Annals of Marine Science
Phase Change Material (PCM) as the Smart Heat-Storing Concept: A Brief Review
Payam Nejat1*, Yashar Fekri2 and Fatemeh Jomehzadeh3
2Department of Mechanical Engineering, Tarbiat Modares University, Tehran, Iran
3Civil Engineering, University Technology Malaysia, UTM- Skudai, Johor, Malaysia
Cite this as
Nejat P, Fekri Y, Jomehzadeh F (2022) Phase Change Material (PCM) as the Smart Heat-Storing Concept: A Brief Review. Ann Mar Sci 6(1): 034-038. DOI: 10.17352/ams.000029Copyright License
©2022 Nejat P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Recently smart materials play an important role in different aspects of the industry due to their specific characteristics. The properties of these materials can change spontaneously in interaction with the immediate surrounding without any external power consumption. Phase change materials are a great division of smart materials with considerable capacity to absorb and release thermal energy during the phase change process. They can also handle temperature regulations since the phase change process occurs at a constant temperature. The implementation of PCMs is one of the potential passive cooling strategies which has received increasing attention in recent years. This paper aims to briefly review the various types of PCMs which can be used for passive cooling in buildings.
Introduction
Smart materials are kind of materials whose characteristics can change in interaction with the surrounding physical conditions, without using any external power. According to the definition, PCMs are considered smart materials that go through a phase change process while the ambient temperature reaches the specific temperatures which trigger the phase change [1]. The smart function of such materials has increased their application in various aspects of the industry. Recently PCMs are growing in the global market of smart materials at a considerable rate of 20% annually [2].
As phase change phenomena happen in PCMs, they are used as thermal energy storage devices due to the high amount of energy that can be stored in the form of latent heat. Since the temperature remains constant during the phase change, PCMs are capable of being implemented as thermal regulators specifically in buildings. They can absorb or release energy when the indoor environmental conditions change which can affect the indoor air temperature and provide occupants with acceptable comfort [3].
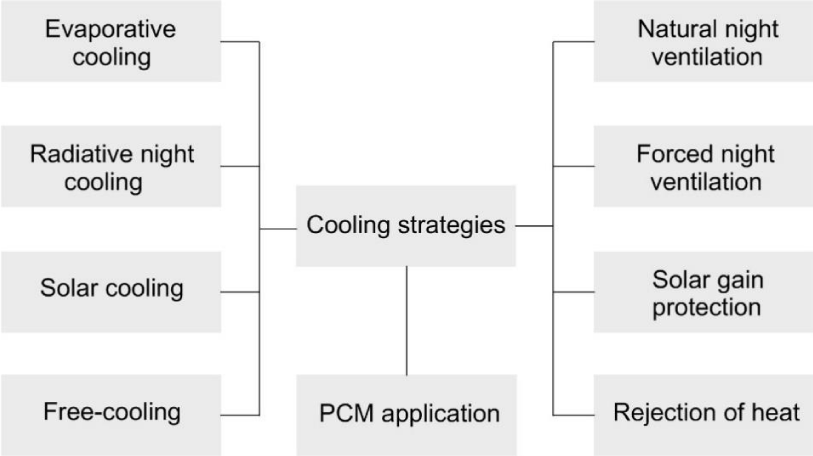
Generally, there are two main strategies for building cooling, namely passive and active. Active cooling techniques include air handling units and packaged thermal air conditioner systems [4]. By the use of methods such as heat modulation, heat dissipation as well as solar and heat protection techniques, passive cooling can be achieved inside the buildings [5]. Heat modulation techniques comprise night ventilation and cooling storage. Different cooling strategies are presented in Figure 1 briefly.
Phase Change Material (PCM) as smart heat-storing materials
Heat-storing smart materials which are also known as phase change materials (PCM) have specific properties which give the ability to store or release thermal energy in a controlled condition by temperature variation [7].
Phase change material can also be named latent heat storage material. The thermal energy is stored in PCM in form of sensible heat (like conventional materials such as concrete etc.) as well as latent heat which the latter has a much higher heat storage capacity than the former. Latent heat is the thermal energy that is released or absorbed when a substance starts to change its phase from solid to liquid or liquid to vapor or vice versa which occurs usually in constant temperature [5,8].
In recent years different studies done in different climates, prove that PCM can have a positive role in building energy management and conservation. The application of PCM for energy saving in the building goes back to 1980 by integrating PCM with gypsum board, plaster, concrete, or other wall covering materials [9].
