Archives of Otolaryngology and Rhinology
Risk factors associated with the diagnosis of Sensorineural Hearing Loss in children
Ileana del Socorro Gutiérrez-Farfán1, Celia Reyes-Legorreta2, Efrén Alatorre-Miguel2, Antonio Verduzco-Mendoza3 and Alfredo Durand-Rivera2*
2Servicio de Neurociencias, Laboratorio de Neuroprotección del Instituto Nacional de Rehabilitación, Mexico City, México
3Servicio de Neurociencias, Laboratorio de Bioacústica del Instituto Nacional de Rehabilitación, Mexico City, México
Cite this as
Gutiérrez-Farfán IDS, Reyes-Legorreta C, Alatorre-Miguel E, Verduzco-Mendoza A, Durand-Rivera A (2018) Risk factors associated with the diagnosis of Sensorineural Hearing Loss in children. Arch Otolaryngol Rhinol 4(4): 092-096. DOI: 10.17352/2455-1759.000084Purpose: The hearing loss is the most frequent sensory alteration of the human being, with numerous medical, social, emotional and cultural implications. It is a multicausal pathology and is related to many risk factors. Identify the risk factors for the appearance of hearing loss in the child.
Method: A sample of 81 children under 3 years of age with severe to profound sensorineural hearing loss was taken, in which the variables studied were the risk factors for hearing loss defined by the Joint Committee on Infant Hearing.
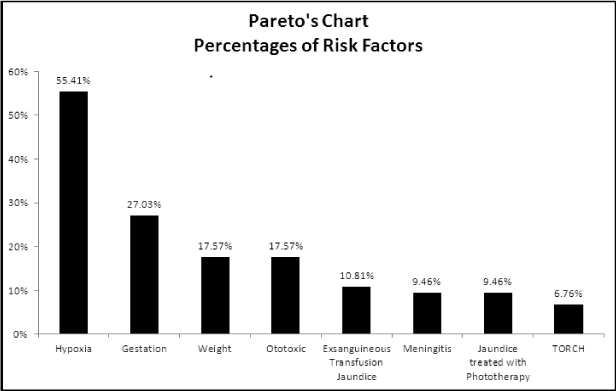
Results: Values of the risk factors were found, with the following percentages: hypoxia (55.41%), gestational age less than 30 weeks (27%), weight less than 1500 grams (17.57%), ototoxic drugs (17.57%), jaundice with exsanguineous-transfusion (10.81%), meningitis (9.46%), jaundice treated with phototherapy (9.46%) and TORCH (6.76%). It was found that patients with hearing loss can present one to four risk factors, while in relation to sex again; the hypoxia risk factor has the highest percentage (male 31.59% and female 37.5%).
Conclusions: It is clear that the patients analyzed have one or several risk factors related to hearing loss. In our case, hypoxia was the factor that most occurred. Therefore, without the realization of a hearing screening for early detection of deafness, the average age of diagnosis is around three years, when parents or educators begin to detect the first manifestations.
Introduction
Hearing loss is a major health problem in the world. Any disturbance in the child's auditory perception at an early age will affect their linguistic and communicative development, their cognitive processes and their social and scholar integration [1]. The first year of life "is a critical period" since language development depends on auditory stimulation before 18 months of age; and then the lack or deficit of hearing can cause important deficiencies, since in the absence of a sensory signal, the morphology and functional properties of the neurons can be altered [2].
The national center of technological excellence in health of Mexico published in April 2009 that hearing loss is the most common congenital abnormality in the newborn and occurs in 3 out of every 1,000 live births, and 20% of these cases have profound hearing loss [3]. Without specific early detection programs, the average age of diagnosis is around three years [4]. Therefore, it is important to implement neonatal hearing screening programs, with which it is intended that hearing impaired children are identified before the third month of life and the diagnosis and treatment are made before the first year of age [5].
Currently, the two tests used and internationally accepted to assess hearing in newborns are transient otoacoustic emissions (TOEs) and brainstem auditory evoked potentials (BAEPs). Both tests have a high specificity and sensitivity in the early detection of hearing loss and are complementary, since the use of both prevents false negatives [6].
Numerous risk factors have been described in the onset of deafness. In 2007, JCIH proposed a single list, given that the indicators associated with congenital/neonatal hearing loss and those associated with progressive late-onset hearing loss overlap and a significant change was introduced in the risk factor for mechanical ventilation time, where is now established an income of more than 5 days in the ICU [7]. There is evidence that only 50% of confirmed cases of hearing loss present a risk factor, which has led to consider that the other 50% do not manifest any identified condition, making early detection difficult [8,9]. On the other hand, it is reported that with reference to the etiology of sensorineural hearing loss, this is unknown in approximately 30% of cases [10] for this reason is necessary to perform Universal Neonatal Auditory Screening (UNAS) [11,12].
