies or ATG induction and TAC and MMF treatment [16]. In another systematic review of 1820 study cases with no induction, where steroids withdrawn at 3 and 6 months post transplantation, Pascual et al., deduced that for at least 3 years following transplantation, renal graft and patient survival were not influenced by steroid withdrawal, however, where patients were on CsA maintenance treatment, the incidence of AR were increased, please see table 1 [17].
Late steroid withdrawal
In a study by Reisman et al., discontinuing of steroids at 7 months in 16 paediatric patients on CsA resulted in a 56% acute rejection rate at 5 years post transplantation. There was, however, improved growth, height and weight in the study cases [18]. Sandrini et al., in a prospective Italian study compared CsA monotherapy and combined CsA-AZA therapy. AZA was introduced at 6 months post transplantation as steroid was being withdrawn. Steroid withdrawal resulted in a high rate of AR in the CsA monotherapy than in the CsA-AZA patient pool [4,19]. Matl et al., published outcomes of follow up period of 12 months post transplantation on 88 patients that step-by-step withdrawal of steroids in 46 patients over a period of 6 months yielded a low AR rate in comparison to the patient pool of 42 who were maintained on standard prednisolone, CsA and AZA, please see table 2 [4,20].
Grinyó et al., in an open pilot study in 1993 withdrew corticosteroids from 26 kidney allograft recipients on CsA and MMF at an average 17 months post transplantation and they did not exhibit any AR episodes as shown in table 2 [21].
Smak Gregoor et al., in a Dutch study composed of 212 kidney transplant recipients compared use of MMF against CNI and steroids where at 6 months after transplantation the first group had CsA withdrawn, the second group had the steroid discontinued while the third group continued with steroid CsA and MMF. In this study patients who withdrew from steroids had lower cholesterol and reduced blood pressure levels and 4% biopsy proven AR against 1.4% in controls at a follow up period of 24 months, refer to table 2 [22].
Opelz et al., in a comparative study of outcomes during a 7 year period under the Collaborative Transplant Study Group, where steroids were withdrawn beyond 6 months post transplantation demonstrated that it was more beneficial to withdraw steroids as graft and patient survival were improved. There was no variation in AR between patients who continued on steroids and those who where withdrawn. Cardiovascular risk was notably reduced [23].
Late stage steroid withdrawal at 5 to 6 months in patients on TAC and MMF maintenance, was reviewed after a 5 year follow up and recommended as being safe with a low risk of allograft failure and AR following studies in 50 patients by Loucaidou et al (2005) [24].
In 2017 Haller et al., carried out a retrospective study on the optimal period for steroid withdraw post transplantation. They evaluated 6070 patients who had received kidney transplants for the first time in the Austrian Dialysis and Transplant Registry. 1131 patients had graft loss, 821 study cases had deceased following withdrawal of steroids within 18 months subsequent to transplantation after induction with anti-IL2. There was a confirmed link to high graft loss in comparison to steroid maintenance [25].
Steroid avoidance
The combined use of polyclonal anti-T cell antibodies, biological agents and CNIs has seen a marked reduction of AR. In order to withdraw steroids at an early stage after transplantation, a strong induction immunosuppression is necessary in order to avoid increased rates of AR. The justification for early withdrawal or avoidance being that if steroid withdrawal is effected several weeks or months after transplantation the likelihood of provoking acute rejection would be increased, please read table 3 [15,26].
Kuypers et al., in a comparison of daclizumab induction, low dose TAC and MMF and steroid withdrawal against standard regime of TAC, MMF and steroids, the patients who were withdrawn from steroids at 5 months had less AR and their graft function was better in study group than in the patients on standard regimen [27].
Polyclonal rabbit antithymocyte globulin was used together with MMF and CsA in living related donor graft where steroids were stopped 5 days after transplantation. 87% of the patients in this study by Mattas et al (2004) did not experience any AR at 12 months post transplantation and kidney function was comparable to control patients on standard therapy without induction [28]. This steroid-free immunosuppressive protocol also demonstrated a high patient and graft survival rates and high acute and chronic rejection free graft survival rates [29].
The rate of AR proven by biopsy at 12 months insignificantly varied in a steroid withdrawal patient study pool (20%) and those on standard immunosuppression (16%) when the effectiveness of basiliximab added to a CsA and MMF was checked in early corticosteroid withdrawal 4 days post transplantation by Vincenti et al., in a study comprising 83 patients. Graft survival was 100% in the steroid withdrawal group [30].
Vincenti et al., in another study comprising of 336 patients with PRA of greater than 20%, complete avoidance of steroids was compared with steroid withdrawal at day 7 and steroid maintenance. Induction for the three study groups was basiliximab while maintenance was CsA and MMF. The results from the study showed a higher biopsy-proven AR rate of 31.5% in the no steroid group followed by 26.1% in the steroid withdrawal group and 14.7% in the standard regimen group. Patient and graft survival rates remained the same in all study groups at 12 months review point [7,31].
While using basiliximab as an induction agent and a CNI and MMF or sirolimus as maintenance, Kumar et al., in a study of 300 kidney transplant recipients compared steroid withdrawal at 2 days versus non-withdrawal and recorded no variation between the 2 groups in AR recipient and graft survival and function except for a slight occurrence of NODAT in the steroid withdrawal group [7,32].
