Archives of Organ Transplantation
Interpretation of Crossmatch reports in a patient with Lupus Nephritis
Shafiq Ahmad Chughtai1, Ajay Kumar Sharma2* and Ahmed Halawa3
2Royal Liverpool University Hospital, Liverpool, UK
3Sheffield Teaching Hospitals, Sheffield Kidney Institute, University of Sheffield, Sheffield, UK
Cite this as
Chughtai SA, Ajay SK, Halawa A (2017) Interpretation of Crossmatch reports in a patient with Lupus Nephritis. Arch Organ Transplant 2(1): 023-029. DOI: 10.17352/2640-7973.000008Acute rejection (AR) is a major issue in renal transplantation which requires hospital admission, invasive investigations, and loss of allograft if it does not resolve with timely treatment. The workup for renal transplantation involves assessing potential recipient’s and donor’s tissue type. To predict the possibility of having a successful transplant offer, calculated panel reactive antibodies (cPRA) are used. The risk AR is predicted by tests such as cytotoxicity crossmatch, flow cytometry crossmatch, and identification of donor-specific antibodies using solid phase assays. Virtual crossmatch can be employed in selected cases to reduce cold ischemia time. None of these tests are to be taken in isolation. It is important to be aware of limitations of every technique. In addition, patient’s serum is tested regularly to determine the presence of antibodies, as antibody titers may fall or new antibodies may surface. Interpretation of the results of these tests can be quite changing. Transplant clinicians need to be aware that autoimmune diseases, blood transfusions, pregnancy or infections can sensitize a recipient and thereby add to a clinical dilemma.
Index Case
A 30 years old gentleman with end-stage renal disease, secondary to lupus nephritis had been on haemodialysis for the last 5 years. There has been no history of blood transfusions or transplantation. Apart from renal failure, he had no major comorbidities and had a normal cardiac workup. He has been offered an organ from a deceased donor pool with a mismatch of 1-0-0. T and B cell cytotoxicity crossmatch was positive and flow cytometry was negative. The Luminex screen did not reveal any donor-specific antibodies.
Lupus nephritis posing clinical dilemma in cross-matching
Lupus nephritis is an autoimmune condition involving antibodies targeting DNA, leading to deposition of immune complexes [1]. Kidneys are afflicted in 50% cases of systemic lupus erythematosus [2], and 10 to 30% of these patients will progress to renal failure [3]. Once these patients develop end-stage renal disease and require renal replacement therapy, their survival is similar to other patients requiring haemodialysis or peritoneal dialysis [4]. Many studies have shown encouraging results of renal allograft survival in patients with lupus nephritis [5]. It has been suggested that the patients with aggressive lupus should be kept on dialysis for at least 3 to 6 months and be commenced on prednisolone up to a dose of 10 mg a day before being considered for transplantation, to maximize the chances that the patient continues to be in remission [6]. Risk of recurrence after renal transplantation is 2 to 11 % [7]. The recurrence in transplant kidney often does not require any modification of immunosuppression [8]. Therefore, it can be concluded that renal transplantation is a safe and effective modality for ESRD secondary to lupus nephritis.
It has been proven that staying on haemodialysis is associated with reduced survival and inferior quality of life [9]. As the number of years spent on dialysis increases, cardiovascular morbidity and mortality rises. With every year spent on dialysis, there is 6% increase in mortality [10]. In dialysis-dependent patients, graft and patient survival are inferior compared with patients who had been dialysis-free prior to transplantation [11].Survival advantage is better in live donor vs deceased donor transplantation [12]. Advancing age has a negative effect on graft survival in deceased donor transplantation [13]. In addition, better HLA matching, low cold ischemia time and donation after brainstem death (rather than donation after cardiac death) is associated with better graft survival [14].
Renal transplantation requires the lifelong need of immunosuppression. Human leucocyte antigens are coded by genes on the short arm of chromosome 6 and are one of the most polymorphic genes known. The product of these genes is represented as class I and class II major histocompatibility molecules. All nucleated cells have class I and antigen presenting cells have class II MHC molecules on their cell membrane [15]. An antigen is a protein, polysaccharide or lipid which can be recognized by the immune system and can initiate an immunological response [16,17].