For the above reasons, in the current study it is proposed to identify independent risk factors for the onset of hearing loss in infants.
Method
An observational analytical study of children with diagnosis of sensorineural hearing loss diagnosed by means of TOEs and BAEPs was carried out.
The work was carried out according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). After the protocol was approved by the INR (Instituto Nacional de Rehabilitación) Ethics and Research Committee and with the signing of the informed consent by the parents or guardians of the children, the sample included 81 Mexican children, in a period of 7 years within the same Institution (INR), all the children was less than 3 years of age with severe to profound sensorineural hearing loss. 32 were female and 49 were male. Of the 81, the risk factors present in each individual for hearing loss were collected from the clinical history.
We excluded children with a family history of hearing loss, suggestive history of genetic hearing loss, cranio-facial malformations and suggestive signs of genetic syndrome.
For the analysis of the data obtained, a Pareto Graph was constructed (Figure 1), where the hearing loss was related to risk factors that are represented in this graph in descending order (from highest to lowest) to highlight the relationship between each category.
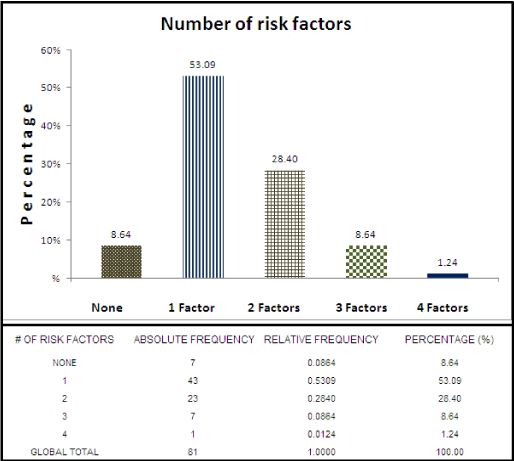
A table was elaborated with the data of the 81 hearing-impaired patients, where 0 to 4 risk factors are present, expressed in absolute, relative frequency and percentage (Table 1).
A graph was made with the number of risk factors per hypoacoustic patient taking the total percentages for each category (Figure 2) and another where risk factors were related in patients with hearing loss with respect to gender, to examine real percentages for both sexes (Figure 3).
Results
There were 43 hearing-impaired patients with a single risk factor, where it was again observed that hypoxia is the most important risk factor with 25 patients (30.86% of the group), while jaundice treated with phototherapy only had one patient (equivalent to a percentage of 1.24%).
Regarding hearing-impaired patients with two risk factors, the total was 23. The combination of hypoxia and gestational age was presented in 6 patients, equivalent to 7.41%. These two are the most important risk factors, as shown in figure 1, and are combined independently with the other factors studied (jaundice treated with phototherapy, ototoxic and weight), each of them was presented with gestational age, and in the case of hypoxia, it was also present with jaundice factors treated with phototherapy, meningitis, ototoxic and TORCH. Finally, there were 4 patients with ototoxic and meningitis, equivalent to a percentage of 4.94%.
On the other hand, there were 7 patients with three risk factors, with the participation of hypoxia and gestational age as the main factors, as well as the presence of jaundice treated with exsanguineous-transfusion, ototoxic drugs, weight, jaundice treated with phototherapy and TORCH.
There was only one patient with four risk factors, and these were: gestational age, hypoxia, jaundice treated with exsanguineous-transfusion and weight, representing 1.24% of the sample.
The table shows that in the sample of 81 patients there were 7 with hearing loss, which did not present any risk factor, representing 8.64% of the sample.
Within the number of risk factors, the highest percentage corresponds to patients with a single factor (53.09%), and subsequently with 2 factors (28.4%), 3 factors (8.64%) and 4 factors (1.24%).
Patients who did not present any risk factor represented a percentage of 8.64%.
The bar graph shows the percentages of risk factors for both the female sex (32 patients) and the male sex (49 patients), and again we can see how the hypoxia risk factor in both sexes is the one that has the greater percentage, being for the masculine ones of 31.59% and for the feminine ones of 37.5%, followed by the gestational age, where it is observed that the percentages were very similar, 16.4% masculine and 16.7% feminine.