The PCM can lessen the vitality requirements for heating, ventilation, and air conditioning (HVAC) frameworks as it is proved that a considerable share of building energy consumption is due to HVAC [10]. It can also decrease indoor temperature variances. The attractive identities of PCM materials are high capacity of thermal energy storage, great heat conductivity, little dilatation, shrinkage amid phase change, and minimum sub-cooling while solidifying. However, they should be non-dangerous and have compound strength. The PCM utilized with the expectation of complimentary cooling ought to have a characteristic temperature running from 15 °C to 30 °C [11].
The working principle of PCM-based free cooling for structures is indicated in Figure 2 which consists of two scenarios of operation:
- In the first scenario, the PCM has a solid phase. The interaction of the high-temperature indoor air with the PCM triggers the heat transfer process in which the PCM gains the excess thermal energy of the air and changes to liquid at a nearly constant temperature. Thus, the air in the vicinity of the PCM loses its thermal energy, and its temperature decreases.
- In the second scenario, airflow with the low temperature inside the building encounters the PCM which is in the liquid phase. Gradually the PCM releases its thermal energy and during the heat exchange process with the air, it goes through the phase change process and turns into a solid at an approximately constant temperature. As a result, the air inside the building gains thermal energy, and its temperature increases [12].
However conventional PCMs have some drawbacks which can limit their application. Various solutions have been proposed to address the challenges [14]. In emerging PCMs the concept of Microencapsulated Phase Change Material (MEPCM) has received increasing attention. In this manner, PCM as the core is covered by a shell (made of other materials) which enables the phase change process inside the small, enclosed volume. These MEPCMs can be conveyed by carrier fluid inside ducts. This technique can solve some of the major challenges of conventional PCMs like thermal instability and low thermal conductivity. In addition, the used shell should be enough strength since volume changes in phase change cycles can destroy the outer covering layer [15].
To overcome the problem of low thermal conductivity, nanomaterials can be integrated with PCMs to increase the effective thermal conductivity due to the specific properties of the additives [14]. These added materials can be in the form of nanotubes, nanorods, nanosheets, etc. Generally, the nanomaterial additives have a considerably high thermal conductivity which can contribute to efficient heat absorption or heat rejection. Combining these materials with PCMs can improve their performance dramatically and as a result, the application of PCMs can be extended [16].
Types of PCMs
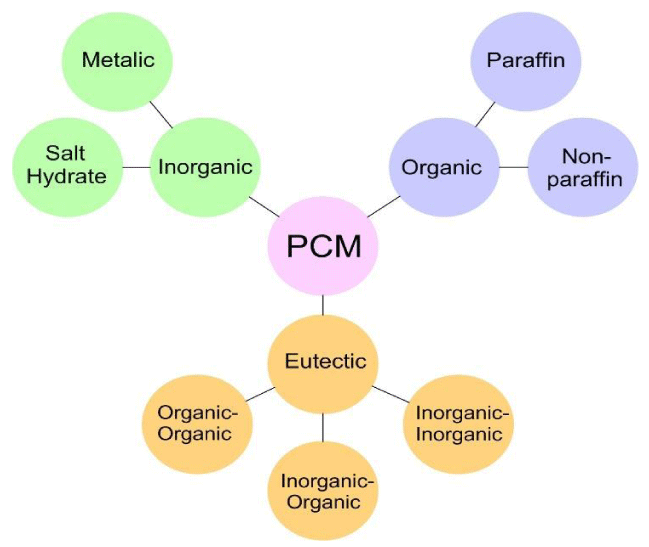
Generally, there is a wide variety of PCM in the market in different temperature ranges. There are various classifications for the PCM in the literature but the most common one is presented in Figure 3 which based on it the PCM can be categorized into three different classes including organic, inorganic, and eutectic [9,17].
Organic phase change materials
Organic PCMs can be classified as paraffin and non-paraffins. Organic PCMs are not usually corrosive and have congruent melting. For building applications with respect to thermal comfort range, organic PCMs with a melting point of 20 °C to 32 °C are potentially applicable [18].
Paraffin: A study conducted by reference [19] illustrated that the range of paraffin’s melting point is from -12 °C to 71 °C which can store 128 kJ/kg to 198 kJ/kg heat. Paraffin is composed of a straight chain Alkanes mixture (CH3-(CH2)-CH3). The process of CH3 chain making crystal can release a high amount of thermal energy or vice versa.