Laftavi et al., carried out a random study on 60 patients who were on a rabbit anti-lymphocyte globulin (rATG) induction and TAC plus MMF immunosuppression. One group withdrew steroids at day 7 while the other group did not withdraw. At a 12 months follow up, there was no variation in graft performance and rate of AR amongst the 2 groups. Interstitial fibrosis was, however, prominent in biopsies at 12 months in the withdrawal group [7,33].
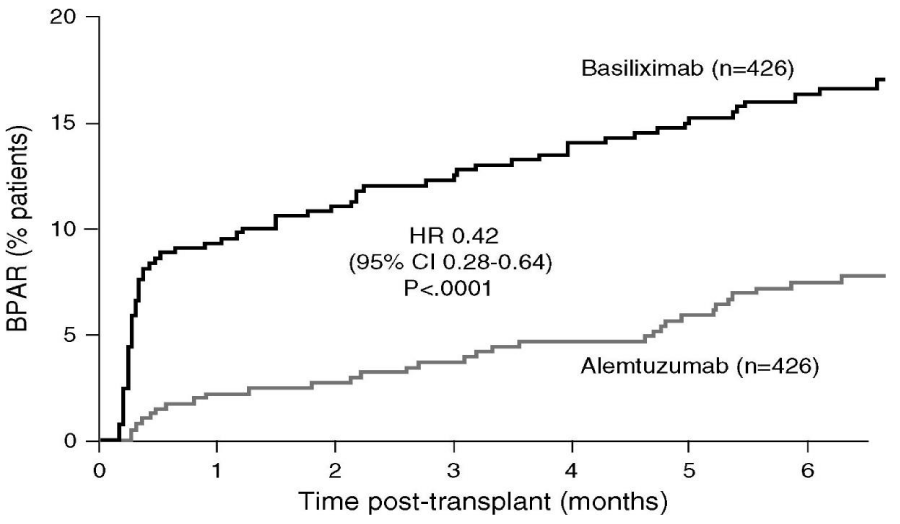
The humanized immunoglobulin IgG1 monoclonal antibody alemtuzumab (Campath-1H) function as an induction agent which can be used in combination with steroid avoidance was studied in the 3C Study (2014) where 426 patients were placed on alemtuzumab induction, and maintenance with low dose TAC, MMF and no steroids while an equal number was assigned to basiliximab treatment and maintenance with standard dose TAC, MMF and steroids. At 6 months, there was a reduction by 58% of biopsy proven AR in the alemtuzumab group, please note figure 1. Graft failure and patient survival did not vary in the two study groups [7,34].
In a similar study utilizing 335 low risk patients, Hanaway et al (2011) carried out a clear cut comparison between alemtuzumab and basiliximab where steroids were withdrawn at day 5. The outcome of this study showed a low biopsy proven acute rejection rate in the alemtuzumab participants after 3 years [7,35].
ter Meulen et al., studied the possibility of withdrawing steroids as early as 3 days following transplantation and while using anti 1L-2Rα induction with TAC and MMF. At 12 months the study revealed no difference in AR confirmed by biopsy between daclizumab group (15%) and control group (14%) [23]. The survival of grafts in the 2 groups was not different at 12 months [2,36].
Complete steroid avoidance was studied by Birkeland et al., in 100 children following a 10 day induction with ATG and CsA and maintenance with MMF. From this study AR was low at 13% and graft survival was 82% at 4 years [37].
Where daclizumab was used by Sarwal et al., instead of steroids in a TAC and MMF protocol, in paediatric graft recipients, no acute rejection or signs of chronic rejection were recorded [38].
In 2008 Umber Burhan et al carried out an analysis of 100 renal transplant recipients who retrospectively had been on TAC and MMF for maintenance immunosuppression. A group of 51 were placed on ATG or daclizumab and long term steroids for induction, while a second group received daclizumab and steroids during the day of operation and day 1. In this study, the 1 year rate of patient survival was calculated at 96% and 94%, respectively for the 2 groups. Graft survival at 1 year was 96% and 94%, respectively. African American and non-African American patients had no significant difference in acute rejection 7% vs 12%. Burhan et al concluded that speedy steroid withdrawal coupled with daclizumab induction and accompanied by maintenance with TAC and MMF creates a conducive patient and graft survival environment in first time transplant recipients with panel-reactive antibodies of below 50%. This protocol accordingly also has a low rate of acute rejection including in African-Americans. The rate at which early steroid withdrawal was being applied, emphasized the importance of safety and in which patient group it should be applied [2].
In addition to the unclearness of the current data, the strategies of steroid withdrawal are now strongly established at most kidney transplant centres and the trend of steroid free protocols started being prominent since 2000 where at least 5% of patients were discharged after transplantation without steroids. This figure increased to at least 23% by 2004 [39].