Regarding the other parameters studied, it can be seen that in male patients the risk factors with the highest percentage in relation to the female sex were: weight (male 12.3% and female 8.3%), meningitis (male 6.8% and female 4.2%) and exsanguineous transfusion jaundice (male 9.6% and female 2.1%). Those of the female sex had higher percentages in the following risk factors: TORCH (female 4.2% and male 4.1%), ototoxic (female 12.5% and male 9.6%) and jaundice with phototherapy (female 10.4% and male 2.7%).
In relation to patients who did not present any risk factor, the male sex had a percentage of 6.8% while in the female sex it was 4.2%.
Discussion
The fetal distress determined by hypoxia constitutes for Iglesias Solis (2011), the first prenatal risk factor [13,14 ]concordant with our results (Table 1). For Martínez Cruz [13], hypoxia at birth is the second factor, although he considers that hearing damage of perinatal origin is of a multicausal etiology, being able to act synergistically more than one factor, such as preterm delivery and low birth weight [13], which also agrees with our results, since we found that hypoxia occurs in relation to other risk factors (Table 1 and Figure 2).
It cannot be determined with certainty whether there is a cause-effect relationship between hypoxia and hearing loss, since some of the patients with a history of hypoxia also had some other proven risk factor associated with it. However, for patients who had only one factor, the highest percentage was for hypoxia (30.86%) of a total with a single factor of 53.09% and if we added hypoxia patients who also had additional risk factors (Table 1) we would obtain 41 patients who presented hypoxia, which would be 50.61% of the total of patients; These data suggest that hypoxia is a very important factor in the development of hearing loss.
Diverse are the investigations that coincide with the results of this study, with respect to the fact that the lower the age of the studied newborns, the risk of hearing loss is proportionately greater, as show the Mexican authors Martínez Cruz et al. (1995). In our results, gestational age was the second risk factor in hearing-impaired patients, representing 24.69%, when taking into account those presenting one or more factors (Figure 1).
Children with extremely low weight manifest secondary presence of cerebral palsy, neuro-developmental disorders, hearing loss and death. In our study, patients weighing less than 1500 g occupied the third place of risk factor (Figure 1).
Risk factors can exert a synergistic effect, especially in patients with lower weight and lower gestational age at birth; who are generally associated, according to our results (Table 1). In these patients, complications are extremely frequent because they remain in the hospital for very long periods [15].
In the group of patients with birth weight less than 1500 g, the etiopathogenesis of hearing loss would be related to a greater disposition to infections, hypoxia phenomena and other pathologies favored by premature birth and low weight. For this reason, these patients receive potentially ototoxic drugs more frequently, thus adding another risk factor to the problem [16] (Table 1, Figure 2).
Several studies mention that ototoxicity during pregnancy is the second most frequent entity present in their research [13,17,] while for Ramos Cruz [18], it is third. In our case, our results exhibit ototoxicity as the fourth risk factor [18] (Figure 1).
Hyperbilirubinemia causes jaundice when the concentration of bilirubin in serum reaches 2-3 mg/dL. The pathophysiology of hearing loss caused by hyperbilirubinemia is not well defined, although its toxicity can affect the cochlea, the auditory nerve and the brainstem [19]. In the present study it was found that the jaundice that requires exsanguineous-transfusion occupied the fifth place among the risk factors present, while the jaundice treated with phototherapy was presented in the seventh place (Figure 1).
There is agreement in our results and those stated by Corujo Santana [20] and Núnez-Batalla et al. [21], in the sense that this risk factor increases if other factors are associated [20-23] (Table 1).
Sensorineural hearing loss is the most common permanent sequel in bacterial meningitis: in different prospective and retrospective studies, the percentage is from 5 to 30% [24-27]; in our results it can be seen that of the factors studied, this occupied the sixth place with a percentage of 9.46% (Figure 1).
The infections grouped in the TORCH term are toxoplasmosis, rubella, cytomegalovirus, and herpes, in addition, syphilis, which cause prenatal acquired sensorineural hearing loss by transplacental transmission from the mother to the fetus, leading to deafness at birth or deferred or progressive development deafness [6].
When analyzing the behavior of this risk factor, it can be observed that it occupied the eighth and last place in relation to the eight factors studied (Figure 1), being also present in association with other risk factors (Table 1).
Regarding gender, our results show a high risk for the female sex, presenting a greater percentage in risk factors of gestational age, hypoxia, TORCH, ototoxic and jaundice treated with phototherapy, than for males, where the highest percentage was by weight, meningitis and exsanguineous-transfusion jaundice (Figure 3). Peruyera et al. [28] and Martínez Cruz [13], highlight the greater risk of the female sex [13,28], Claro Almeida et al. [17] and Ramos Cruz [18], also coincide with a greater incidence of risk factors for hearing loss in females [18].
