Archives of Otolaryngology and Rhinology
Paraneoplastic Neurological Syndrome with Demyelinating Polyradiculoneuropathy in Tonsil Cancer patient
Tena Simunjak1*, Boris Filipovic2, Irena Pirkl2 and Boris Simunjak2
2University Hospital "Sveti Duh", Department of Otorhinolaryngology, Head and Neck surgery, Zagreb, Croatia
Cite this as
Simunjak T, Filipovic B, Pirkl I, Simunjak B (2019) Paraneoplastic Neurological Syndrome with Demyelinating Polyradiculoneuropathy in Tonsil Cancer patient. Arch Otolaryngol Rhinol 5(3): 065-068. DOI: 10.17352/2455-1759.000100Objective: To describe a rare case of a paraneoplastic neurological syndrome associated with tonsillar squamous cell carcinoma.
Design: Case report.
Patient: A 51-year-old female patient with paraneoplastic neurological disorder characterized by rapid and progressive bilateral facial paresis, diplopia, arm and leg weakness and paresthesia.
Interventions: Symptomatic therapy for pain, surgery and radiotherapy for cancer.
Main outcome measures: Medical file, radiological imaging, EMNG, immunological findings and surgical report.
Results: Patient neurological status drastically improved in early postoperative period (after primary surgical intervention). Only mild right facial paresis persisted upon release from hospital. Patient is scheduled for radiotherapy.
Conclusion: This is to our knowledge first case of paraneoplastic neurogical syndrome with demyelinating polyradiculoneuropathy associated with tonsillar carcinoma. In a case of an early staged carcinoma, symptomatic therapy and surgery alleviated or slowed progression of symptoms.
Introduction
A paraneoplastic syndrome (PNS) consists of a group of symptoms presenting in a cancer patients. It is believed that they are mediated by tumor metabolites, hormones or are a result of body immune response mediated threw onconeural antibodies to the tumor and not by its local or metastatic effects.Syndrome is present in 1 to 7.4% all cancer patients and it can precede, follow or coexist with tumor [1,2].
Mostly the mechanisms of PNS are not well known, but ectopic hormone production is probably the most common mechanism [3]. Different studies have tried to identify specific antibodies associated with neurological disorders as a part of PNS. The first well identified antibodies were against voltage gated calcium cannels (anti MuSK, anti N-AChR) in LEMS. However nervous system (peripheral and central) can be affected by onconeural nuclear antibody in PNS such as: anti Hu, Yo1, Ri, anti-neuronal nuclear antibodies type 1, Purkinje-cell antibodies, anti-neuronal nuclear antibodies type II, anti-Ma, anti-Tr, anti-amphiphysin, collapsin response mediator protein-5, N-methyl D-aspartate antibodies, voltage gated potassium channel antibodies, double-stranded DNA antibodies, extractable nuclear antigen antibodies and anti-neutrophil cytoplasmic antibodies. Up to now this antibodies have been associated with different neurological symptoms (subacute sensory neuropathy, encephalopathy, opsoclonus-myoclonus syndrome.), or different type and localization of carcinoma.
In a case of primary head and neck cancers symptoms of PNS may be endocrine, dermatological, neurological, hematological, rheumatologic or ocular nature [4]. The most common hystopathological type causing PNS is squamous cell carcinoma but also other types such as lymphoma, thyroid carcinoma, melanoma etc. have been reported [1].
Although PNS is recognized as a clinical entity, it has been rarely described in addition to primary head and neck tumors, and barely few times with primary tonsil cancer.
To the best of our knowledge this is first case of paraneoplastic neurogical syndrome with demyelinating polyradiculoneuropathy associated with squamous cell carcinoma of the tonsil.