Summary review of randomised control trials (RCT)
Pascual et al. in 2009, managed to systematically review all these RCTs to analyze their impact on short and long-lived consequences. The steroid sparing techniques had no impact on graft loss or mortality inclusive of death. Patients on conventional steroid therapy had a lower graft loss risk in comparison to those of steroid sparing protocols. There was a high AR rate in the steroid-sparing groups. The frequency of acute rejection was even higher following steroid withdrawal or avoidance than with conventional CsA treatment. As a result of steroid sparing and withdrawal strategies a decrease in the drug requirement for hypertensives, hyperlipidaemia, cholesterol, new onset diabetes after transplantation (NODAT) and cataract. Steroid avoidance managed to reduce cardiovascular episodes. Patients on TAC and CsA had the same antihypertensive and cholesterol reduction need while the need to use medication to treat hyperlipidaemia was prominent with TAC. NODAT reduction for treatment was only noticeable with CsA. Steroid sparing patients on CsA had a reduced infection rate. TAC has been suggested to be more protective than CsA since there was an increased AR rate in the studies utilizing CsA. Patients on steroid avoidance showed less prevalence of NODAT requiring any treatment than those on steroid withdrawal [40].
A review by Haller et al., evaluated the benefit and harms of steroid withdrawal or avoidance for kidney transplant recipients. They reviewed data from 48 RCTs (n= 7803 kidney transplant recipients) of which 3 RCTs (n=346) were children. The review compared steroid withdrawal against steroid maintenance in 24 out of 45 studies in adults, steroid avoidance against steroid maintenance in 18 out of 45 studies in adults, and steroid avoidance against steroid withdrawal in 3 out of 45 studies in adults.
Under the steroid withdrawal against steroid maintenance strategy, steroids were withdrawn at 3 months (8 studies), at 6 months (8 studies) at 12 months (1 study) and past 12 months after transplantation (1 study).
In the steroid avoidance against steroid maintenance strategy, no steroids were administered before and after the transplantation (2 studies), steroids were withdrawn until day seven after transplantation (12 studies), and between day 8 and 14 (2 studies).
Finally in the steroid avoidance against steroid withdrawal strategy (3 studies), there was steroid withdrawal up till the seventh day after transplantation in the avoidance group and steroid withdrawal within 3 and 6 months subsequent to transplantation in the steroid withdrawal group.
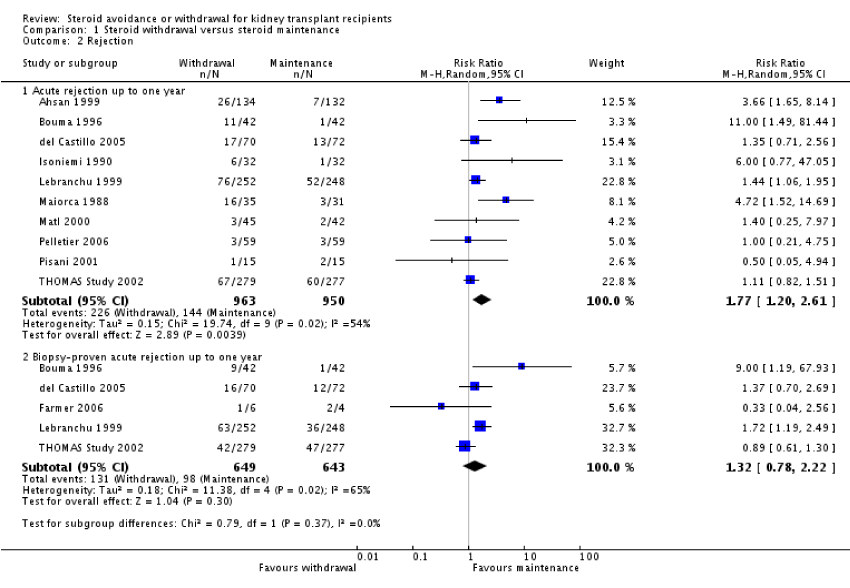
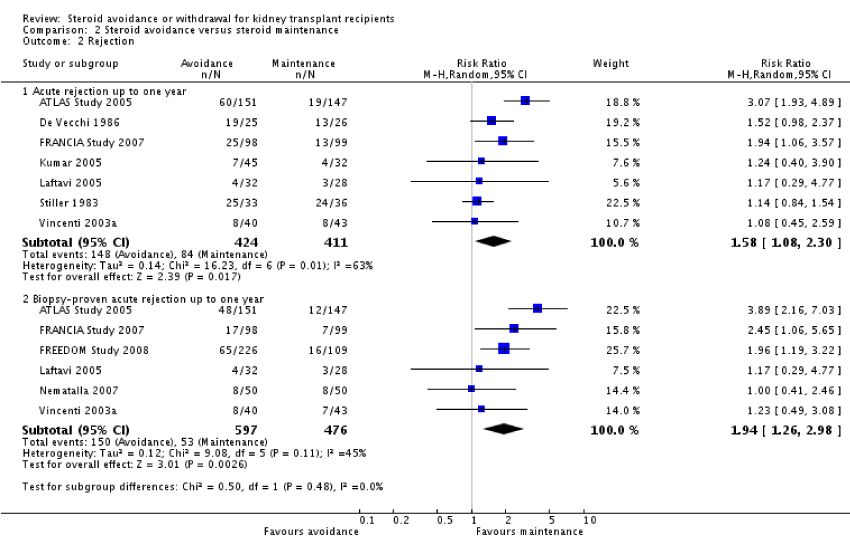
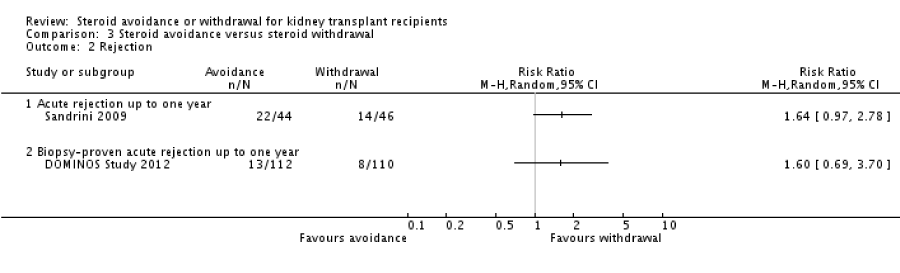
Patient mortality and graft loss was not significantly different in the steroid withdrawal versus steroid maintenance study and in the steroid avoidance versus steroid maintenance group. The chances of AR were highly increased in recipients who received steroid therapy for less than 14 days following transplantation (figure 2) and also in patients who were taken off steroid treatment at a subsequent time after transplantation (figure 3). No evidence was established to show any difference in the comparative groups for adverse or harmful events like malignancy and infection. The outcomes were measured within year one up to five years post kidney transplantation. In this review, most of the studies compared steroid withdrawal against steroid maintenance. Pediatric transplantation studies consisted of steroid withdrawal versus steroid maintenance with death, graft loss and rejection. There were limited studies for the comparison of steroid avoidance against steroid withdrawal with death, graft loss and AR (figure 4).