For paraffin, increasing the chain length leads to an increase in latent heat of fusion and melting point. Paraffin can be considered a safe, reliable, cheap, and non-corrosive material which has very important factors for application in buildings. Moreover, from a chemical point of view, they are inert and stable (lower than 500 °C) and their volume does not change considerably when changing the phase. In Table 1, there is a list of some paraffin with their properties [20]. Apart from their appropriate characteristics like congruent melting they have some unsatisfactory properties; they are nearly flammable, they have low heat conductivity, and they are not compatible with plastics.
Non-paraffins: The category of non-paraffin organic PCM has the vastest varieties in types and properties. Unlike the paraffin group which has similar characteristics, non-paraffins have a wide variety of their own properties. Therefore they are considered the largest candidate category for thermal storage applications [17]. Two studies conducted by references of [22,23] concluded that alcohols, esters, glycols, and fatty acids are appropriate non-paraffin PCMs some of them are presented in Table 2.
One of the disadvantages of non-paraffin PCMs is flammability which limits them to exposure to flame, high temperature, and oxidizing substances. In this group, fatty acids appear to have the highest potential to be implemented practically. Since it shows many satisfactory properties which include: economic view (availability and price), high latent heat, low supercooling, no phase segregation, and different melting temperature [24].
Moreover, this type demonstrates reproducible freezing and melting with no supercooling during freezing [17]. Generally, fatty acids can be explained by following the formula CH3(CH2)2nCOOH. However, in comparison with available paraffin, they are nearly two times more expensive. In addition, they are corrosive to some extent. The advantages and disadvantages of organic PCMs are presented in Table 3 [17].
Inorganic phase change materials (salt hydrates)
Compared with organic PCMs, inorganic PCMs have a higher heat of fusion per unit mass with lower cost and most of them are not flammable. But on the other side, they have super cooling and decomposition which overshadow their advantages [18,26]. In this category salt hydrates are the most important types and a lot of studies have been done on them for thermal storage applications due to their high latent heat per unit mass. Table 4 shows the latent heat and melting points of some inorganic PCMs and Table 5 illustrates the advantages and disadvantages of inorganic types [17].
Eutectics
A eutectic PCM consists of at least two other PCMs so during freezing they form a blend crystal [27]. This mixture can consist of organic with organic, inorganic with inorganic, and organic with inorganic some of them are indicated in Table 6 [18]. The separation of the components is very unlikely because they mostly change the phase without segregation (due to freezing to an intimate mixture of crystals) and during melting all components change to liquid simultaneously.
Selection criteria
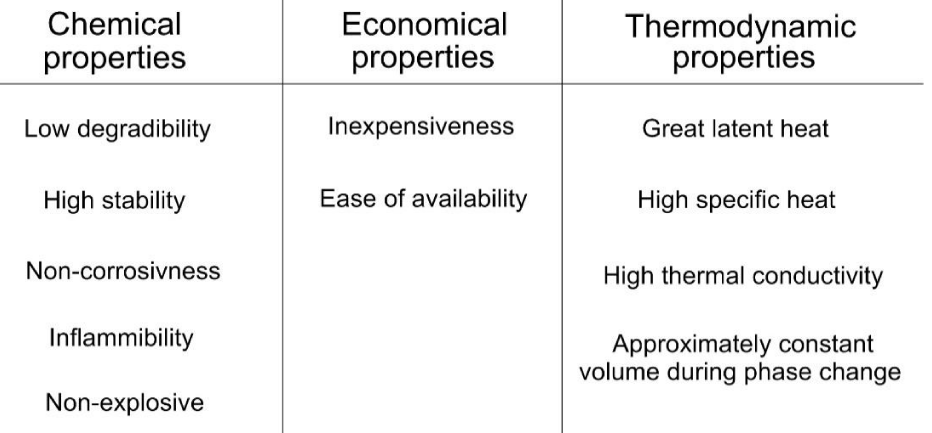
As stated in previous investigations issues related to HVAC account for a great proportion of energy consumption in buildings [28] and PCMs are of high potential to decrease the required energy. To be applied for cooling purposes in buildings the PCMs should have some desirable qualifications. For instance, from an economic aspect they should have reasonable prices and availability in the market, from a heat transfer view, they should have high thermal conductivity to absorb and release heat fast from the surrounding environment [17] [29,30] (Figure 4).
From a thermodynamic point, they should have high heat of fusion per unit mass to be able to store a high amount of thermal energy. In addition, they should not chemically be flammable, toxic, corrosive, or unstable. All stated properties are important for PCMs to be easily incorporated with building components and passive cooling strategies. So, with respect to all those properties, the PCM in Figure 4 can be employed for the cooling system in the building.