Case Presentation
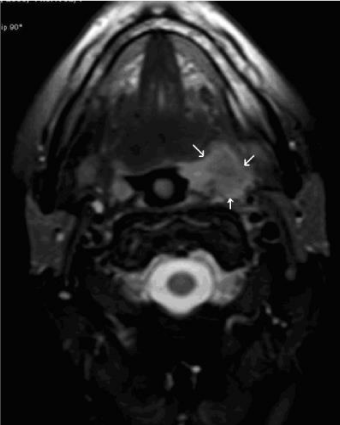
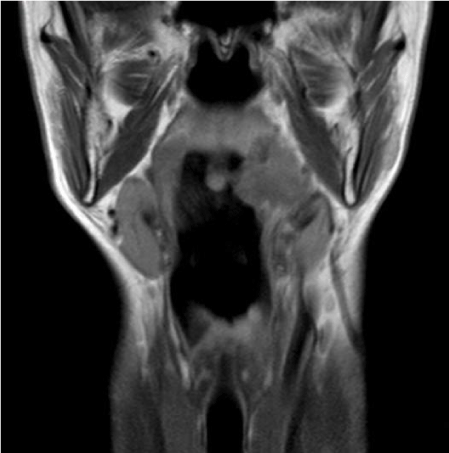
In this case, we present a 51-year old female patient with a paraneoplastic neurological syndrome associated with left tonsillar carcinoma. The patient condition started with diffuse and very intensive not localized pain, paresthesia in arms and legs and general malaise. She had lost 15 kg in body weight in one year time. She was diagnosed by her physician with angina and treated with antibiotics for 3 days. In that period she developed right peripheral facial paresis. Upon admission to hospital and neurological examination she experienced arm and leg muscle weakness which was enabling her to walk by herself and intensity of her pain worsened (visual analog scale 9), myotatic reflexes were absent on both legs. Right peripheral facial paresis was House-Brackmann 4. During the next several days she developed left peripheral facial paresis and diplopia. The neurologist performed several tests. Results of routine laboratory test were all within normal limits. The EMNG showed loss of action potential in face muscles; also inability to produce sensory potential in n. medianus, radialis and suralis. Upon ophthalmological examination (Lancaster test) right paresis of n. VI was diagnosed. She was referred to our department of ENT because of the unclear medical status and possible tonsillitis. ENT examination revealed an exophytic process of left tonsil which is expanding in front to the palatoglossal arch up to the superior pole of the tonsil (mucous layer to the uvula was free of the process), descend to the tonsillolingual sulcus as shown in Figure 1 and Figure 2. Hypopharynx and endolaryngeal examination revealed no lesions. There was no lymph node enlargement in the neck at that time. She had no prior medical illness.
Classical commando operation was performed, including soft-tissue resection en bloc with segmental mandibulectomy and modified radical neck dissection of the left side. The simple and effective closure was used for the reconstruction of the pharyngeal defect. Histopathological diagnosis was squamous cell carcinoma grade 3. ICU recovery was prolonged due to neurological symptoms. The tracheotomy cannula was removed on day six with complete recovery of her voice, swallowing and appearance. Thirty days after surgery the patient was discharged from hospital and postoperative radiotherapy was initiated. Patient neurological status drastically improved in the early postoperative period (after primary surgical intervention). Only mild right facial paresis persisted upon release from the hospital.
Discussion
Paraneoplastic neuropathies and cases of PNS appear more often with certain malignant conditions described in the literature (mostly small-cell-lung-cancer (SCLC), breast-cancer, ovarian-cancer, Hodgkin’s or non-Hodgkin’s lymphoma) but they have been rarely described in cases of primary head and neck tumors. Those syndromes have significant clinical relevance. Firstly because they can antedate the initial diagnosis or relapse of systemic cancer. Secondly, if not diagnosed properly and on time they may inflict certain disability that may not be reversible when treatment is started. And third, they have the wide specter of differential diagnosis [5]. Therefore it is inevitable that timely diagnosis must be provided.
L.W.J. Baijens and J.J. Manni [1], described 4 different histological types of head and neck cancer (nasopharyngeal carcinoma, B-cell lymphoma of the nasopharynx, hypopharyngeal carcinoma, supraglottic carcinoma) accompanied by different types of paraneoplastic syndromes(erythrodermia, Schwartz-Bartter syndrome, paraneoplastic polyarthritis and anti-Hu positive encephalomyelitis)
Two most recognized neurological PNS in primary head and neck cancers are cerebellar degeneration and Eaton-Lambert myasthenic syndrome. Eaton Lambert myasthenic syndrome (LEMS) is a neurological disorder characterized by muscle weakness of the limb caused by the antibodies against voltage-gated calcium channels. Several authors reported Lambert Eaton syndrome with squamous cell carcinoma of the larynx and complete regression of syndrome following surgical removal of the tumor [4,6,7].
Up to now to our knowledge PNS tie-up with tonsillar carcinoma was reported in literature in six different cases, but neither one was associated with demyelinating polyradiculoneuropathy. According to the literature there is:
1. One case of lymphoepithelial carcinoma of the tonsil and the tongue base associated with paraneoplastic cerebellar degeneration(PCD) [8], PCD is a condition characterized by subacute symptoms of vertigo, nystagmus, ocular dysmetria, and opsoclonus. Symptoms are usually the first sign of malignancy and in about half of patients onconeural antibodies are found [9]. Athough, in this case, the patient, like ours, had no positive onconeural antibodies. But the clinical presentation of PCD was typical and the patient condition improved, like in our case, after treating the tumor.