The issue of timing of steroids withdrawal and how long the outcomes be observed are critical factors in evaluating the advantages and harms of steroid withdrawal in kidney transplant recipients. A cut off of 14 days was common in steroid withdrawal and steroid avoidance, however, most of the steroid avoidance groups administered steroids for at least seven days or less while the bulk of withdrawal studies phased out steroids from three to six months post transplantation. Most studies had a short follow up period following steroid withdrawal or avoidance thus inhibiting finalization concerning lifelong complications for recipient and graft survival. AR has a major influence on graft survival and it is well known that recurrence of AR in most cases leads to allograft loss. In this review, AR occurred generally in the first year after transplantation, however, the harms of steroid withdrawal may stay undetected for up to five years. Therefore, the harmful after effects following steroid withdrawal on lifelong graft survival could not be dismissed on the basis of these studies. Equally so there is a need to build more evidence on adverse effects including infections. There is a general agreement amongst 3 studies on steroid withdrawal and 4 studies on steroid avoidance in patients on CsA with or without AZA that there is a high rate of AR in recipients withdrawn from steroids in comparison to those who were not taken off steroids [1]. Mudge in 2016 commented on the Haller et al 2016 review, that both steroid avoidance and withdrawal result in higher rate of AR [41].
Low steroid dose maintenance
Vlachopanos et al., proposed giving the recipient intravenous methylprednisolone in three doses of 500, 250 and 250 mg daily during the kidney transplant operation, and postoperatively on day 1 and 2. This is followed by an accelerated reduction of the methylprednisolone to 20mg oral per day for up to 2-4 weeks. Finally, the steroid dose is decreased to achieve 4 mg per day at 3 months and maintained at the same dose if no acute rejection is experienced [7]. Maintenance therapy is still commonly practiced in kidney transplant recipients. Woodle et al in the Astellas Corticosteroid Withdrawal Study comprising of 386 patients with Panel reactive antibodies of less than 25%, designed a strategy where one study group had steroids discontinued at 7 days following transplantation and the second group gradually reduced to 5mg per day at 6 months. In this study 68% of the patients were on ATG induction while 32% were on IL-2 receptor blocking monoclonal antibodies. All patients were on TAC and MMF maintenance. There results of the study after 5 years showed no variation in patient and graft survival with the group that continued steroid intake having a low biopsy proven AR. In the steroid withdrawal arm, the patients inducted with ATG had an insignificant reduction of AR rate than with IL-2 receptor blocking monoclonal antibodies. While creatinine clearances were the same between the two study groups at 5 years, there was a very high prevelance of chronic allograft nephropathy in the steroid withdrawal group than in the patients that continued with steroid treatment. Very early withdrawal had an insignificant variation in new onset diabetes mellitus, hypertension total cholesterol and low density lipoprotein but had a significant improvement in serum triglycerides. Bone fractures and avascular necrosis were lowered while subcapsular cataracts were increased [7,42].
The conclusion that patients on steroid avoidance or withdrawal had a statistically high AR rate compared to those patients maintained on steroids was reached in a meta-analysis study by Knight et al., comprising of 5637 patients. In this study allograft function was improved in patients on steroid continuation. Hypertension, new onset diabetes mellitus and hypercholesterolemia were decreased with steroid avoidance [7,43].
