Archives of Organ Transplantation
Split/Reduced Liver Transplantation “IMSS”: The First Two Cases and Literature Overview
Pierre Jean Aurelus1*, Hermilo De La Cruz Yáñez1, Alfonso Yamamoto Nagano1, Roberto Ortiz Galván1, Nicolás Fernández Mezo1 and Jacques Baulieux2
2Former President of French Surgical Academy and Emerita Professor of Medicine of Lyon University, México
Cite this as
Aurelus PJ, De La Cruz Yáñez H, Nagano AY, Galván RO, Mezo NF, et al. (2017) Split/Reduced Liver Transplantation “IMSS”: The First Two Cases and Literature Overview. Arch Organ Transplant 2(1): 009-014. DOI: 10.17352/2640-7973.000005Introduction: The term Split/Liver Transplantation involved the ex vivo division of an adult cadaver liver into a pediatric allograft and a remnant adult allograft. The efforts were an attempt to satisfy an increasing demand for pediatric cadaver allografts that had resulted in prolonged waiting periods and a wait-list mortality of approximately 50% at major pediatric referral centers. The main of this work is given to know our experience in two different cases and encouraged at the adult surgeons to confide and accept the right allograft of this technique for an adult patient.
Cases: We performed two reduced procedures by the split liver technique. The first case was a procurement of a male donor of 33 years’ old with diagnosis of cerebral death due to aneurysm rupture, and the recipient was a five years’ old girl with the diagnosis of biliary atresia. For the second case, we had a male donor of 8 years’ old with diagnosis of cerebral death secondary to arteriovenous malformation and the recipient was a 2.9 year´s old girl, with biliary atresia.
Conclusion: There are no differences in complications between split in cadaveric donor to living liver donor. However for a good outcome, it is important to have a good donor like it is for a good recipient. In our center, the split liver transplantation is uncommon and there is a clear need for better training of surgeons and for improved sharing of information about this needed procedure.
Introduction
Alternative methods are required to permit an increase in the number of grafts, Split/liver transplantation (SLT), it is considered one of them, and it is defined as the division of a deceased donor’s liver into two different parts functionally independent, and the transplantation of each of them into a different recipient [1-3]. Transplantation of partial-liver allograft for children was advocate by Smith in 1969 and was initially performed through the surgical reduction of a larger child or adult cadaver allograft, termed reduced liver transplantation by Bismuth, Houssin and Broelsh et al. in 1984 [1,3]. The term SLT was simultaneously reported by Pichlmayr et al. and Bismuth et al. in 1989. Their technique involved the ex vivo division of an adult cadaver liver into a pediatric allograft and a remnant adult allograft [1-4]. These efforts were an attempt to satisfy an increasing demand for pediatric cadaver allografts that had resulted in prolonged waiting periods and a wait-list mortality of approximately 50% at major pediatric referral centers [1-3].
This technique was performed in selected centers in Europe and the United States to decrease the pediatric waiting mortality, and at the same time to avoid discarding the right side of the liver [1-3,5]. Since, there has been a general agreement on anatomic classification made by Couinaud and accept across Asian, European and North American Transplant communities [6,7]. In our center, with the increasing pediatric and adult waiting list, a clinical group and surgeons of IMSS (Instituto Mexicano del Seguro Social) were performed in this technique, in Croix Rousse and Mére et Enfant Hospitals (les hospices civils) in Lyon/France, in an attempt to reduce the waiting list of patients who need a liver transplant in the country, specially (IMSS). The main of this work is given to know our experience in two different cases and encouraged at the adult surgeons to confide and accept the right allograft for an adult patient.