Authors such as Peñazola et al. [29] reported 62% prevalence in males [29], as well as Ferreira et al. [9] with a similar prevalence [9].
According to the results of this study of the 81 patients studied, 74 of them (91.4%) have at least one risk factor for neonatal hearing loss. The incidence of this disease in patients with risk factors is ten to twenty times higher than in the general population. Of all the patients with sensorineural hearing loss seven of them (8.6%) did not present any risk factor. However, patients without a risk factor have the same probability of suffering hearing loss at birth [30].
The association of the greatest number of risk factors with the presence of hearing loss suggests that the aforementioned risk factors may exert a synergistic effect, especially in children with lower birth weight and lower gestational age. In these children the complications are extremely frequent, because they remain in the hospital for very long periods.
Conclusions
The results of the study show that approximately half of the patients analyzed had several associated risk factors, making it difficult to accurately determine the etiology of the hearing loss. However, we can observe that the most predominant risk factors to cause hearing loss are hypoxia, followed by gestational age and low birth weight. What is demonstrated is the existence of a relationship between morbidity in patients (given by the number of risk factors) and the appearance of hearing loss.
- Campaña de detección precoz de la sordera (1990) Madrid, FIAPAS. Link: https://goo.gl/M1Wh6R
- Martínez CG, Valdez GM (2003) Detección oportuna de la hipoacusia en el niño. Acta Pediatrica Mexicana 24: 176-180. Link: https://goo.gl/zXFSup
- Centro Nacional de Excelencia Tecnológica en Salud (CENETEC-SALUD). (2009). Implantes Cocleares. Gaceta, Segunda Época, Año1, No 1, abril de. Link: https://goo.gl/VMupP9
- Martin JAM, Bentzen O, Colley JRT, Hennebert D, Holm C, et al. (1981) Childhood deafness in the European Community. Scandinavian Audiology 10: 165-174. Link: https://goo.gl/3EkYXf
- Comisión para la Detección Precoz de la Hipoacusia Infantil (CODEPEH) (1999) Programa para la detección precoz, el tratamiento y la prevención de la hipoacusia infantil. Anales Españoles de Pediatría 51: 336-344. Link: https://goo.gl/BbWzns
- Núñez-Batalla F, Trinidad-Ramos G, Sequí-Canet JM, Alzina V, Jáudenes-Casaubón C (2012) Risk factors for sensorineural hearing loss in children. Acta Otorrinolaringológica Española 63: 382-390. Link: https://goo.gl/w526Lv
- Trinidad-Ramos G, de Aguilar VA, Jáudenes-Casaubón C, Núñez-Batalla F, Sequí-Canet JM (2010) Recomendaciones de la Comisión para la Detección Precoz de la Hipoacusia (CODEPEH) para 2010. Acta Otorrinolaringológica Española 61: 69-77. Link: https://goo.gl/vkaRQm
- Cardemil F (2012) Aspectos éticos en el tamizaje de hipoacusia neonatal en Chile. Revista de Otorrinolaringología y Cirugía de Cabeza y Cuello, 72: 249-260. Link: https://goo.gl/Nf5M9f
- Ferreira R, Basile L, Munyo A, Añazo G (2003) Emisiones otoacústicas en recién nacidos con factores de riesgo auditivo. Archivos de Pediatría del Uruguay 74: 197-202. Link: https://goo.gl/PPNrdT
- Peñaranda A, Mendieta JC, Perdomo JA, Aparicio ML, Marín LM, et al. (2012) Beneficios económicos del implante coclear para la hipoacusia sensorineural profunda. Revista Panamericana de Salud Pública 31: 325-331. Link: https://goo.gl/R665qR
- González L, Fernández JM, Torres MI (2012) Current status of the programs for detection of hearing loss in children younger than six months in Cali. Colombia Médica 43: 73-81. Link: https://goo.gl/xQw5wq
- Cañete O and Torrente M (2011) Evaluación del programa de detección precoz de hipoacusia en recién nacidos prematuros (RNPE), experiencia Hospital Padre Hurtado. Revista de Otorrinolaringología y Cirugía de Cabeza y Cuello 71: 117-122. Link: https://goo.gl/NgS4f5
- Martínez-Cruz CF, Poblano A, Fernández-Carrocera LA, Garza-Morales S (1995) Factores de riesgo para la hipoacusia y hallazgos audiométricos en una población preescolar egresada de cuidados intensivos neonatales. Salud Pública de México 37: 205-210. Link: https://goo.gl/pSqFF6