The basic purpose of MHC molecules is to differentiate self-antigens from non-self. Within the self-antigens, it will identify all abnormal cells that have turned malignant or are infected with viruses/bacteria and initiate process to kill them. Recognition of self-antigens is done by CD8 T cells as they interact with class I MHC molecules on all nucleated cells. CD4 T cells interact with MHC class II molecules represented on antigen presenting cells. Antigen presenting cells engulf, process and present foreign antigens on their cell surface via MHC class II molecules. These cells are involved in presenting antigenic components of pathogens such as bacteria and fungi to CD4 T cells. Central to the immune system are CD4 T cells that orchestrate a comprehensive antigen- specific immunological effector response involving recruitment of CD8 T cells, natural Killer cells, endothelial activation, cytokine production, clonal proliferation of T and B cells, and transformation of B cells into plasma cells. This results in some of the T and B cells transforming into memory cells [18]. Memory cells can cause delayed antibody production on exposure to antigens.
Sensitizing events such as blood transfusion, transplantation or pregnancy results in exposure to a non-self-antigen which results in antigen-specific T and B cell proliferation with resultant memory cells and antibody production. In the absence of sensitizing event, antibodies may exist due to molecular mimicry between pathogens and HLA epitopes. Epitopes are shared between different antigens (also called cross-reactive groups or CREG), therefore, it is possible to acquire an antibody against an epitope that is reactive to more than one HLA antigens [19].
The process of transplantation involves exposure to a non-self-antigen and initiation of immunological response. In the context of a preformed antibody, it will result in hyperacute rejection. In case of T and B memory cells, accelerated rejection will occur. Hence pre-transplant crossmatch is done to identify the level of sensitization recipient has specifically with regards to potential donor [20].
Organ allocation is done based on a point based matching system in the UK. Patients are prioritized based on a scoring system based on time on waiting list, sensitization, HLA matching and age matching. HLA matching is considered a key factor determining graft outcome both in live and deceased donor transplantation. With increasing mismatch, the probability of graft failure due to immunological reasons increases. This effect is much more pronounced when a mismatch is at HLA-DR antigens than at mismatch at HLA-B, and even lesser when there is HLA-A mismatch [21]. Index patient has 1-0-0 mismatch which essentially means he has only one HLA mismatch at HLA A loci, and thus in UK allocation system, this will be considered as Tier D allocation [22].
Assessing sensitization of recipient, Panel Reactive Assay (PRA) and calculated Panel Reactive assay (c-PRA)
PRA and cPRA quantify sensitization amongst recipients. In PRA, the donor pool is created by taking T and B lymphocytes from local volunteers. Cells from each donor are placed in wells and recipient serum added followed by addition of complement. If in a 50-cell panel, 30 cells are destroyed, PRA is considered as 60%. This means 60% of local donors would be unsuitable and, if transplanted, will result in hyperacute rejection. Thus, PRA is the percentage of unacceptable donors for a specific recipient in given population and is a guide to potential chances of getting an organ from local pool of donors [23].
There were problems with PRA. Local donor pool does not represent true donor population. There were variations from centre to centre. This led to the introduction of c-PRA, proposed by UNOS in 2009. Unacceptable antigens were defined by solid phase assays. This information is uploaded to a computer programme which compares these unacceptable antigens against last 10,000 donors. For example, if an antibody exists against HLA A2 which is present in 47% of Caucasians, c-PRA will be 47%. This means 47% of the national donor pool is unsuitable for a recipient with HLAA2. This gave a better estimate of sensitization status and chances of a prospective recipient being considered for [23].
Approach to recipient
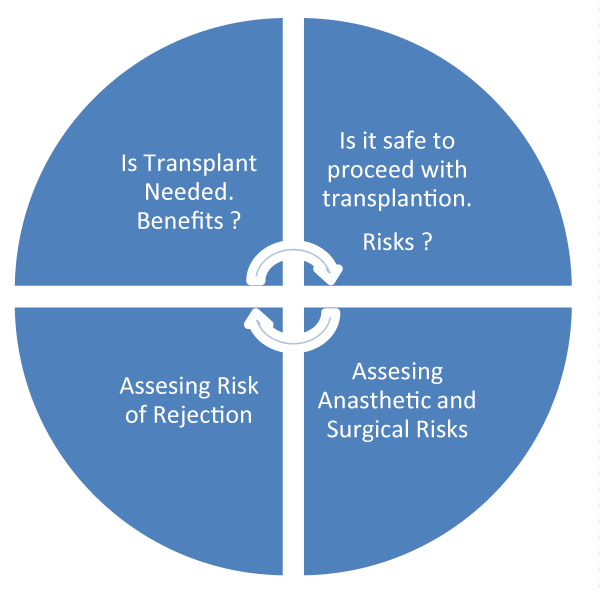
The decision to consider a patient for transplantation involves careful risk and benefit assessment. Decision making involves patient’s wishes and expectations as well as a multidisciplinary approach. Quality of life, anaesthetic risks and potential for surgical complications and co-morbidities are crucial factors in decision-making, figure 1.