Conclusion
Currently, energy is considered an international important issue in the world. The building sector accounts for one-third of total energy consumption in the world and 8% of direct CO2 emissions. A considerable share of building energy consumption is related to HVAC systems with nearly two third. Passive cooling methods are sustainable techniques that can provide appropriate thermal comfort conditions for occupants without consuming fossil fuels and threatening the environment. Generally, there is a wide variety of PCM in the market in different temperature ranges. There are various classifications for the PCM in the literature but the most common can be categorized into three different classes organic, inorganic, and eutectic. Organic PCMs are categorized as paraffin and non-paraffins. Organic PCMs are not usually corrosive and have congruent melting. For building applications with respect to thermal comfort range, organic PCMs with a melting point of 20 °C to 32 °C are potentially applicable. Compared with organic PCMs, inorganic PCMs have a higher heat of fusion per unit mass with lower cost and most of them are not flammable. But on the other side, they have super cooling and decomposition which overshadow their advantages. In this category salt hydrates are the most important types and a lot of studies have been done on them for thermal storage applications due to their high latent heat per unit mass. A eutectic PCM consists of at least two other PCMs so during freezing they form a blend crystal. This mixture can consist of organic with organic, inorganic with inorganic, and organic with inorganic. The separation of the components is very unlikely because they mostly change the phase without segregation (due to freezing to an intimate mixture of crystals) and during melting all components change to liquid simultaneously. Finally, to be applied for cooling purposes in buildings the PCMs should have some desirable qualifications such as reasonable price and availability in the market (economical aspect), and high thermal conductivity to absorb and release heat fast from the surrounding environment (heat transfer aspect).
- Orsini F, Marrone P, Santini S, Sguerri L, Asdrubali F, Baldinelli G, Bianchi F, Presciutti A. Smart Materials: Cementitious Mortars and PCM Mechanical and Thermal Characterization. Materials (Basel). 2021 Jul 27;14(15):4163. doi: 10.3390/ma14154163. PMID: 34361356; PMCID: PMC8347051.
- Long F, Cheng Y, Ren Y, Wang J, Li Z, Sun A. Latest Advances in Development of Smart Phase Change Material for Soft Actuators. Adv Eng Mater 2022; 24:2100863. https://doi.org/10.1002/ADEM.202100863.
- Ali Husein H, Hama A, Sarhang Jalal Y. Effects of phase change materials on reducing energy consumption in residential buildings: case study of park view apartments in erbil city. Manag Appl Sci Technol. 11. https://doi.org/10.14456/ITJEMAST.2020.158.
- Gan PY, Komiyama R, Li Z. A low carbon society outlook for Malaysia to 2035. Renew Sustain Energy Rev 2013;21:432–43. https://doi.org/10.1016/j.rser.2012.12.041.
- Rao Z, Wang S, Zhang Z. Energy saving latent heat storage and environmental friendly humidity-controlled materials for indoor climate. Renew Sustain Energy Rev 2012;16:3136–45. https://doi.org/10.1016/j.rser.2012.01.053.
- Souayfane F, Fardoun F, Biwole P. Phase Change Materials ( PCM ) for cooling applications in buildings : A review. Energy Build 2016. https://doi.org/10.1016/j.enbuild.
- Salunkhe PB, Shembekar PS. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew Sustain Energy Rev. 2012; 16:5603–16. https://doi.org/10.1016/j.rser.2012.05.037.
- Rathod MK, Banerjee J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew Sustain Energy Rev. 2013; 18:246–58. https://doi.org/10.1016/j.rser.2012.10.022.
- Anisur MRR, Mahfuz MHH, Kibria MAA, Saidur R, Metselaar IHSCHSC, Mahlia TMIMI. Curbing global warming with phase change materials for energy storage. Renew Sustain Energy Rev. 2013; 18:23–30. https://doi.org/10.1016/j.rser.2012.10.014.
- Nejat P, Jomehzadeh F, Abd Majid MZ Bin, Mohd Yusof MB, Zeynali I. Windcatcher as sustainable passive cooling solution for natural ventilation in hot humid climate of Malaysia. IOP Conf Ser Mater Sci Eng. 2019; 620. https://doi.org/10.1088/1757-899X/620/1/012087.
- Raj VAA, Velraj R. Review on free cooling of buildings using phase change materials. Renew Sustain Energy Rev. 2010; 14:2819–29. https://doi.org/10.1016/j.rser.2010.07.004.