- Iglesias Solís M, Estevez Regró AS, Quesada Rodríguez GL, Santana Alvarez J (2011) Frecuencia de los factores de riesgo de la hipoacusia neurosensorial infantil severa y profunda en la provincia de Camagüey. VII Congreso de la Sociedad Cubana de Otorrinolaringología y Cirugía de Cabeza y Cuello. Reunión Especial Societas ORL Latinas. La Habana, Cuba
- Acero Hoyos L and Alprecht Quiroz P (2008) Prevalencia de factores de riesgo para hipoacusia neonatal en la maternidad Enrique C. Sotomayor de Guayaquil, en el periodo de enero a junio del. Link: https://goo.gl/Z1T4Eq
- Robertson CM, Tyebkhan JM, Peliowski A, Etches PC, Cheung PY (2006) Ototoxic drugs and sensorineural hearing loss following severe neonatal respiratory failure. Acta Paediatrica 95: 214-223. Link: https://goo.gl/1hB6yp
- Claro Almeida K, Bueno González E, Cardona Iglesias L (2011) Detección precoz de pérdida auditiva en niños con factores de riesgo en la provincia de Guantánamo. VII Congreso de la Sociedad Cubana de Otorrinolaringología y Cirugía de Cabeza y Cuello. Reunión Especial Societas ORL Latinas. Habana, Cuba. Link: https://goo.gl/LzZ9oZ
- Ramos Cruz M (2011) Pesquisaje de afecciones auditivas en niños preescolares. VII Congreso de la Sociedad Cubana de Otorrinolaringología y Cirugía de Cabeza y Cuello. Reunión Especial Societas ORL Latinas. La Habana, Cuba.
- Kaga K, Kitazumi E, Kodama K (1979) Auditory brainstem responses of kernicterus infants. Pediatric Otorhinolaryngology 1: 255-264. Link: https://goo.gl/pN2hF2
- Corujo Santana C (2014) Evaluación de la hiperbilirrubinemia como factor de riesgo de hipoacusia neurosensorial en el programa de screening universal de hipoacusia infantil del Complejo Hospitalario Universitario Insular Materno Infantil de Gran Canaria entre los años 2007 al 2011. Tesis para obtener el Título de Doctorado. España: Universidad de las Palmas de Gran Canaria. Link: https://goo.gl/M1PLDi
- Núñez-Batalla F, Carro-Fernández P, Antuña-León ME, González-Trelles T (2008) Incidencia de hipoacusia secundaria a hiperbilirrubinemia en un programa de cribado auditivo neonatal universal basado en otoemisiones acústicas y potenciales evocados auditivos. Acta Otorrinolaringológica Española 59: 108-113. Link: https://goo.gl/F46XW4
- Shapiro SM (2003) Bilirubin toxicity in the developing nervous system. Pediatric Neurology 29: 410-421. Link: https://goo.gl/ehCeKW
- De Vries LS, Lary S, Dubowitz LMS (1985) Relationship of serum bilirubin levels to otoxicity and deafness in high-risk low-birth-weight-infants. Pediatrics 76: 351-354. Link: https://goo.gl/2PrBVP
- Feigin RD, Mc Cracken GH Jr, Klein JO (1992) Diagnosis and management of meningitis. Pediatric Infections Disease 11: 785-814. Link: https://goo.gl/7HXV7s
- Bogacz, Jaime y colaboradores (1985) Los potenciales evocados en el hombre. Ed. El Ateneo, Editorial. Argentina. Link: https://goo.gl/agp9aa
- Cohen BA, Schenk VA, Sweeney DB (1988) Meningitis-related hearing loss evaluated with evoked potencials. Pediatric Neurology 4: 18-22. Link: https://goo.gl/iQVZDa
- Charuvanij A, Visudhiphan P, Chiemchanya S (1990) Sensorioneural hearing loss in children recovered from purulent meningitis. Journal of the Medical Association of Thailand 73: 253-257. Link: https://goo.gl/awgb7z
- Peruyera Moreira IA (2002) Factores de riesgo para la hipoacusia neurosensorial en la población infantil pediátrica. Link: https://goo.gl/4zTvkd
- Peñazola-López YR, Castillo-Maya G, García-Pedroza F, Sánchez-López H (2004) Hipoacusia-sordera asociada a condiciones perinatales adversas según registro en Unidad Especializada de la ciudad de México. Análisis en función del peso al nacimiento. Acta Otorrinolaringológica Española 56: 262-269.
- Rivera T and Cobeta I (2001) Screening auditivo en niños con factores de riesgo de hipoacusia. Acta Otorrinolaringológica Española 52: 447-52. Link: https://goo.gl/aFjTLL
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