Cases
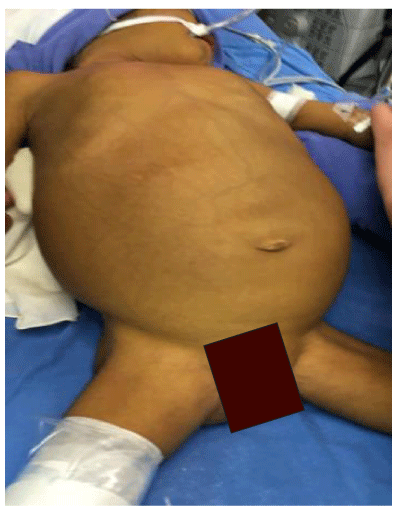
September to November 2017 in IMSS, we performed two reduced procedures by the split technique. For the first case, we performed a procurement of a male donor of 33 years’ old with body weight 109-kg and the diagnosis of cerebral death due to an aneurysm rupture. Others characteristics as donor are finding in table 1 and the recipient was a five years’ old girl with the diagnosis of biliary atresia with Kasai procedure. For The second case, in the same hospital (CMNSXXI/Pediatric Hospital Dr Silvestre Frenk F), we had a male donor of 8 years’ old, weight 27 kg with the diagnosis of cerebral death secondary to arteriovenous malformation and the recipient was a 2.9 year´s old girl, weight 9.6-kg with diagnosis of biliary atresia with Kasai procedure. She had respiratory distress due to ascites, hypersplenism and hepatomegaly. This recipient was classified like child C.
Discussion
Technical considerations
The bipartition technique requires only standard surgical facilities with no specialized equipment and have performed concomitant with additional abdominal and thoracic organ procurements [2,4]. Casually in our first donor for splitting, the procurement was multy-organic.
Types of split
Infants have the highest wait-list mortality of all liver transplantation candidates [5,6]. Deceased donor split-liver transplantation, a technique that provides both an adult and pediatric graft, might be the best way to decrease this disproportionate mortality [4,6]. Yet concern for an increased risk to adult split recipients has discouraged its widespread adoption [6,8,9]. We aimed to determine the current risk of graft failure recipients after split-liver transplantation. Adult/child vs adult/adult: the term split SL includes 2 different entities. The first is the adult /child (ACSL) that generates 2 differently sized grafts: 1 graft including the Couinaud segments II and III, suitable for transplantation into a small child generally not exceeding 30 kg in weight, and the other graft includes segments I and IV to VIII, suitable for an adult transplant [2,4,6,7]. In our cases, the split procedures were planning for children and adults.
The second entity of split is represented by adult/adult SL (AASL) that generates 2 similarly sized grafts to be transplanted into adults or large infants exceeding 25 to 30 kg in weight. In this different technique, segments I to IV constitute the left graft and segments V to VIII, the right [6,7,10]. Since, Azoulay (2001) reports the largest series of AASL collect over 6 years by Paris Paul Brousse Hospital in Paris. Finally, the Third entity: Ex situ vs IS (in situ); as mentioned above, both in ACSL and in AASL, the division of the liver may be performed IS while the heart is still beating and before flushing all the organs[1-3,6]. The main advantages of the IS technique are that ischemia time is shorter and that optimal control of bleeding from the cut surface of both grafts may be performed during the splitting procedure itself, so that minimal bleeding is expected after implantation in the recipient[ 1,3-5,11]. On the other hand, the IS technique significantly increases the operation time on the donor, thus impacting the organization of the procedure both for the donor hospital and for the teams involved in procuring other organs [1,3,4]. In our cases, we reduced the liver using the technique of the SLT adult/child with back table to separate the liver (ex-situ) and we organized how to perform this procedure of in situ to minimize bleeding after recipient implantation.