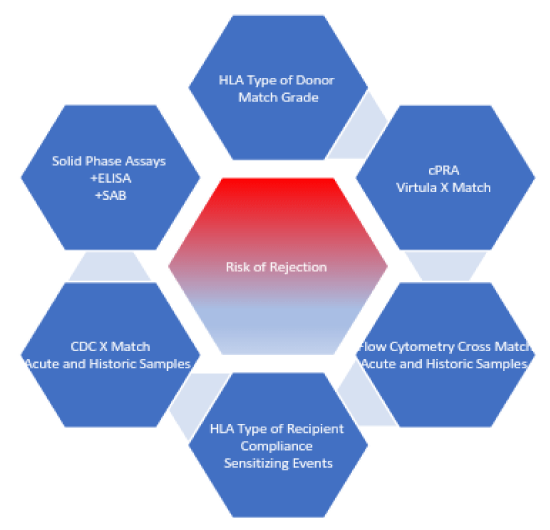
The following is the stepwise approach to immunological risk stratification, please refer to figure 2:
1. Define patient’s tissue type: This involves HLA typing which is commonly done using DNA based techniques [23].
2. Define patient’s antibodies to HLA antigens: This will give us information in regards to unacceptable antigens against which prospective recipient has antibodies. These measurements are performed using solid phase assays (ELISA or single antigen beads) [23]. Titres are expressed as mean fluorescence value or mean channel shifts [24,25].
3. What is patient’s c-PRA: Patient’s chances of getting a cadaveric organ depend on his or her level of sensitization. A score of >80% is considered highly sensitized [26].
4. Assess recent and historic donor-specific antibodies: Patients are regularly screened at 3 months intervals. Sensitizing events can happen, new antibodies may form or titres may rise or fall with time. Solid phase assays and Luminex screening are used to assess the development of new antibodies against HLA antigens [20].
5. Patient receives an offer based on point-based system: Points are given on the basis of a level of sensitization, matching grade, age matching and time on the list [22]. In the UK, national organ allocation is guided by point based system.
6. Assess risk of rejection: When an offer is considered from a donor, the risk of AR is to be calculated. This is based on the following approach.
a. How long the patient had been on the list and what is cPRA?
b. What is mismatching grade?
c. Based on recipient’s HLA type, sensitization status and unacceptable antigen (compared with the donor), is crossmatch needed or transplant can proceed with virtual crossmatch? In case of doing virtual cross match, actual crossmatch follows after transplantation.
d. What are the results of CDC and flow cytometry crossmatch in acute and historic samples?
e. What is the DSA screen of a recipient in acute and historic samples?
7. No result is considered in isolation: Recipient is stratified into three groups based on their risk of rejection, standard, intermediate or high risk. The decision is made whether to proceed with transplantation or not based on individual patient’s circumstances and urgency of transplantation.
Complement Dependent Cytotoxicity (CDC)
In CDC crossmatch, donor lymphocytes are added to recipient serum and complement is added. In the presence of preformed donor-specific antibodies, complement cascade will be activated resulting in the formation of membrane attack complex, cell lysis and death. Generally, 20% cell destruction is considered positive [24]. CDC crossmatch is done separately on T and B cells. Positive CDC T cell crossmatch reflects presence of donor-specific antibodies (DSA) against MHC class I antigen. Positive B cells cross match reflects the presence of DSA against MHC class I and II antigens. CDC cross match is done on both acute and historic samples.
Limitation of CDC Cross-match
CDC can be falsely positive in 20% or falsely negative in 4% [25].
The reasons for falsely negative tests are:
The low titer of antibody.
Low expression of antigen on the cell surface.
Non-complement fixing antibodies [24].
Falsely positive CDC tests are seen in the presence of:
IgM auto antibodies [24].
Serum that reacts with lymphocytes but not with HLA I or HLA II antigens on solid phase assays is also reflective of autoantibodies [23].