2. Three cases of dermatomyositis paraneoplastic syndrome before symptomatic tonsillar squamous cell carcinoma
a.) Botsios et al., [10], reported 1 case of a patient developing dermatomyositis 8 months before the diagnosis of tonsillar squamous cell carcinoma (SCC) Like in our case, onconeural antibodies were absent
b.) A report from Korea described 2 separate cases of paraneoplastic dermatomyositis presenting 1 year and 2 years before diagnosis of SCC [11].
c.) A case from Maryland [12], reported a 63-year-old man with dermatomyositis as a paraneoplastic syndrome developing more than a year before clinical manifestations of SCC.
3. Janus et al., [13] reported the case of a tonsillar SCC associated with paraneoplastic thoracic spine tractopathy who initially presented with weakness and spastic gait.
4. The case of paraneoplastic sensory neuropathy due to pharynx and tonsillar SCC, also with no detected onconeural antibodies [14].
5. Wright et al., [15], reported an uncommon paraneoplastic syndrome of digital necrosis associated with a locally advanced squamous cell carcinoma of the tonsil.
6. Also anti-Mal 1 and 2 onconeural antibodies against intracellular protein present in testes but also trough brain regions, were reported in a patient with tonsillar carcinoma and PNS encephalitis characterized by narcolepsy, sleep behavior disorder and gaze palsy [16].
Our case-patient is to our knowledge first case of the paraneoplastic neurological syndrome with demyelinating polyradiculoneuropathy associated with tonsillar carcinoma. According to the reviewed literature paraneoplastic demyelinating neuropathy has been, until now, reported with adenocarcinoma of the pancreas, colon and liver, breast cancer, melanoma, renal cell carcinoma, hematological malignancies mostly NHL and HL [5,17].
In our case, patient first developed diffuse and very intensive not localized pain, paresthesia in arms and legs and general malaise, bilateral neck pain, followed by right peripheral facial paresis (HBS4), muscle arm and leg weakness. During the next several days left peripheral facial paresis and diplopia appeared (right paresis of n. VI) and, patients pain intensity worsened (it was scored 9 on visual analog scale). All that indicates sings of acute demyelinating polyradiculoneuropathy
In some patients with this kind of PNS onconeural anti-Ma2, anti-CV2/CRMP-5 anti-Hu or, anti-alpha-enolase antibodies are postitiv [5,18-20].
Although the presence of onconeural antibodies has often a high diagnostic specificity, an important fact is that these antibodies are not present in many types of paraneoplastic neuropathies and the correct diagnosis remains on a high degree of clinical suspicion [5,21].
Despite missing onconeural antibodies in our patient (Hu, Yo, Ri- negative; anti N-AChR, anti MuSK-negative), the substantially slowed disease progression after tumor resection and responsiveness to adjuvant radiotherapy largely exclude other differential diagnoses.
Thereupon, an international panel of neurologists interested in PNS proposed to define a neurological syndrome as paraneoplastic: "definite" and "possible“ according to the presence or absence of certain criteria [22,23].
According to those criteria our patient encompasses the flowing two (which is enough to meet the diagnosis):
1. A non-classical syndrome that resolves or significantly improves after cancer treatment without concomitant immunotherapy, provided that the syndrome is not susceptible to spontaneous remission.
2. A non-classical neurological syndrome, no onconeural antibodies, and cancer present within two years of diagnosis
Conclusion
From this case report we highlight that:
1. Acute demyelinating polyradiculoneuropathy can be associated with tonsillar carcinoma, so both neurologists and otorhinolaryngologists must be aware of such a presentation.
2. Early surgical intervention with symptomatic therapy may possibly alleviate or slow progression of neurological symptoms.