Logistical consideration
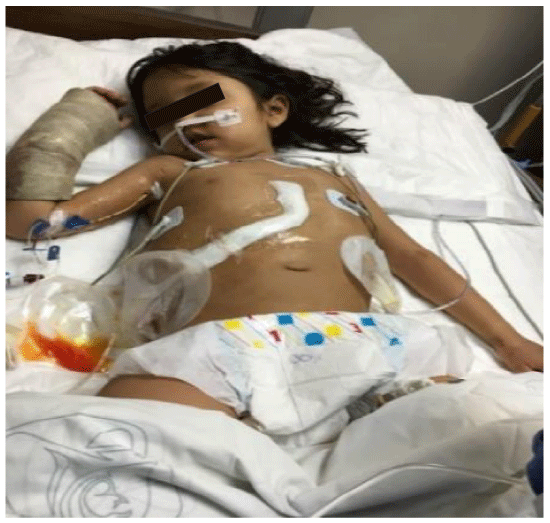
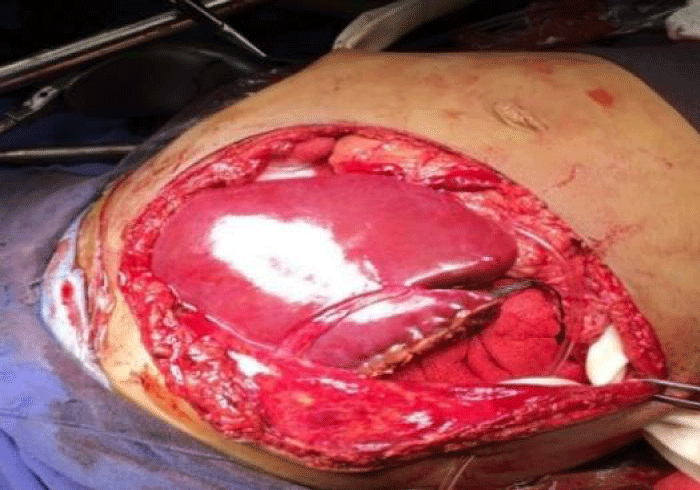
Donor and recipient selection are the most important for the successful use of partial grafts, the main reason that we presented this work with 2 different receptors [4,11,12]. However, a good donor selection criterion include ABO compatibility, age, liver function, sizemacth, absent/ scant arrest period, vasopressor requirements, serum sodium concentration and brief donor hospitalization [11-13]. Split liver transplantation entails greater requirements in term of time, material and human resources than conventional whole organ liver transplantation and, unavoidably, a learning curve. Favorable liver graft allocation policies and collaborative coordination among centers represent the basis for a rational, extensive use of this donor source [5,12,13]. In our donors we had used area transportation by the large distance, we had a directly communication with the coordination and the Hospital (Pediatric Hospital CMNSXXI, IMSS). The first recipient had a better weight and child B than the other recipient with child C (Figures 1-4).
Donor Selection
Preoperative evaluation
SLT may not be performed safely with all donors. Essential in the success of SLT is the proper selection of a cadaveric liver graft to split. Tissue injury may arise not only from the stress of cold ischemia and perfusion but from manipulation during dissection as well as parenchyma transection [4,5,12]. The young hemodynamically stable potential organ donor with acceptable vasopressor support and a short hospital stay (in intensive care unit= ICU) seems to be most suitable for a split procedure. Indeed, poor donor selection has been recognized as a cause of unfavorable outcomes [3,4,13,14]. Marginal donors are not suitable for splitting [1,3]. An emerging donor source for SLT are recipients with familial amyloid polyneuropathy (FAP) whose liver explants may be used in another recipient in what is referred to as “domino liver transplantation”, this procedure has been performed since 1995, domino SLT (splitting an FAP live donor liver for two recipients) has been reported from France and two cases have been performed in Japan to date [3,14].
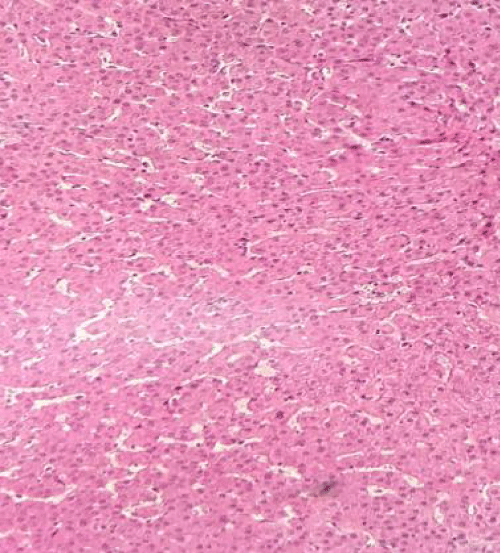
Appropriate donor and recipient selection are critical to the success of SLT; typically, donor selection is restricted to optimal candidates with respect: age is intended to be kept < 50 years for graft splitting because the liver’s regeneration capacity is compromised by aging [1,2,4,9,11]. ABO compatibility, size match, liver enzymes no more than double the normal, minimal inotropic support requirements, absent/scant arrest period, no macroscopic evidence of hepatic steatoses or less than 20% hepatic steatosis if a biopsy was taken and serum sodium concentration. In ours donors, we had a biopsy in the first donor with 10 % of steatosis and the little donor had not steatosis. Most widely used criteria are reported in table 2 (table 1 is our patients) [1,2,9,11].