Positive CDC crossmatch in historic samples but negative CDC in acute samples means presence of detectable DSA in past. Antibody titres may fall over time and in the presence of memory cells, subsequent exposure to the antigen will lead to accelerated rejection due to reactivation of memory cells into plasma cells [20]. Therefore, the levels in both acute and historic samples are to be examined carefully. In addition, it is important to regularly inform the transplant lab about any potential sensitizing event, such as pregnancy, transfusion, transplantation, infection, vaccination or treatment with immunological agents such as rituximab which can influence crossmatch and result in the development of antibodies [20].
Antibodies are either IgM or IgG antibodies. IgM antibodies are often autoantibodies and are commonly detected in sera of patients with autoimmune disorders such as systemic lupus erythromatosis [23]. These antibodies are considered clinically less significant and limited number of studies have implicated them in rejection [26,27]. Non-HLA antibodies include MICA, angiotensin type I receptor antibody, anti-vimentin and anti-collagen antibody. Non-HLA antibodies can be identified by Luminex solid phase assays [27]. Non-HLA antibodies are not routinely tested for in a pretransplant crossmatch.
Antibodies are either IgM or IgG antibodies. IgM antibodies are often autoantibodies and are commonly detected in sera of patients with autoimmune disorders such as systemic lupus erythromatosis [23]. These antibodies are considered clinically less significant and limited number of studies have implicated them in rejection [26,27]. Non-HLA antibodies include MICA, angiotensin type I receptor antibody, anti-vimentin and anti-collagen antibody. Non-HLA antibodies can be identified by Luminex solid phase assays [27]. Non-HLA antibodies are not routinely tested for in a pretransplant crossmatch. Since index patient has CDC positive crossmatch for both B and T cells, the chances are that he has pre-formed antibodies against T and B cells (MHC Class I and Class II Abs), which is an absolute contraindication to transplantation. If this test is not false positive, options are to consider desensitization, considering paired pool or to redefine unacceptable antigens based on MFI values [23]. There is a possibility that this test in index case is a false positive test. As this patient has an autoimmune disorder which is notorious to be associated with IgM antibodies, it is important to exclude autoantibodies before refusing this organ [23].
The Sensitivity of CDC crossmatch can be enhanced by following techniques-
Antihuman Globulin (AHG)- Antihuman globulin is an antibody against the human Ig G. If the recipient has a low titre of DSA, CDC will be negative. By adding AHG, antibodies bound to donor lymphocytes will have AHG bound to their surface. This will increase the number of Fc receptors which then can bind complement component 1 and initiate cell lysis [24]. Thus, anti-human globulin promotes complement fixation and improves the sensitivity of CDC test [23].
Auto-crossmatch and Dithiothreitol (DTT) treatment- There are autoantibodies which are mostly IgM type. These are still considered clinically insignificant. In auto crossmatch, recipient serum is cross matched against recipient’s own lymphocytes [24]. Positive autocross match means presence of IgM autoantibodies. In addition, if the original CDC crossmatch is positive, by adding DTT, di-sulphide bonds can be disrupted between IgM antibodies thus preventing false positive result [24], Phosphate buffer saline is added to overcome the dilutional effect.
Amos Wash- As the name suggest, Amos wash involves removing the recipient serum after incubation with the donor lymphocytes. If antibodies are present, they are bound to lymphocyte cell membrane. By removing the serum, the anti-complement factors are removed that can cause false negative results by inhibiting the formation of complement [28].
Prolong Incubation and heating the sera- If the antibody titres are low, by prolonged incubation the sensitivity of CDC crossmatch is increased. IgM antibodies are reactive at 4 degrees Celsius and its activity can be removed by heating the sera at 55 degrees Celsius [23].
Flow cytometry crossmatch
Flow cytometry crossmatch is more sensitive than CDC crossmatch. It is routinely used in a pre-transplant cross match. The test involves mixing donor lymphocytes and recipient sera which would result in binding of antibodies to lymphocytes. Then fluorescein-labelled antibody against IgG antibodies is added. This fluorescent labelled antibody will bind IgG antibody, which if present, is already bound to T cells and B cells. Unlike CDC, flow cytometry crossmatch will detect donor-specific antibodies before they can cause cell death. There is no addition of complement. Fluorescein-labelled dyes are used to differentiate T and B cells. The cells are then run through a flow cytometer, which will identify antibody bound B and T cells. The strength of antibodies is represented as a number of channel shifts of mean fluorescence above the baseline [24,25], Like CDC cross match, positive T cell flow cytometry crossmatch means DSA against MHC I receptors and positive B cell crossmatch means DSA against MHC I and II receptors. T cells have a low density of MHC class I molecules. B cells have a high density of MHC class I molecules as well as MHC class II molecules on a cell surface.