Steroid minimization and high immunological risk
Information relating to application of steroid avoidance in patients who are at an increased immunological risk is still scares. Outcomes on the basis of rATG induction with steroid withdrawal at day 4 post transplantation in African Americans maintained on TAC and Sirolimus or MMF have been analysed in two studies by Saull et al., and Haririan et al. For these high immunological risk studies, the AR rates were moderate at 13-14% with a remarkably improved graft and patient survival being recorded. However, there is still need for further verification on the consistence of this type of management protocol [44-47].
Another trial included high immunological risk patients who had PRA of greater than 20% or subsequent transplantations and on alemtuzumab, TAC and no steroids or, alternatively, on rATG, TAC, MMF and steroids for 5 days. The outcome showed a higher AR rate of 18% with alemtuzumab and 37.5% in the rATG participants after 12.4 months. Allograft survival was equal in both groups at 87%. These results confirm that steroid avoidance is not an appropriate alternative for high immunological risk patients, refer to table 4 [48].
Augustine et al. 2010, and Gaber et al. 1996, recommended high risk recipients for instance those with delayed graft function or donor reactive cellular immunity to be identified at the time of transplantation for steroid maintenance [49-51]. Recurrence of some glomerulopathies following steroid avoidance has been noted to be higher than with steroid continuation. Patient and graft survival was not affected in patients inducted on rATG and maintained on TAC and MMF [44,52]. In a study comprising 124 kidney transplant recipients, of whom 91% were on rATG induction Visger et al., also had the same results as shown by Kuklan [44,53]. Generally in patients where the initial cause of kidney disease is IgA nephropathy, steroid avoidance is not recommended.
Steroid minimization in children
Steroid minimization concepts are of special interest in paediatric patients due to impediment of growth that is linked to steroid usage on a long term basis. In a study of 196 children where steroids were withdrawn at day 4 in patients on daclizumab induction, and TAC, MMF maintenance against a control group on TAC, MMF and steroid regimen, Grenda et al., showed improved growth in the children’s growth at 6 months post transplantation. The survival rates of patients and allograft including graft function were not divergent in the study arms [7,54]. Hocker et al., also achieved identical results in a study involving 42 moderate to high risk children on CsA, MMF immunosuppression maintenance and had steroid withdrawal at 12 months post transplantation, please refer to table 4 [7,55].
A study by Chavers et al., showed enhanced growth in children who had been subjected to a low dose intravenous steroids up to day 5 following kidney transplantation and maintained on CsA and MMF, refer to table 4. The biopsy proven AR at 2 years was recorded as moderate at 19%. Besides the presence of leucopenia and Epstein-Barr infections, other paramaters in the steroid avoidance group of the study were not affected [44,56].
Zero biopsy proven AR rate at 13 months following transplantation was attained in a study by Li et al., following use of a comparatively high rATG induction dose, steroid avoidance TAC and MMF [44,57]. Wittenhagen et al., in a retrospective study, detailed a lesser number of biopsy proven AR of 9% at 12 months post transplantation in paediatric patients inducted on rATG and maintained on various immunosuppression regimens [58].
Steroid minimization in the elderly and other conditions
Steroid minimization is considered as being non-beneficial to elderly patients since the rates of AR are already reduced in elderly persons. Steroid withdrawal in the elderly needs to be cautiously and carefully applied as it may lead to bad outcomes including harsh AR. Additionally, due to their limited life expectancy steroid minimization advantages on cardiovascular disease becomes irrelevant [7,59]. In their review Yarlagadda et al (2009) reported that ischemic injury in kidney transplant recipients with delayed graft function and prolonged cold ischemia time is linked to acute rejection. This makes this category of patients not attractive for steroid minimization [7,60].
Novel immunotherapy and steroid avoidance
A few trials have been carried out on novel immunosuppresion protocols that do not include steroids and CNI. 89 low to moderate immunological risk patients were placed randomly under three study categories of differing immunosuppression regimens consisting of either belatacept with MMF, belatacept with sirolimus and TAC with MMF while they were all on rATG induction with intravenous steroids to 4 days post transplantation. After a 12 months study the biopsy proven AR rate for belatacept/MMF was 14%; for belatacept/sirolimus this figure was 4% and for control TAC/MMF this incidence was 3%. Steroid-free immunosuppression may, therefore, not be achieved through CNI-free protocols [61-63].
Conclusions
The goal of this evaluation was to provide an up to date evidence of steroid avoidance and withdrawal in kidney transplant recipients for our newly established kidney transplantation unit. Earlier reports from the CsA era on steroid free immunosuppression were adverse due to increased rates of AR, however, this old information is no longer applicable since strategies have been refined with more potent medications for induction and maintenance. While the use of steroid free strategies has now been applied extensively, the review has confirmed total steroid avoidance or very early withdrawal protocols as the most preferred and is considered to be effective by using lymphocyte depleting induction which reduce the immunological risk of graft failure and AR. Most RCTs registered high AR rates in cases where steroids were withdrawn in comparison to where steroids were maintained. Early AR, however, have no effect on patient and graft survival. In practice patient selection is most critical for successful outcomes and steroid avoidance is not appropriate for high immunological risk patients, recipients with delayed graft function, prolonged cold ischemia time, cardiac death donation, black patients and those with a history of glomerulonephritis. Low immunological risk recipients like first transplant cases with low panel reactive antibodies are more appropriate candidates for steroid minimization. The adverse effects of steroids are well documented, however, steroid minimization has been associated with an unclear reduction of these adverse effects. The main argument to be addressed is whether the harmful effects of AR that can be reversed in a small number of patients to maintain the gains associated with steroid avoidance. This review has given an insight on the way forward in choosing a suitable steroid withdrawal protocol for our unit in view of the complexity, unclearness and incompleteness surrounding the issue of steroid avoidance or withdrawal. It is, therefore, relevant to apply a strategy which utilizes effective induction agents like rATG or alemtuzumab along with intraoperative intravenous steroids, and postoperative maintenance therapy of a low steroid dose tapered at 4 weeks and then further reduced to a very small dose for an indefinite period in combination with a calcineurin inhibitor TAC and MMF. Transplant recipients with low immunological risk are ideal candidates for early steroid withdrawal.