Donor liver anatomy: specifically variations of the hepatic artery, portal vein and biliary anatomy precluding splitting are the macroscopic appearance of the donor liver. Since, the body weight, degree of illness of the potential recipient, the availability of an experienced surgeon and a number of logistical considerations had been required to an adequate preoperative valuation [9,11]. In the opinion of the group Yale New Haven Center, donor selection criteria and evaluation of the donor liver intraoperatively by an experienced surgeon are the most important ones to initiate the process. After a decision is made that the donor liver is suitable for splitting, logistical considerations, including decreasing the ischemia time and assignment of teams to perform recipient operations [2,4,5].
Intraoperative Evaluation. Before procurement procedure, it is important to ensure that the donor had adequate nutrition because liver glycogen stores are depleted within 8-12 hours of fasting. Therefore, some surgeons routinely used N-acetyl-cysteine 150 mg/kg IV as an oxygen free radical scavenger 1-3 hours before procurement [4,5]. Macroscopic inspection is a requirement to evaluate the liver in abdominal cavity after incising, the examination should include: consistency of the liver, deciding the fat content by finger printing test, and, if necessary, obtain a liver frozen section biopsy to evaluate steatosis. Macrovacuolar steatosis when > 20% impacts adversely on liver transplant outcomes. Transection of the common, most liver grafts may be divided for implantation into 2 recipients; only under exceptional anatomic circumstances should liver splitting be aborted [4,5,15].
Recipient Selection
Split liver recipient selection is even more relevant that good donor selection. With such strategy, many centers have managed to significantly shorten waiting time and decrease waiting list mortality of pediatric candidates without compromising the adult donor poor; now, it is our project in our center Pediatric to diffuse the message in all centers of liver transplantation in Mexico, like it has been projected that splitting all cadaver donor livers in the USA could provide grafts for all pediatric candidates in the entire country [3,10-15]. Specific eventual complications derived from implantation of a segmental graft: cut surface bleeding, small-for-size syndrome vascular, biliary complications and segment IV hypoperfusion. Recipient graft variables include graft fraction, graft mass, graft type, hepatic artery reconstruction ex vivo prior to transplantation, cold and warm ischemia period, biliary drainage and using Roux limb [2,5,11].
The needed for a critical graft parenchymal mass has been discussed previously [1,2,5,16]. In our recipients a preoperative evaluation included a completed history and physical examination, an abdominal computed tomography scan and angiogram. The use of split liver grafts for severely ill patients is under revision and the outcome is not good, like it is in our case number two. The minimal graft/recipient weight ratio of 0.8 suffices to cope with the metabolic needs of the recipient without developing small-for-size syndrome [2,12,16,17]. In this syndrome, it is important to understand the concept of functional graft size: compromised venous drainage of the graft, severity of the portal hypertension of the recipient, technical complications such as bile leak and infectious complications immediately after transplant facilitate the development of small-for-size syndrome even if the graft/recipient weight ratio is >0.8 (2,4,17).
Since, definitive differences were seen in hepatic hemodynamics between survivors and patients with graft failure. Sugimoto et al. reported the hemodynamic features in the patients with graft failure were (1) extremely increased PVPV(portal venous peak velocity; cm/sec) on postoperative day 1; (2) rapidly deteriorated PVPV in the early postoperative period; (3) reciprocally increased HAPSV( hepatic arterial peak systolic velocity; cm/sec); and (4) increased SAPI (splenic arterial pulsatility index) indicating portal hypertension [13,14,17,18]. Status I United Network for Organ Sharing candidates must be evaluated carefully because poorer outcomes have been observed among these recipients. A clear example, it had in our second recipient patient [5,6]. The model for End –Stage Liver Disease (MELD) score reflects not only the probability of death on the waiting list, but also the severity of End-Stage liver disease[ 9,13,16].