A negative CDC crossmatch and positive flow cytometry crossmatch is because of following reasons [24].
Low titres of DSA.
Low expression of antigen on cell surface.
Non-complement fixing antibodies.
Limitation of flow cytometry crossmatch
Flow cytometry crossmatch is more sensitive methods of detecting DSAs. In other words, the clinical significance of DSAs determined by flow cytometry depends on patient’s history and results of CDC and Luminex screen. In unsensitized recipients, an isolated positive flow cytometry crossmatch in the presence of negative CDC crossmatch and no DSA is considered clinically insignificant and transplant can proceed. In sensitized recipients, who have negative CDC crossmatch and positive flow cytometry cross match, the results can be due to a low level of DSA and memory cells. Therefore, isolated flow cytometry crossmatch is considered significant in sensitized recipients since the incidence of AR is high and graft survival is low at one year in sensitized recipients having transplantation with a positive flow cytometry crossmatch [23]. Like CDC crossmatch, flow cytometry cross match should not be reviewed in isolation.
Patients with nonspecific immunoglobulins and on rituximab can have false positive flow cytometry crossmatch. By pretreating lymphocytes with pronase, which is a nonspecific peptidase, false positive flow cytometry results can be reduced.
Flow cytometry cross match is specifically important in following circumstances.
Positive flow cytometry and negative CDC in sensitized recipients is considered clinically significant as it may represent presence of memory cells [24].
In elderly recipients and with marginal donor kidneys, because of reduced immunity in these patients and relatively poor graft reserves [23].
A weak positive test is considered significant only in the presence of sensitization history or presence of DSA [23].
Flow cytometry test should always be considered with recipient history, age, level of sensitization and presence or absence of DSA levels.
Solid phase assays
Solid phase assays do not need donor lymphocytes (not cell based like CDC and flow cytometry) and they are the most sensitive method of determining HLA antibodies. They can differentiate HLA class I from class II antibodies. Their sensitivity can be increased up to a single antigen bead level where a single antibody can be detected [25].
Purified class I or class II HLA molecules are made and represented as a combination of different or a single HLA antigen.
HLA antigens are then represented on solid phase media platforms (ELISA) or microbeads [25].
Enzyme-linked immune absorbent assay (ELISA): ELISA is the least sensitive of solid phase assay and uses an antibody (bound to an enzyme) against preformed DSAs. If preformed DSA are present, then they are already bound to receptors on donor’s lymphocytes. If an antihuman antibody is added to this solution, it will bind to DSAs attached to donor’s lymphocytes. The antihuman antibody can be modified to have specific enzymatic activity. Enzyme substrate is added to this solution. Chemical reaction sets in that results in a signal, commonly a colour change which is picked up by optical density reading, resulting in detection of DSAs. ELISA is 10% more sensitive than AHG CDC crossmatch [25].
Single Antigen Beads Assay: When HLA molecules are represented on microbeads, the presence of antibodies can be detected by two methods.
These microbeads can be run through a traditional flow cytometer or a suspension array on a Luminex platform [25].
In Luminex microbeads, each bead represents a different HLA antigen. Recipient serum is added to these beads and 100 different beads can be combined in a single test [23].This is the most sensitive method of determining anti HLA antibodies. Single- antigen bead (SAB) can determine HLA type at allelic antigen level [29].
In terms of sensitivity, solid phase assays are the most sensitive and CDC is the least sensitive test [30].
Prozone phenomenon is seen when the presence of antibodies is far more in number than antigen. This results in a coating of all antigen antibody binding site, failure of bridging between the antigens and loss of agglutination [31].
Limitation of solid phase assay
Detection of the very low level of DSA which may be clinically irrelevant.
Detection of false positive anti HLA antibodies due to denatured proteins on beads [30].
The output of solid phase assay is optical density readouts and fluorescence assays. There are considerable inter-laboratory variations as to what threshold should be considered positive.
Number of alleles discovered are growing in thousands and not all of them can be represented or checked on solid phase assays.
Prozone phenomenon
High concentration of antibodies (IgM) can cause complement activation and deposition of complement proteins (C1) on the beads. This prevents binding of HLA antibody on HLA beads via prozone effect (less antigen binding sites available for binding resulting in false negative result). This can be treated with dilution or addition of DTT [32].
Epitope sharing amongst different beads can cause antibodies to be distributed to different beads reducing the MFI on a specific bead resulting in a false negative result [33].