- Faraj K, Khaled M, Faraj J, Hachem F, Castelain C. Phase change material thermal energy storage systems for cooling applications in buildings : A review. Renew Sustain Energy Rev. 2019; 109579. https://doi.org/10.1016/j.rser.2019.109579.
- Mousavi S, Rismanchi B, Brey S, Aye L. PCM embedded radiant chilled ceiling : A state-of-the-art review. Renew Sustain Energy Rev. 2021; 151:111601. https://doi.org/10.1016/j.rser.2021.111601.
- Nazir H, Batool M, Bolivar FJ, Isaza-ruiz M, Xu X, Vignarooban K . International Journal of Heat and Mass Transfer Recent developments in phase change materials for energy storage applications : A review.Int J Heat Mass Trans. 2019; 129:491523.https://doi.org/10.1016/j.ijheatmasstransfer.2018.09.126.
- Alam TE, Dhau JS, Goswami DY, Stefanakos E. Macroencapsulation and characterization of phase change materials for latent heat thermal energy storage systems. Appl Energy. 2015; 154:92–101. https://doi.org/10.1016/j.apenergy.2015.04.086.
- Angayarkanni SA, Philip J. Review on thermal properties of nanofluids: Recent developments. Adv Colloid Interface Sci. 2015 Nov;225:146-76. doi: 10.1016/j.cis.2015.08.014. Epub 2015 Sep 3. PMID: 26391519.
- Sharma A, Tyagi VV, Chen CR, Buddhi D. Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev. 2009; 13:318-345. https://doi.org/10.1016/j.rser.2007.10.005.
- Tyagi VV, Buddhi D. PCM thermal storage in buildings: A state of art. Renew Sustain Energy Rev. 2007; 11:1146-1166. https://doi.org/10.1016/j.rser.2005.10.002.
- Peng S, Fuchs A, Wirts R. Polymeric phase change composites for thermal energy storage. J Appl Ied Polym Er. 2004; 98.
- Hale D, Hoover M. O’Neill M. Phase change materials hand book. 1971.
- Tyagi V V, Chopra K, Kalidasan B, Chauhan A, Stritih U, Anand S. Phase change material based advance solar thermal energy storage systems for building heating and cooling applications : A prospective research approach. Sustain Energy Technol Assessments. 2021; 47:101318. https://doi.org/10.1016/j.seta.2021.101318.
- Abhat. Development of a modular heat exchanger with an integrated latent heat storage 1981.
- Buddhi D, Sawhney R. Proceedings on thermal energy storage and energy conversion 1994.
- Sarı A. Form-stable paraffin/high density polyethylene composites as solid–liquid phase change material for thermal energy storage: preparation and thermal properties. Energy Convers Manag. 2004; 45:2033-42. https://doi.org/10.1016/j.enconman.2003.10.022.
- David D, Johannes K, Roux J-J, Kuznik F, David D, Johannes K. A review on phase change materials integrated in building walls. Renew Sustain Energy Rev. 2011; 15:379-391. https://doi.org/10.1016/j.rser.2010.08.019.
- Baetens R, Jelle BP, Gustavsen A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010; 42:1361-1368. https://doi.org/10.1016/j.enbuild.2010.03.026.
- Osterman E, Tyagi VV, Butala V, Rahim NA, Stritih U. Review of PCM based cooling technologies for buildings. 2012; 49: 37-49. https://doi.org/10.1016/j.enbuild.2012.03.022.
- Nejat P, Hussen HM, Fadli F, Chaudhry HN, Calautit J, Jomehzadeh F. Indoor environmental quality (IEQ) analysis of a two-sided windcatcher integrated with anti-short-circuit device for lowwind conditions. Processes. 2020; 8. https://doi.org/10.3390/pr8070840.
- Khudhair AM, Farid MM. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers Manag. 2004; 45:263-275. https://doi.org/10.1016/S0196-8904(03)00131-6.
- Cabeza LFF, Castell A, Barreneche C, Gracia A De, Fernández AII, de Gracia A. Materials used as PCM in thermal energy storage in buildings: A review. Renew Sustain Energy Rev. 2011; 15:1675–95. https://doi.org/10.1016/j.rser.2010.11.018.
- Solé A, Miró L, Barreneche C, Martorell I, Cabeza LF. Review of the T-history method to determine thermophysical properties of phase change materials (PCM). Renew Sustain Energy Rev. 2013; 26:425–36. https://doi.org/10.1016/j.rser.2013.05.066.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