Graft mass
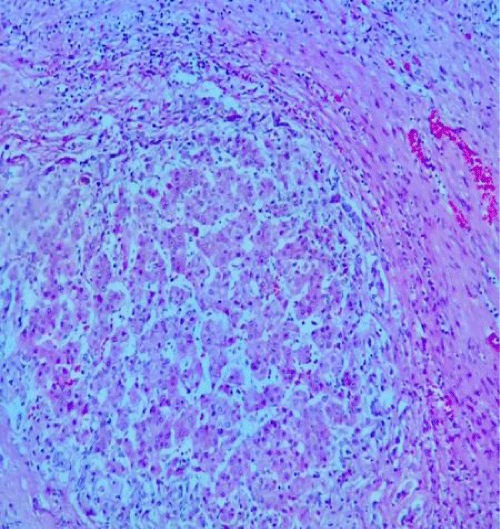
Adequate graft mass has been extensively explored in living donor liver transplantation with minimal graft thresholds advocated. However, these data are not directly applicable to SLT because parenchyma quality and immediate function of living donor grafts exceeds that of cadavers [1,6,11]. The preference is graft mass of at least 1% recipient body weight have been used with success [6, 15-17]. Inadequate graft mass for the recipient manifests as a pattern of dysfunction associated with portal hypertension with prolonged cholestasis and gradual recovery [17,18]. One adult cadaver liver can be divided in two nearly equal sized grafts by the splitting along the middle hepatic vein for transplanting into two larger individuals, left –side 400 cc graft can be created with segments I-6V or leaving the caudate lobe (II, IV), recipient weighing equal o inferior 80 kg, right side 800-10000-cc grafts (segments I, V-VIII or V-VIII) are considered suitable [6,11,18]. Termed small-for-size syndrome that reveals a pattern of diffuse ischemic injury characterized by hepatocyte ballooning, steatosis, centrilobular necrosis and parenchymal cholestasis that may be misinterpreted as preservation injury [1,6,11]. The exact mechanism leading to injury of a small for size graft after transplantation remains unknown. It has been suggested that excessive portal flow secondary to relative portal hypertension may be the cause and that portal decompression may improve graft survival [6,17,18].
Split liver procedure
After the procurement the split liver is lengthened by 90 to 120 minutes. The ideal and most important sharing pattern was originally described by Bismuth et al. in 1989; the principal concept of this sharing pattern is its avoidance of multiples branches that would need to be reconstructed in recipient [2,6]. The classical description of SLT consists of division of the liver along the umbilical fissure (falciform ligament) into the left lateral segments II and III, this graft can be transplanted in pediatric recipient is approximately 250 cc in volume. The right lobe together with the medial and caudate lobes segments (segments I,IV-VIII), can be transplanted to an adult, it is approximately 1100 cc based on the Couinaud classification and accepted across Asian, European, and North American transplant communities[ 2,6,7,11 ]. This classification and the one refined by Bismuth and used as references for partial-organ allografts, in our two patients in this study we performed the same classification. This generates a graft for a small child and an adult or bigger child, respectively or bigger child respectively. And in the split for two adults, this would entail transection of the liver near the main lobar fissure to generate two hemi liver grafts [3,5,7,11].
The increased use of split-liver transplant from deceased donors for pediatric recipients hassled to the selection of some recipients weighing more than 30-kg for whom the left lateral lobe (segments II and III) was usually considered too small [9-11]. The left lobe frequently has single branch of the portal vein, hepatic duct and venous outflow that is a common channel of the left and middle hepatic veins (Figure 5). The right lobe often has a single right hepatic artery, but multiples branches are commonly seen in the venous drainage, hepatic duct and portal vein. The left-sided graft, the right-sided graft retains the remaining main branches, including the common hepatic duct, main portal vein and vena cava [6,11,18]. Split liver transplantation for two adults is technically feasible [13,16,17,19].
Split liver graft implantation
The technique was selected as indicated for each split graft-patient couple with percutaneous Veno-venous bypass installed as needed. The implantation was adapted as necessary using preservation of the native vena cava, arterial or venous grafts, or the prevention of kinking of the venous anastomosis. The left graft is rotated through 180° of sagittal orientation so that the hilar structures are brought into an anterior and medial location coaxial with the native liver pedicle [8,13-15].
Postoperative care
The usual post-transplant care for the center depends of every liver transplantation center. Even though, the recipient could receive in UCI. In our case graft perfusion was checked by Doppler ultrasound examination daily in the intensive care unit (UCI). Patients whose partial thromboplastin time was less than 1.3 times control or whose platelet count was more than 30,000/ were anticoagulated with heparin [8,13,14].
Complication in split liver
Pediatric liver, by the 1980s, had become the standard treatment for infants or children suffering from life-threatening End-Stage liver disease [14]. Biliary complications still remain a significant source of patient morbidity and mortality in liver transplantation. Split liver procedures that divide a cadaver organ into a small left graft for a child and a larger right graft for an adult have reduced the graft shortage for children and could even eliminate the need for elective living donors in this population. Split liver procedures for two adult recipients however, are still uncommon [13,20].