The results of solid phase assays should not be considered in isolation and should be reviewed with patient’s sensitivity status and CDC/Flow cytometry crossmatch results.
Virtual crossmatch
For virtual crossmatch, recipient’s antibody screening is taken into consideration against donor HLA type. It is mandatory for a recipient to give serum at 3 monthly intervals. In virtual crossmatch, DSAs from all previous serum are taken into consideration as antibody titres can vary with time [25]. Virtual crossmatch done in the right situation is reliable and reduces cold ischemia time.
Limitations of virtual crossmatch [25]
The recipient may have alleles specific antibodies which can lead to false positive virtual crossmatch (variations in alleles are common within HLA antigens).
Null alleles are not expressed on the cell surface but are inherited. DNA typing can identify them. In the virtual crossmatch, it will come as a potential problem.
Very low level of antibodies might be detected which may not be clinically significant.
The discovered HLA antigens are increasing every day and not every one of them is represented on solid phase assay. Therefore, this can cause a false negative result.
If virtual crossmatch is done, the proper crossmatch should follow transplantation [25].
Application to index patients
Lupus Nephritis is an autoimmune disorder which is associated with antibodies against DNA [2]. In addition, strong association exist between lupus and presence of IgM autoantibodies [20]. A positive T & B cell CDC crossmatch represents the presence of MHC Class I and II antibodies which are complement fixing and are causing significant cell death in donor lymphocytes. However, a flow cytometry crossmatch which is more sensitive test is negative for B and T cells. There has been no donor-directed antibodies in recent and historic serum, which is tested using solid phase assays and is by far the most sensitive method of antibody testing. Therefore, our patients is unlikely to be sensitized. Thus, we can conclude that CDC crossmatch is likely to be a false positive test because of following reasons.
Patient has lupus nephritis which is associated with IgM autoantibodies [23]. This increases the likelihood of false positive CDC crossmatch.
Non-HLA antibody can also cause a positive cross-match [34].
Flow cytometry cross match which is more sensitive than CDC crossmatch is negative.
There are no donor-specific antibodies by solid phase assays in recent and historic samples.
Therefore, we need to exclude IgM antibodies to ensure it is not false positive result. IgM antibodies can be excluded by doing autocross match, cross match with DTT and saline buffer solution or by heating the sera to 55 degrees Celsius before repeating the crossmatch, which inactivates IgM antibodies. If the presence of IgM autoantibodies is confirmed by above mentioned methods, then it is safe to proceed with the transplant. In the presence of negative T and B CDC (DTT) cross match, negative T and B flow cytometry crossmatch, no sensitizing history, no donor specific antibodies on solid phase assays in recent and historic samples and 1-0-0 mismatch, this is a low immunological risk transplantation. The patient can have an induction with basiliximab. Maintenance immunosuppression should include CNI inhibitor, an anti-proliferative agent and steroids as per KDIGO 2012 guidelines.
If there is any deterioration in graft function (serum creatinine >25% above baseline), formal assessment of graft dysfunction should be done. This includes a proper clinical examination, specifical assessment of recipient’s fluid balance, the presence of infection, any graft tenderness, compliance to medications, investigations to exclude infections such as full blood count, CMV, EBV and BK virus PCR. Ultrasound scan of allograft to exclude perinephric collection, ureteric obstruction and graft perfusion should be undertaken. Trough levels of immunosuppressive medication, such as cyclosporine, tacrolimus or rapamycin should be monitored to exclude toxicity. In addition, patient’s drug history should be reviewed to exclude the recent use of any nephrotoxic agent. If AR is suspected, donor-specific antibodies should also be sent. It is now established that pre-formed as well as de novo donor-specific antibodies are associated with higher incidence of transplant glomerulopathy and acute/chronic antibody-mediated rejection [35]. Allograft biopsy is considered if no other cause of graft dysfunction is identified. If de novo donor specific antibody rise is associated with graft dysfunction and biopsy shows features of antibody mediated rejection, treatment should be commenced and maintenance immunosuppression be escalated. If graft function improves following treatment of rejection, regular follow-up and surveillance of DSA is recommended [36,37].