- Haller MC, Royuela A, Nagler EV, Pascual J, Webster AC, et al. (2016) Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev 22: CD005632. Link: https://goo.gl/Sqcgqk
- Burhan U, Guilfoyle G, Superdock KR, Keith R, Badosa F, et al. (2008) Steroid Withdrawal After Kidney Transplantation with Daclizumab Induction. Dialysis & Transplantation 37: 314-319. Link: https://goo.gl/xoS3hx
- Augustine JJ, Hricik DE (2006) Steroid sparing in kidney transplantation: changing paradigms, improving outcomes, and remaining questions. Clinical Journal of the American Society of Nephrology 1: 1080-1089. Link: https://goo.gl/85jqG7
- Grinyó JM, Cruzado JM, (2005) Steroid or calcineurin inhibitor-sparing immunosuppressive protocols:strategies to prevent organ rejection. Kidney Transplantation 146: 30-42. Link: https://goo.gl/uSDnEx
- Cattaneo D, Perico N, Gaspari F, Gotti E, Remuzzi G, et al. (2002) Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation. Kidney International 62: 1060-1067. Link: https://goo.gl/hCmro3
- Almawi WY, Melemedjian OK, Reider MJ (1999) an alternate mechanism of glucocorticoid anti-proliferative effect: promotion of a Th2 cytokine-secreting profile. Clinical Transplantation 13: 365-374. Link: https://goo.gl/Z4b7kN
- Vlachopanos G, Bridson JM, Sharma A, Halawa A (2016) Corticosteroid minimization in renal transplantation: careful patient selection enables feasibility. World J Transplant 6: 759-766. Link: https://goo.gl/AEM7mu
- Stratta RJ, Armbrust MJ, Oh CS et al. (1988) Withdrawal of steroid immunosuppression in renal transplant recipients. Transplantation 45: 323-328.
- Vanrenterghem Y, Lebranchu Y, Hené R, Oppenheimer F, Ekberg H (2000) Steroid Dosing Study Group. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 70: 1352-1359. Link: https://goo.gl/rpSDpZ
- Sinclair NR (1992) Low dose steroid therapy in cyclosporine-treated renal transplantation recipients with well-functioning grafts. The Canadian Multiccentre Transplant Study Group. CMAJ 147: 645-657. Link: https://goo.gl/8xrVjL
- Hricik DE, O'Toole MA, Schulak, Herson J (1993) Steroid free Immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. Journal of American Society of Nephrology 4: 1300-1305. Link: https://goo.gl/MB43df
- Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, et al. (1999) Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil-a prospective randomized study. Steroid Withdrawal Study Group. Transplantation 68: 1865-1874. Link: https://goo.gl/sCdMK8
- Hricik DE, Knauss TC, Bodziak KA, Weigel K, Rodriguez V, et al. (2003) Withdrawal of steroid therapy in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation 76: 938-942. Link: https://goo.gl/Sw71iT
- Hricik DE, Whalen CC, Lantman J, Bartucci MR, Moir EJ, et al. (1992) Withdrawal of steroids after renal transplantation - clinical predictors of outcome. Transplantation 53: 41-45. Link: https://goo.gl/7TN2ms
- Vanrenterghem Y, van Hooff JP, Squifflet JP, Salmela K, Rigotti P,et al. (2005) Minimization of immunosuppressive therapy after renal transplantation: results of a randomized controlled trial. Am J Transplant 5: 87-95. Link: https://goo.gl/LybjKb
- Pascual J, Royuela A, Galeano C, Crespo M, Zamora J (2011) Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrology Dialysis Transplantation 27: 825-832. Link: https://goo.gl/qLng1M
- Pascual J, Galeano C, Royuela A, Zamora J. (2010) A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation 90: 343-349. Link: https://goo.gl/jicdoh
- Reisman L, Lieberman KV, Burrows L, et al. (1990) Follow up of cyclosporine-treated paediatric renal allograft recipients after cessation of prednisolone. Transplantation 49: 76-80.