By a meta-analysis of studies concerning pediatric liver transplants to compare patient/graft survival and incidence of surgical complications between whole liver transplantation(WLT) and technical variant liver transplantation(TVLT). There was no significant difference in five-year graft survival rate between the two groups. When it comes to complications, the results suggested a lower incidence of portal vein thrombosis (PVT) and biliary complication (BC) in the WLT group. The incidence of artery thrombosis (HAT) was comparable between the groups [5,16,20,21]. Collectively, these results show that WLT is associated with a better outcome when compared to TVLT. Pediatric and small-sized donor organs are a scare resource, making it difficult to find adequate grafts for small-sized transplant candidates. Some centers have reported that with the successful application of TVLT, the time on the waitlist and pre-transplantation mortality has been reduced dramatically without compromising patient outcomes [13,16,21]. In this work: we had a good outcome with the recipient of child B in the first three months of transplant, the recipient child C had a good donor, in fact by its hemodynamic instability she presented a grade V of the Clavien-Dindo classification due to disseminate intravascular-coagulation (Figure 5).
However, in our center it is an obligation to perform this technique in pediatric transplantation by the large number of patients in the waitlist vs the poor rate of donation less donor. On the other hand the SLT in adult, using the extended right hepatic lobe, does not notably differ from WLT with regard to initial graft function, postoperative complication, or patient and graft survival. Based on this, the liver can be considered a paired organ, and mandatory splitting of good-quality livers can be recommended (J Am Coll Sur 2002; 195:648-65è.c 2002 by the American College of Surgeons). In this study we had not observed difference outcomes between split in cadaveric donor to living liver donor [10,14,16]. The risk of graft failure is now similar between split and whole-liver recipient in the vast majority of cases, demonstrates that the expansion of split-liver allocation might be possible without increasing the overall risk of long-term graft failure in adult recipients. Additional prospective analysis should examine if selection bias might account for the possible increase in risk for recipients with hepatocellular carcinoma of designated status, the surgical technique should be realizing by a group of experience surgeon [8,11,15]. Like we had observed in our first two patients, the selection of recipient and donor is so important like the surgical technique for split liver transplantation, Majella Doyle et al. in a study demonstrated excellent outcomes in adult and pediatric recipients using carefully selected donors; they recommend escalation of the use of split liver transplants to expand the donor pool for cadaveric liver transplantation [19].
Conclusion
Liver Pediatric Transplant, by the 1980s, had become the standard treatment for infants or children suffering from life-threatening End-Stage liver disease. Outcomes and complication rates can be improved by rigid selection criteria for donors and recipients, particularly for the smaller left graft, and possibly also by in insitu splitting in cadaver donors. Like mentioned literature review, there are no differences in complications between split in cadaveric donor to living liver donor. However for a well outcome, it is important to have a good donor like it is for a good recipient, example child B recipients, not like we had in our case 2. The most important benefit of this procedure is obtained from the elimination of the patient from the waiting list, decreasing the chances of pre-transplantation mortality. Since, we observed in both cases in this work that the end result is also highly dependent on the optimal choice of donor and recipients. In our center, the split liver transplantation is uncommon and there is a clear need for better training of surgeons and for improved sharing of information about this needed procedure.