Conclusions
There has been a gradual increase in deceased donor transplantation activity [38]. Due to a mismatch between demand and supply, more marginal kidneys and high immunological risk transplants are attempted. Predicting the risk of rejection is not easy. Consideration is to be given to recipient’s history of sensitization, CDC and flow cytometry crossmatch and Luminex screening results. It has to be kept in mind that a high immunological risk transplant may be justifiable for a recipient who is running out of access options. By stratification of the risk of AR, immunosuppressive regimens can be tailored to minimize morbidity and mortality of AR and optimize the immunosuppressive medications accordingly. A careful interpretation of clinical background, the range of cross match tests and cPRA is required for avoiding inordinate exclusion of a good donor, to perform a successful renal transplant. This would need comprehensive immunological assessment of a prospective recipient including a multi-disciplinary discussion between transplant immunologist, transplant surgeon and nephrologist. Each of these tests does have inherent strengths and limitations. No single test is good enough in isolation [39,40].
- O’Flynn J, Flierman R, van der Pol P, Rops A, Satchell SC, et al. (2011) Nucleosomes and C1q bound to glomerular endothelial cells serve as targets for autoantibodies and determine complement activation. Mol Immunol 49: 75-83. Link: https://goo.gl/cXn6Vk
- Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, et al. (1993) Systemic lupus erythematosus: clinical and immunological patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine 72: 113-124. Link: https://goo.gl/qGxS2D
- Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN (2010) Review: Lupus Nephritis: pathogenic features, epidemiology and guide to therapeutic decisions. Lupus 19: 557-574. Link: https://goo.gl/skSUcP
- Moroni G, Tantardini F, Ponticelli C (2003) Renal replacement therapy in lupus nephritis. J Nephrol 16: 787-791. Link: https://goo.gl/jKKnPt
- Lionaki S, Kapitsinou PP, Iniotaki A, Kostakis A, Moutsopoulos HM, et al. (2008) Kidney transplantation in lupus patients: a case-control study from a single centre. Lupus17: 670-675. Link: https://goo.gl/kQUj5A
- Ponticelli C, Moroni G (2005) Renal transplantation in lupus nephritis. Lupus 14: 95-98. Link: https://goo.gl/omWrh4
- Burgos PI, Perkins EL, Pons-Estel GJ, Kendrick SA, Liu JM, et al. (2009) Risk factors and impact of recurrent lupus nephritis in patients with systemic lupus erythematosus undergoing renal transplantation: data from single US institution. Arthritis Rheum 60: 2757-2766. Link: https://goo.gl/WKfFME
- Ponticelli C, Moroni G, Glassock RJl (2011) Recurrence of secondary glomerular disease after renal transplant. Clin J Am Soc Nephrol 6: 1214-1221. Link: https://goo.gl/NWwXGV
- Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ (1998) Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with haemodialysis during long term follow-up. J Am Soc Nephrol 9: 2135-2141. Link: https://goo.gl/7V2j7C
- Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG (2000) Vintage, nutritional status, and survival in haemodialysis patients. Kidney Int 57: 1176-1181. Link: https://goo.gl/qxMv3o
- Herwig-Ulf Meier-Kriesche, Friedrich K Port, Akinlolu O Ojo, Steven M Rudich, Julie A Hanson, et al. (2000) Effect of waiting time on renal transplant outcome. Kidney Int 58: 1311-1317. Link: https://goo.gl/Ss83Jh
- Port FK, Dykstra DM, Merion RM, Robert A Wolfe (2005) Trends and results for organ donation and transplantation in the United states, 2004. Am J Transplant 5: 843-849. Link: https://goo.gl/pPwsd9
- Orsenigo E, Socci C, Carlucci M, V Zuber, P Fiorina et al. (2005) Multivariate Analysis of factors Affecting Patient and Graft Survival After Renal transplant. Transplant Proc 37: 2461-2463. Link: https://goo.gl/NWBP1Q
- Opelz G (1998) The benefit of exchanging donor kidneys among transplant centers. N Engl J Med 318: 1289-1292. Link: https://goo.gl/r5HvUt
- Shiina T, Inoko H, Kulski JK (2004) An update of the HLA genomic region, locus information and disease associations: 2004.Tissue Antigens 64: 631-649. Link: https://goo.gl/roncwL
- Richards S, Watanabe C, Santos L, Craxton A, Clark EA (2008) Regulation of B-cell entry into the cell cycle. Immunol Rev 224: 183-200. Link: https://goo.gl/iWNk8j
- Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ (2000) B-cell activation by T-cell-independent type 2 antigen as an integral part of the humoral immune response to pathogenic microorganism. Immunol Rev 176: 154-1570. Link: https://goo.gl/kvZ1KX
- Hivroz C, Chemin K, Tourret M, Armelle Bohineust (2012) Cross talk between T lymphocytes and Dendritic Cells. Crit Rev Immunol 32: 139-155. Link: https://goo.gl/beFLvS
- Lee PC, Lee PH, Shaw CK, SK Takemoto, DW Gjertson, et al. (1998) HLA epitopes for kidney allocation. Transplant Proc 30: 3496-3497. Link: https://goo.gl/sbWYem
- British Transplant Society (2014) Guidelines for detection and characterization of clinically relevant antibody*/-es in allo-transplantation. Report number: 3. Link: https://goo.gl/2Bsj1d
- Doxiadis II, de Fijter JW, Mallat MJ, Haasnoot GW, Ringers J, et al. (2007) Simpler and equitable allocation of kidneys from post-mortem donors primarily based on full HLA-DR compatibility. Transplantation 83: 1207-1213. Link: https://goo.gl/23vAZu
- National Health Service Blood and Transplantation. Kidney Transplantation: Deceased Donor Organ Allocation. Report number: 186/6,2017.
