Archives of Otolaryngology and Rhinology
Sinusitis-Induced Guillain-Barré Syndrome
Omer J Ungar1*, Anat Wangier1, Nurit Omer2 and Ilan Koren1
2Department of Otolaryngology, Head & Neck and Maxillofacial Surgery, Tel Aviv Sourasky Medical Center, “Sackler” Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Cite this as
Ungar OJ, Wangier A, Omer N, Koren I (2018) Sinusitis-Induced Guillain-Barré Syndrome. Arch Otolaryngol Rhinol 4(4): 079-081. DOI: 10.17352/2455-1759.000081Introduction
Guillain-Barré syndrome (GBS) is a heterogeneous, relatively uncommon, post-infectious, immune-mediated polyradiculoneuropathy. It is estimated to affect 1.1-1.8/100,000/year in Europe and North America [1]. Historically, GBS was considered to be a single disorder, but it is currently classified into six clinically distinct subtypes. It can manifest as cranial nerve involvement, including bilateral facial palsy, which is observed in 45-75% of cases [2]. In most instances, bilateral facial palsy or facial diplegia (FD) manifests either as bilateral Bell's palsy or as part of the presentation of GBS [3].
Plasmapheresis or the administration of intravenous immunoglobulin (IVIG) are the gold standard therapies for the demyelinating form of GBS and probably for the other subtypes as well, and they reportedly shorten the course of the disease [4]. Despite recent progress in therapeutic management, GBS still results in an in-hospital mortality rate of over 2.5% and a >9% need for endotracheal intubation, which is known to be a predictor of mortality [5]. We describe the clinical presentation, radiologic findings and management of a unique case of acute pansinusitis-induced GBS with isolated FD.
Case Report
An otherwise healthy 41-year-old male presented to the emergency room with rapidly progressive FD of 2 days duration. A detailed anamnesis revealed a recent diagnosis of acute rhinosinusitis that had been treated empirically with oral azithromycin for 3 days, with no significant improvement in nasal congestion, purulent nasal discharge or facial pain. One day prior to admission, he noticed that his speech was altered because his tongue felt numb. He also found it difficult to move his mouth and close his eyes. He denied dysphagia, visual disturbances of any kind or other sensory deficit. There was neither history of rash or arthritis in the past nor any evidence of those symptoms during this episode.
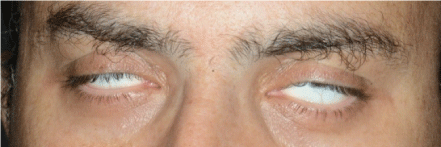
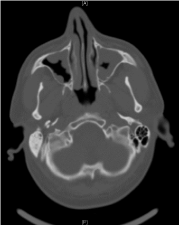
The admission neurologic examination confirmed FD (House Brackmann 6/6) and Bell’s phenomenon (Figure 1), with no concomitant meningeal signs. Facial sensation, gag reflex and ocular reflex were intact, as were tendon reflexes and plantar response. No other motor or sensory deficits were observed. The results of the blood analysis (general chemistry and complete blood count) were within normal limits for all parameters, including C-reactive protein (2.39 mg/L). A brain computerized tomography (CT) scan demonstrated normal brain parenchyma, without any evidence of increased intracranial pressure, space-occupying lesions, bleeding or infarcts. There was, however, considerable mucosal thickening of all paranasal sinuses (Figure 2), which was not present in a previous head CT scan performed 8 years earlier due to minor head trauma. A lumbar puncture was performed, and opening pressure was 140 mm H2O. Seven erythrocytes and a single leukocyte were present per high magnification field is 40. Cerebrospinal fluid (CSF) analysis showed a glucose level of 95 mg/dL and a protein level of 78 mg/dL. The patient was admitted to the Neurology Department and plasmapheresis was initiated under a working diagnosis of GBS. An extensive laboratory work-up was performed to exclude an alternative cause for facial diplegia. The results of HBV, HCV, HIV, HTLV-1, HSV-1, HSV-2, VZV, EBV, EBNA, CMV, mycoplasma pneumoniae, Borrelia Burgdorferi and VDRL serologies were negative. The results of a complement system analysis (C3, C4), ANCA, RF, ANA, LAC and ACE levels were normal as well. Beta 2 glycoprotein and anticardiolipin antibodies (IgM and IgG each) were within normal limits. There were no signs of porphyria in the blood, urine or stool analyses. A chest X-ray ruled out common symptoms of sarcoidosis. Electromyography (EMG) revealed bilateral facial nerve axonal damage, without any other signs of peripheral polyneuropathy.
An otolaryngologic examination revealed clinical signs of sinusitis, including viscous pus draining from the left frontal recess, which was clearly visible on endoscopic examination. Empirical treatment consisting of intravenous amoxicillin and clavulanate was initiated. Further questioning of the patient’s past medical history failed elicit a potential predisposition for GBS, recent diarrhea, upper respiratory tract infections or febrile episodes. An ophthalmological examination excluded involvement of cranial nerves II, III, IV and VI. A fiberoptic endoscopic evaluation of swallowing (FEES) was also within normal limits. He was diagnosed as having acute sinusitis-induced GBS since it was the only infectious source that had been identified after a detailed work-up.