- Yersiz Hasan, Renz John F, Busuttil Ronald W (2004) Split-Liver Transplantation: Past, Present and Future. Transplantation reviews 18: 164-170. Link: https://goo.gl/UeOMQC
- Colledan Michele (2005) Split liver transplantation: technique and results. Transplantation Reviews 19: 221-231. Link: https://goo.gl/MwY01F
- Chen Chao-Long, De Villa Vanessa H (2002) Split Liver Transplantation. Asian J Surg 25: 285-290. Link: https://goo.gl/OoO8qD
- Emre S, Umman V (2011) Split Liver Transplantation: An Overview. Transplantation Proceedings 43: 884-887. Link: https://goo.gl/ZatYbx
- Ferla F, Lauterio A, Di Sandro, SI Mangoni, C Poli, et al. (2014) Split-Liver Full-Left Full-Right/proposal for an operative Protocol. Transplantation proceedings 46: 2279-2282. Link: https://goo.gl/AepLl1
- Valdecasas-Garcia JC (2009) Split and living donor liver transplantation. Digestive and Liver Disease Supplements 3: 93-95. Link: https://goo.gl/6LdHwC
- Abradelo M, Sanabria R, Caso O, Álvaro E, Moreno E, et al. (2012) Split Liver Transplantation: Where? When? How? Transplantation Proceedings 44: 1513-1516. Link: https://goo.gl/xJWm1w
- Cauley Ryan P, Vakili Khashayar, Fullington Nora, et al. (2013) Deceased-Donor Split Liver Transplantation in Adult Recipients: Is the learning Curve Over? J Am Coll Surg 217: 672-684. Link: https://goo.gl/7sYkyY
- Aseni Paola, De Feo Tulia María, De Carlis Luciano, Valente U et al. (2014) A Prospective Policy Development to Increase Split-Liver Transplantation for 2 adult Recipients. Ann. Surg 259: 157-165. Link: https://goo.gl/0y7PkK
- Campos B. Daniel, Botha Jean F (2012) Strategies to optimize donor safety with smaller grafts for adult-to-adult living donor liver transplantation 17: 230-234. Link: https://goo.gl/c7cQCT
- Huanqiu Liu, Ruijun Li, Jinling Fu, Qianyan He, Ji Li (2016) Technical Skills Required in Split Liver Transplantation. Ann Transplant 21: 408-415. Link: https://goo.gl/6My03r
- Hashimoto K, Fujiki M, Quintini C, Aucejo FN, Uso TD (2016) Split liver transplantation in adults. World J Gastroenterol 33: 7500-7506. Link: https://goo.gl/y6yiiZ
- Azoulay Daniel, Castaing Denis, Adam Rene, Savier Eric, Valérie Delvart, et al. (2001) Split –Liver Transplantation for Two Adult Recipients: Feasibility and Long-Term outcomes. Ann. Surg 223: 565-574. Link: https://goo.gl/ONW5Vy
- Ye Hui, Zhao Qiang, Wang Yufang, Wang Dongping, Zhouying Zheng, et al. (2015) Outcomes of Technical Variant Liver Transplantation versus Whole Liver Transplantation for Pediatric Patients: A Meta-Analysis. PloS One Journal 2-13. Link: https://goo.gl/5w8RfS
- Fondevila Constantino, Jiménez-Galanes Santos, Valdecasas Juan Carlos García (2009) Como incrementar el número de trasplantes hepáticos. Gastroenterol Hepatol 32: 519-530. Link: https://goo.gl/LY0chr
- Broering DC, Topp S, Schaefer U, Fischer L, Gundlach M, et al. (2002) Split Liver Transplantation and Risk to the Adult Recipient: Analysis Using Matched Pairs. J Am Coll Surg 195: 648-657. Link: https://goo.gl/4myWP1
- Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, et al. (2005) Effect of intraportal infusión to improve small for size graft injury in living donor adult liver transplantation. Transplant International 18: 923-928. Link: https://goo.gl/UFm5bq
- Sugimoto H, Kaneko T, Hirota M, Nagasaka T, Kobayashi T, et al. (2004) Critical Progressive Small-Graft Injury Caused by Intrasinusoidal Pressure Elevation Following Living Donor Liver Transplantation. Transpl Proc 36: 2750-2756. Link: https://goo.gl/opbOLr
- Doyle Majella, Maynard Erin, Lin Yiing, Vachharajani Neeta, Shenoy Surendra, et al. (2013) Outcomes with Split Liver Transplantation Are Equivalent to those with Organ Transplantation. J Am Coll Surg 217: 102-112. Link: https://goo.gl/BtJ3fA
- Vasavada Bhavin, Chen Chao Long, Zakaria Muhammad (2014) Using low graft/Recipient´s Body Ratio Graft With Portal Flow Modulation an Effective Way to Preve nt Small-for-Size Syndrome in Living-Donor Liver Transplant: A retrospective Analysis. Exp Clin Transplant 5: 437-442. Link: https://goo.gl/wzPmJb
- Castaldo Eric T, Austin Mary T, Pinson Wright C, Pinson, Chari Ravi S (2007) Management of the bile duct anastomosis and its complications after Liver Transplantation. Transplantation Reviews 21: 26-33. Link: https://goo.gl/SViG3X
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