- Sandrini S, Maiorca R, Scolari F, Cancarini G, Setti G, et al. (2000) A prospective randomized trial on azathioprine addition to cyclosporine versus cyclosporine monotherapy at steroid withdrawal, 6 months after renal transplantation. Transplantation 69: 1861-1867. Link: https://goo.gl/ipgMui
- Matl I, Lácha J, Lodererová A, Símová M, Teplan V, et al. (2000) Withdrawal of steroids from triple?drug therapy in kidney transplant patients. Nephrology Dialysis Transplantation 15: 1041-1045. Link: https://goo.gl/EC26ii
- Grinyo JM, Gil-Vernet S, Serón D, et al. (1997) Steroid withdrawal in mycophenolate mofetil-treated renal allograft recipients. Transplantation 63: 1688-1690. Link: https://goo.gl/xa98kR
- Smak Gregoor PJ, de Sévaux RG, Ligtenberg G, Andries J. Hoitsma†, Ronald J. Hené, et al. (2002) Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. Journal of the American Society of Nephrology 13: 1365-1373. Link: https://goo.gl/8tQrqp
- Opelz G, Dohler B, Laux G (2005) Collaborative Transplant Study. Longterm prospective study of steroid withdrawal in kidney and heart transplant recipients. Am J Transplant 5: 720-728. Link: https://goo.gl/EWb56G
- Loucaidou M, Borrows R, Cairns T, Griffith M, Hakim N, et al. (2005) Late steroid withdrawal for renal transplant recipients on tacrolimus and MMF is safe. In Transplantation proceedings 37: 1795-1796. Link: https://goo.gl/Gdq6MW
- Haller MC, Kammer M, Kainz A, Baer HJ, Heinze G, et al. (2017) Steroid withdrawal after renal transplantation: a retrospective cohort study. BMC Medicine 15: 8. Link: https://goo.gl/fowKBP
- Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, et al. (2005) Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation 80: 1734-1741. Link: https://goo.gl/urM81R
- Kuypers DR, Evenepoel P, Maes B, Coosemans W, Pirenne J, et al. (2003) The use of an anti?CD25 monoclonal antibody and mycophenolate mofetil enables the use of a low?dose tacrolimus and early withdrawal of steroids in renal transplant recipients. Clinical transplantation 17: 234-241. Link: https://goo.gl/saZwQW
- Matas AJ, Ramcharan T, Paraskevas S, Gillingham KJ, Dunn DL, et al. (2001) Rapid discontinuation of steroids in living donor kidney transplantation: a pilot study. Am J of Transplantation 1: 278-283. Link: https://goo.gl/G5X13g
- Matas AJ, Kandaswamy R, Humar A, Payne WD, Dunn DL, et al. (2004) Long-term immunosuppression, without maintenance prednisone, after kidney transplantation. Annals of surgery 240: 510-517. Link: https://goo.gl/eSmYCK
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Roza A (2003) Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. Am J Transplant 3: 306-311. Link: https://goo.gl/TsVBAB
- Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, et al. (2008) A randomized, multicenter study of steroid avoidance, earlysteroid withdrawal orstandard steroid therapy in kidney transplant recipients. Am.J. Transplant 8: 307-316. Link: https://goo.gl/JYZYLB
- Kumar MS, Heiftes M, Moritz MJ, Saeed MI, Khan SM, et al. (2006) Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation 81: 832-839.
- Laftavi MR, Stephan R, Stefanick B, Kohli R, Dagher F, et al. (2005) Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery 137: 364-371. Link: https://goo.gl/pnm5Kx
- 3C Study Collaborative Group, Haynes R, Harden P, Judge P, Blackwell L, et al. (2014) Alemtuzumab-based induction treatment versus basiliximab-based induction treatment in kidney transplantation (the 3C Study): a randomized trial. Lancet 384: 1684-1690. Link: https://goo.gl/GW1ZXX
- Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, et al. (2011) alemtuzumab induction in renal transplantation. N. Engl J Med 364: 1909-1919. Link: https://goo.gl/a723is
- te Meulen CG, Riemsdijk IV, Hené RJ, Christiaans MH, Borm GF, et al. (2004) Steroid-Withdrawal at 3 Days After Renal Transplantation with Anti?IL?2 Receptor ? Therapy: A Prospective, Randomized, Multicenter Study. American journal of transplantation 4: 803-810. Link: https://goo.gl/uuK2xd
- Birkeland SA (2001) Steroid-free immunosuppression in renal transplantation: a long-term follow-up of 100 consecutive patients. Transplantation 71: 1089-1090. Link: https://goo.gl/BCtDbE
- Sarwal MM, Yorgin PD, Alexander S, Millan MT, Belson A, et al. (2001) Promising early outcomes with a novel, complete steroid avoidance immunosuppression protocol in pediatric renal transplantation. Transplantation 72: 13-21. Link: https://goo.gl/sx7Eg2
- Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, et al. (2006) Immunosuppression: evolution in practice and trends, 1994-2004. American Journal of Transplantation. 6: 1111-31. Link: https://goo.gl/jk3DNM
- Pascual J, Zamora J, Galeano C, Royuela A, Quereda C, et al. (2009) Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 21: CD005632. Link: https://goo.gl/wR66Qe
- Mudge DW. (2016) Avoiding or stopping steroids in kidney transplantation recipients:sounds good but does it work? Cochrane Database Syst Rev Link: https://goo.gl/wLiX6A
- Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, et al. (2008) Astellas Corticosteroid Withdrawal Study Group. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564-577. Link: https://goo.gl/T91nBu
- Knight SR, Morris PJ (2010) Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 89: 1-14. Link: https://goo.gl/KMJLtW
- Naesens M, Berger S, Biancone L, Crespo M, Djamali A, et al. (2016) Lyphocyte-depleting induction and steroid minimization after kidney transplantation: A review. Nefrologia 36: 469-480. Link: https://goo.gl/3vW5QB
- Saull HE, Enderby CY, Gonwa TA, Wadei HM (2015) Comparison of alemtuzumab vs. antithymocyte globulin induction therapy in primary non-sensitized renal transplant patients treated with rapid steroid withdrawal. Clin Transpl 29: 573-580. Link: https://goo.gl/VfB3wQ
- Haririan A, Sillix DH, Morawski K, El Amm JM, Garnick J, et al. (2006) Short term experience with early steroid withdrawal in African-American renal recipients. Am J Transpl 6: 2396-2402. Link: https://goo.gl/T75RSt
- Zeng X, El-Amm JM, Doshi MD, Singh A, Morawski K, et al. (2007) Intermetiate-term outcomes with eraly steroid withdrawal in African American renal transplant recipients undergoing surveillance biopsy. Surgery 42: 538-544. Link: https://goo.gl/Jsrk2Y
- Thomas PG, Woodside KJ, Lappin JA, Vaidya S, Rajaraman S, et al. (2007) Alemtuzumab (Campath 1H) induction with tacroplimus monotherapy is safe for high immunological risk renal transplantation. Transplantation 83: 1509-1512
- Augustine J. Avoiding steroids can be a successful strategy after kidney transplantation…Kidney news online. www.kidneynews.org/kidney-news. Link: https://goo.gl/mz84QP
- Gaber LW, Gaber AO, Hathaway DK, Vera SR, Shokouh-Amiri MH (1996) Routine early biopsy of allografts with delayed function: correlation of histopathology and transplant outcome. Clin transplant 6: 629-34. Link: https://goo.gl/9eDs4p
- Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, et al. (2005) Pre?Transplant IFN?? ELISPOTs Are Associated with Post?Transplant Renal Function in African American Renal Transplant Recipients. Am J of transplantation 5: 1971-1975. Link: https://goo.gl/VA3mpm
- Kukla A, Chen E, Spong R, Weber M, El-Shahawi Y, et al. (2011) Recurrent glomerulonephritis under rapid discontinuation of steroids. Transplantation 91: 1386-1391. Link: https://goo.gl/P6ezGd
- Von Visger JR, Guna YY, Andreoni KA, Bhatt UY. Nori US, et al. (2014) the risk of recurrent IgA nephropathy in a steroid free protocol and other modifying immunosuppression. Clin Transpl 28: 845-854. Link: https://goo.gl/QJzRdY
- Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, et al. (2010) A randomized trial to assess the impact of early steroid withdrawal on growth in paediatric renal transplantation: the Twist study. Am J Transplant 10: 828-836. Link: https://goo.gl/k6SnUV
- Hocker B, Weber LT, Ferneberg R, Drube J, John U, et al. (2010) Impoved growth and cardiovascular risk after late steroid withdrawal: 2 year results of a prospective, randomized trial in paediatric renal transplantation. Nephrol Dial Transplant 25: 617-624. Link: https://goo.gl/UfiqWh
- Chavers BM, Chang YC, Gillingham KJ, Matas A (2009) Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results. Transplantation 88: 237-241. Link: https://goo.gl/rnUcS5
- Wittenhagen P, Theisson HC, Baudier F, Pedersen EB, Neland M (2014) Long term experience of steroid free pediatric renal transplantation: effects on graft function, body mass index, and longitudinal growth. Pediatr Transpl 18: 35-41. Link: https://goo.gl/QTXnWp
- Meier-Kriesche HU, Srinivas TR, Kaplan B (2001) Interaction between acute rejection and recipient age on long term renal allograft survival. Transplant Proc 33: 3425-3426. Link: https://goo.gl/rq5aKq
- Yarlagadda SG, Coca SG, Formica RN, Poggio ED, Parikh CR. (2009) Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039-1047. Link: https://goo.gl/nk1ViQ
- Refaie AF, Mahmoud KM, Ismail AM, Sheashaa HA, Kamal AI, et al. (2011) Alemtuzumab preconditioning allows steroid-calcineurin-free regimen in live-donor kidney transplant. Exp Clin Transpl 5: 295-301. Link: https://goo.gl/vcwxjF
- Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, et al. (2011) Immunosuppression with belatacept-based, corticosteroid avoiding regimens in denovo kidney transplant recipients. Am J Transpl 11: 66-76. Link: https://goo.gl/mmU7Eg
- Kirk AK, Guasch A, Xu H, Cheeseman J, Mead SI, et al. (2014) Renal Transplantation using belatacept without maintenance steroids or calcineurium inhibitors. Am J Transpl 14: 1142-1151. Link: https://goo.gl/8HA7pf
- Li L, Chaudhuri A, Chen A, Zhao X, Bezchinsky M, et al. (2010) efficacy and safety of thymoglobulin induction as an alternative approach for steroid-free maintenance immunosuppression in pediatric renal transplantation. Transplantation 90: 1516-1520. Link: https://goo.gl/mDfx5K
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