- Cecka JM, Rajalingam R, Zhang J and Reed E (2010) Histocompatibility testing, Cross matching, and Immune Monitoring. In: Danovitch GM (eds.) Handbook of Kidney Transplantation.5th Edition. Philadelphia: Lippincott Williams & Wilkins p.36-60.
- Mulley WR, Kanellis J (2011) Understanding crossmatch testing in organ transplantation: A case based guide for general nephrologist. Nephrology 16: 125-133. Link: https://goo.gl/tPDcFC
- Tinckam KJ (2012) Basic Histocompatibility Testing Methods. Chandraker A, Sayegh MH, Singh AK. (eds.) Core Concepts in Renal Transplantation. United states of America. Springer 21-42.
- Cecka JM (2010) Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant 10: 26-29. Link: https://goo.gl/UNfZ4j
- Dragun D, Philippe A, Catar R (2012) Role of non-HLA antibodies in organ transplantation. Curr Opin Organ Transplant 17: 440-445. Link: https://goo.gl/7HJ7Ba
- Gebel HM, Bray RA (2000) Sensitization and sensitivity: defining the unsensitized patient. Transplantation 69: 1370–1374. Link: https://goo.gl/PVnHgm
- Gebel HM, Bray RA, Nickerson P (2003) Pre-Transplant Assessment of Donor-Reactive, HLA-Specific Antibodies in Renal Transplantation: Contraindication vs. Risk. Am J Transplant 3: 1488-1500. Link: https://goo.gl/YM4ECM
- Middleton D, Jones J, Lowe D (2014) Nothing’s Perfect: The art of defining HLA specific antibodies. Transpl Immunol 30: 115-121. Link: https://goo.gl/o6Na1p
- Gillet P, Mori M, Esbroeck MV, Jef Van den Ende, Jan Jacobs (2009) Assessment of the prozone effect in malaria rapid diagnostic tests. Malar J 8: 271. Link: https://goo.gl/eiRHo7
- Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, et al. (2002) All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation 74: 1192-1194. Link: https://goo.gl/RKfk3V
- Willcombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, et al. (2012) De novo DQ donor specific antibodies are associated with a significant risk of antibody mediated rejection and transplant glomerulopathy. Transplantation 94: 172-177. Link: https://goo.gl/UR8Dse
- Weinstock C, Schnaidt M (2013) The complement-mediated prozone effect in the Luminex single-antigen bead assay and its impact on HLA antibody determination in patient sera. Int J Immunogenet 40: 171–177. Link: https://goo.gl/TYmc1C
- Phanish MK (2016) Immunological risk in assessment of human leukocyte antigen antibody testing kidney transplantation. Indian J Nephrol 26: 80-85. Link: https://goo.gl/riwskv
- Zhang Q, Reed EF (2016) The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol 12: 484-495. Link: https://goo.gl/GznzYq
- Worthington JE, Martin S, Al-Husseini DM, Dyer PA, Johnson RW (2003) Post transplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation 75: 1034-1040. Link: https://goo.gl/nGY81Q
- Loupy A, Hill GS, Jordan SC (2012) The impact of donor specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348-357. Link: https://goo.gl/p3r9Q9
- Lionaki S, Panagiotellis K, Iniotaki A, John N Boletis (2013) Incidence and clinical significance of de novo donor specific antibodies after kidney transplantation. Clin Dev Immunol 2013: 849835. Link: https://goo.gl/Fy6v71
- Annual report on kidney transplantation 2015/2016.NHS Blood and Transplantation.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