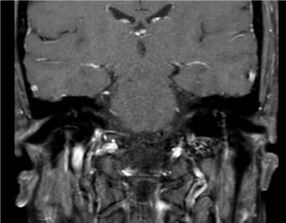
Five days of plasmapheresis yielded no clinical facial motor improvement. A second fiberoptic examination performed prior to discharge ruled out any pus or mucosal congestion in the nasal cavity. After 5 days of intravenous amoxicillin and clavulanate, he was discharged with an additional 5-day oral regimen, which provided subjective sinonasal relief. He was seen two weeks later and underwent another MRI scan which showed normal paranasal mucosal structures free of any pathological findings suggestive of sinusitis. There was bilateral improvement of his facial nerve movements (Figure 3). He was symptom free and had no facial nerve weakness at the 3-month follow-up.
Discussion
Facial diplegia is a rare condition that occurs mainly in the context of GBS. The patient we describe was diagnosed as having GBS by means of a typical CSF laboratory analysis, including albuminocytological dissociation (elevated protein level without increase in cell count) and an EMG characteristic of bilateral facial nerve axonal damage, which is considered to be a GBS variant. Most GBS cases are associated with a clinically apparent antecedent infection. A meticulous search for a GBS-provoking infection in this patient revealed no clinical sign or symptom other than pansinusitis. Respiratory, gastrointestinal and urinary tract infections were excluded by anamnesis, physical examination, laboratory results (including urinary, blood and CSF analysis) and radiologic imaging.
Cranial nerve palsy is a well-known manifestation of complicated acute sinusitis, through cavernous sinus thrombosis (CST). The mechanism is thought to be via hematogenous spread from the paranasal sinuses through the facial venous plexus, or via direct infection from the sphenoid sinus to the adjacent cavernous sinus [6]. The typical symptoms of CST are fever, proptosis, ptosis, chemosis and orbital (III, IV and VI) cranial nerve palsy. Facial nerve palsy can not, however, be explained by the CST mechanism, due to the lack of an anatomical relationship between the cavernous sinus and the facial nerve. There is only one publication on a complicated frontal sinusitis-induced unilateral facial paresis by means of subdural empyema causing a mass effect at the upper level of the brainstem [7]. This mechanism was ruled out in the current case: our patient had no fever and showed no signs of general deterioration or meningeal disease. Moreover, his head CT did not reveal any intracranial abnormalities. We found three few publications linking acute sinusitis to GBS [8,9], of which only one was written in English [9]. This case report is unique case of isolated FD that was secondary to GBS and provoked by acute pansinusitis. There were no other signs of cranial nerve involvement, with the FEES and ophthalmic findings within normal limits. We did not perform Schirmer’s test to assess the greater superficial petrosal nerve.
Conclusion
GBS is not an uncommon pathology and one that is associated with significant morbidity and mortality. The otorhinolaryngologist might be the first healthcare provider to evaluate a patient with sinusitis-induced GBS. We recommend a high index of suspicion and that GBS should be considered in the differential diagnosis of every patient who presents with FD.
- McGrogan A, Madle GC, Seaman HE, de Vries CS (2009) The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology 32: 150-163. Link: https://goo.gl/szAWiL
- D'Amore A, Viglianesi A, Cavallaro T (2012) Guillain-barré syndrome associated with acute onset bilateral facial nerve palsies. A case report and literature review. Neuroradiol J 25: 665-670. Link: https://goo.gl/tmTRU8
- Khlebtovsky A, Saban T, Steiner I (2013) Unusual and benign course of idiopathic facial diplegia. J Clin Neurosci 20: 904-905. Link: https://goo.gl/J7sgu9
- Xiao J, Simard AR, Shi FD, Hao J (2013) Strategies in the management of Guillain-Barré syndrome. Clin Rev Allergy Immunol.
- Alshekhlee A, Hussain Z, Sultan B, Katirji B (2008) Guillain-Barré syndrome: incidence and mortality rates in US hospitals. Neurology 70: 1608-1613. Link: https://goo.gl/daueu5
- Pavlovich P, Looi A, Rootman J (2006) Septic thrombosis of the cavernous sinus: two different mechanisms. Orbit 25: 39-43. Link: https://goo.gl/N63yLg
- Holland AA, Morriss M, Glasier PC, Stavinoha PL (2012) Complicated subdural empyema in an adolescent. Arch Clin Neuropsychol 28: 81-91. Link: https://goo.gl/fPqnN5
- Haug AK, Rothhammer V, Scherer EQ, Pickhard AC (2013) [Guillain-Barré syndrome with dysphagia after frontal sinusitis]. [Article in German]. HNO 61: 52-54.
- Chaudhary A, Ramchand T, Frohman LP, Liu JK, Eloy JA (2012) Miller Fisher variant of Guillain-Barré syndrome masquerading as acute sphenoid sinusitis with orbital apex syndrome. Laryngoscope 122: 970-972. Link: https://goo.gl/s1RX6y
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