- Baijens LWJ, Manni JJ (2006) Paraneoplastic syndromes in patients with primary malignancies of the head and neck. Four cases and a review of the literature. Eur Arch Oto-Rhino-Laryngology. 263: 32-36. Link: https://bit.ly/32uXZBt
- Gulya AJ (1993) Neurologic Paraneoplastic Syndromes With Neurotologic Manifestations. Laryngoscope 103: 754. Link: https://bit.ly/2NZd6Qc
- Minotti AM, Kountakis SE, Stiernberg CM (1994) Paraneoplastic syndromes in patients with head and neck cancer. Am J Otolaryngol 15: 336-343. Link: https://bit.ly/32y0cfc
- Ferlito A, Rinaldo A, Bishop JA, Hunt JL, Vander Poorten V, et al. (2016) Paraneoplastic syndromes in patients with laryngeal neuroendocrine carcinomas: clinical manifestations and prognostic significance. Eur Arch Oto-Rhino-Laryngology 273: 533–536. Link: https://bit.ly/2O8KVhM
- Antoine JC, Camdessanché JP (2017) Paraneoplastic neuropathies. Curr Opin Neurol 30: 513–520. Link: https://bit.ly/2XNpW8E
- Shipley E, Krim E, Deminière C, Lagueny A (2008) Syndrome myasthénique de Lambert-Eaton associé à un carcinome épidermoïde de la corde vocale. Rev Neurol (Paris) 164: 72-76. Link: https://bit.ly/2JNdc8a
- Medina JE, Moran M, Goepfert H (1984) Oat cell carcinoma of the larynx and Eaton-Lambert syndrome. Arch Otolaryngol 110: 123-126. Link: https://bit.ly/2Lo3f4D
- Henke C, Rieger J, Hartmann S, Middendorp M, Steinmetz H, et al. (2013) Paraneoplastic cerebellar degeneration associated with lymphoepithelial carcinoma of the tonsil. BMC Neurol 13: 147. Link: https://bit.ly/2Lpg2ni
- Shams’ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, et al. (2003) Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain 126: 1409-1418. Link: https://bit.ly/2XNnAqC
- Botsios C, Ostuni P, Boscolo-Rizzo P, Da Mosto MC, Punzi L, et al. (2003) Dermatomyositis and malignancy of the pharynx in Caucasian patients: Report of two observations. Rheumatol Int 23: 309-311. Link: https://bit.ly/32nLgQS
- Kim SW, Shim JS, Eun YG, Kwon KH (2010) Tonsillar Squamous Cell Carcinoma Associated with Dermatomyositis: The First 2 Cases in Korea. Yonsei Med J 51: 605. Link: https://bit.ly/2YWSpW6
- Adi AH, Alturkmani H, Spock T, Williams Yohannes P, Wargo S, et al. (2015) Dermatomyositis paraneoplastic syndrome before symptomatic tonsillar squamous cell carcinoma: A case report. Andersen P, editor. Head Neck 37: E1–3. Link: https://bit.ly/2XRjr01
- Janus JR, Chinnadurai S, Moore EJ (2013) Case report: Paraneoplastic neurologic syndrome associated with squamous cell carcinoma of the tonsil. Ear Nose Throat J 92: E13. Link: https://bit.ly/2JKVv9A
- Pericot I, Río J, Jaén A, Crespo F, Codina A (2001) Paraneoplastic sensory neuropathy due to pharynx and tonsil squamous cell carcinoma. Neurologi 16: 237-239. Link: https://bit.ly/2JA2MtU
- Wright JR, Gudelis S (2002) Digital necrosis associated with squamous cell carcinoma of the tonsil. Head Neck 24: 1019-1021. Link: https://bit.ly/2SlH5Re
- Adams C, McKeon A, Silber M, Kumar R (2011) PSP-like syndrome with REM sleep behavior disorder and narcolepsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Mov Disord 26: S328.
- Koike H, Sobue G (2013) Paraneoplastic neuropathy. Handb Clin Neurol 115: 713-726. Link: https://bit.ly/2LmRdIw
- Samarasekera S, Rajabally YA (2011) Demyelinating neuropathy with anti-CRMP5 antibodies predating diagnosis of breast carcinoma: Favorable outcome after cancer therapy. Muscle Nerve 43: 764–766. Link: https://bit.ly/2NWw5ed
- Ju W, Qi B, Wang X, Yang Y (2017) Anti-Ma2–associated limbic encephalitis with coexisting chronic inflammatory demyelinating polyneuropathy in a patient with non-Hodgkin lymphoma. Medicine (Baltimore) 96: e8228. Link: https://bit.ly/2Y4PGg9
- Tojo K, Tokuda T, Yazaki M, Oide T, Nakamura A, et al. (2004) Paraneoplastic Sensorimotor Neuropathy and Encephalopathy Associated with Anti-α-Enolase Antibody in a Case of Gastric Adenocarcinoma. Eur Neurol 51: 231-233. Link: https://bit.ly/2LVn6HD
- Raspotnig M, Vedeler CA, Storstein A (2011) Onconeural antibodies in patients with neurological symptoms: detection and clinical significance. Acta Neurol Scand 124: 83-88. Link: https://bit.ly/2XTllNB
- Graus F (2004) Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 75: 1135-1140. Link: https://bit.ly/2LqvAHy
- Antoine JC, Camdessanché JP (2007) Peripheral nervous system involvement in patients with cancer. Lancet Neurol 6: 75–86. Link: https://bit.ly/2xPnqQb
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
