Annals of Marine Science
Biochemical composition of Meretrix meretrix in the Bakkhali river Estuary, Cox’s Bazar, Bangladesh
Joydeb Chowdhury, Mohammad Saydul Islam Sarkar, Md. Ashraful Azam Khan and Md. Simul Bhuyan*
Cite this as
Chowdhury J, Islam Sarkar MS, Khan MAA, Bhuyan MS (2019) Biochemical composition of Meretrix meretrix in the Bakkhali river Estuary, Cox’s Bazar, Bangladesh. Ann Mar Sci 3(1): 018-024. DOI: 10.17352/ams.000016The present study was conducted in the Bakkhali river estuary, Cox’s Bazar, Bangladesh from September 2012 to July 2013. Percentage of protein was the main component of the biochemical composition in the mussels followed by carbohydrate, ash, and lipid. The proximate composition of Meretrix Meretrix revealed that it contained protein (12.184-14.291%), lipid (0.721-0.922%), ash (2.435-3.201%), moisture (77.0-78.9%) and carbohydrate (4.914-5.907%). The mineral content of the Meretrix Meretrix were Ca (0.601-0.801 mg/g), Fe (0.070-0.099 mg/g) and phosphorus (0.300-0.794 mg/g). In the present investigation, the seasonal changes of protein and lipid were found to follow a similar pattern. Hence, a significant positive correlation was found between the percentage of protein and lipid in Meretrix meretrix. A positive relationship was found between the percentage of protein and lipid, protein and ash and, lipid and ash. A significant inverse correlation was found between protein and carbohydrates, carbohydrate and lipid and between carbohydrate and ash content of dry tissue of Meretrix meretrix (p<0.01). Further study on Meretrix meretrix should be carried out since their acceptability to the coastal dwelling community.
Introduction
The clam, Meretrix meretrix, is a delicious, cheaper, and protein-rich food source for people residing along with the coast [1,2]. It has been traditionally used as medicine and raw material for village industries [3]. These edible bivalves are filter feeders with high conversion efficiency and high levels of biochemical constituents. Due to high protein content and the high nutritive value, several species of clams were studied [4-7]. Some important features like high protein content, low calorific values, low fat/ cholesterol profile and lower proportions of saturated fat, the presence of good lipids, significant amounts of omega-3-fatty acids, dietary essential amino acids, vitamin B12 and several important minerals such as iron, zinc and copper make the calm a good food [8,9].
The biochemical analysis is the percentage of water, protein, lipids, carbohydrate, and minerals required for human [10]. This nutritive value of edible living organisms is needed for consumption and marketing [11]. The proximate composition is an important tool to identify the quality of meat and to understand the changes in the nutritive value in the clams during the gametogenesis [12].
Various factors like spawning season, fecundity, and depth of culture area affect the proximate composition of Meretrix meretrix [11,13-17]. Growth of body size, maturation of the clam increase in the protein, lipid, and carbohydrate (glycogen) contents in calm [18]. The season played a significant role in the lower concentration of fat and it happens during spawning just after the release of gametes [19-21]. Similar observations had been conducted by Pease [22] and Durve and Bal [20], in oysters and Joshi and Bal [19], in K. Marmorata. Giese et al. [23] observed that lipid levels were relatively stable for most of the year except for the small variation in the gonads; the lipid reached a peak value at the time of most active formation of gametes.
Biochemical composition of bivalves studied by various researchers [12,19,24-32].
Meretrix meretrix abounds along the coastal region from St. Martin’s Island, Bakkhali river estuary, Sahaparirdip, Teknaf, Cox’s Bazar, Moheskhali, Kutubdia in Cox’s Bazar district. Galachipa, Kalapara in Patuakhali district. Amtoli, Borguna, and Paterghata in Borguna district. Mongla port, Saronkhola in Bagherhat district. Dakop, Koira, Paikgacha in Khulna and Satkhira district. The availability and high nutrients contents compelled us to know the biochemical composition of Meretrix meretrix.
Materials and Methods
Study area
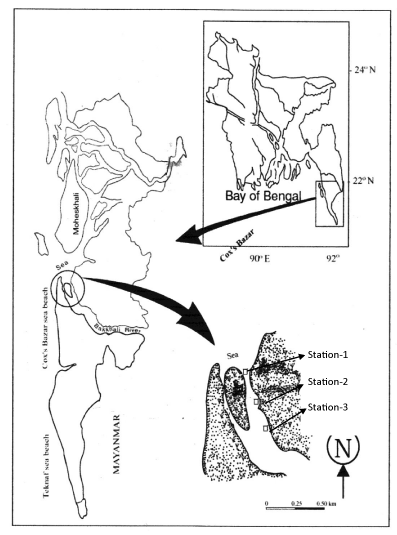
The study was carried out in the Bakkhali river estuary, Cox’s Bazar district (Figure 1). The global positions of four stations were between latitude (N) 21°26’50’’ to 21°32’50’’ and longitude (E) 91°65’30’’ to 91°90’30’’. Almost 2 sides of three stations were open to the Moheshkhali channel and another west side was surrounded by Cox’s Bazar town. Meretrix meretrix is found as sub-soil animals. The species inhabits in the sand and muddy sand bottom in the intertidal and sub-tidal water to a depth of about 20m in the Indo-west pacific seas [33].
Sample collection and processing
Meretrix meretrix samples were collected from the Bakkhali river estuary. Immediately after collection, the clams were transferred to the laboratory of Faculty of Marine Sciences and Fisheries in live condition. Then the clams were acclimatized with filtered aerated estuarine water in the plastic containers. The clams were adopted for 48 hours and water was changed 3 times in a day during this period [26]. Then clams were washed completely and the shell was opened. Matured and immature clams were identified and weighed after removing the excess moisture. Finally, the meat was extracted and stored at 4°C for further analyses [27].
Protein determination
The nitrogen was estimated by the micro-Kjeldahl method [34] and the amount of crude protein was calculated by multiplying nitrogen value by 6.25. The fat was extracted in solvent ether from samples of dried meat by using Soxhlet apparatus. Glycogen was estimated by the method of Kemp and Heijningen [35], using Engel’s colorimeter. The amount of glycogen was calculated by multiplying the glucose value by factor 0.927. The results are expressed in percentage of dry weight.
The protein content of the feedstuff is obtained by estimating the nitrogen content of the materials and multiplying the nitrogen value by 6.25 this is referred to as crude protein content. According to Kjeldahl methods-proteins are hydrolyzed to amino and with H2SO4. Further heating decomposes the amino acid releasing-Ammonia which immediately trapped on (NH4)2SO4 and water. Micro Kjeldahl Method [36-38], was to determine the crude protein.
% of Nitrogen =V×N×0.014×100/wt of sample
Where, V= Volume of HCl
N= Normality of HCl
% of Crude protein = % of Nitrogen×Conversion factor
Lipid determination: Fat is examined with low boiling organic solvent (petroleum ether/ diethyl ether, xylene) by soxhlet extraction and the extract thus obtained weighed after recovery of the solvent. Crude fat was determined through Sox let Extraction Technique [39,40], using hexane (65ºC-70ºC) as the solvent.
% of crude fat = (Corrected weight of fat÷Weight of sample)×100
Ash determination: Ash is the residues of the inorganic matter (mineral) of the sample after burning. If the sample in a muffle furnace at 600ºc the organic matter is evaporating and residues are called ash. Ash content of each feed was estimated by following incineration Method [39].
% of ash = (Weight of ash÷Weight of sample)×100
Moisture determination: Moisture is an important, debutante of the nutrient of the feed or feed ingredients. It is necessary to know the moisture contents of the feed because it has an important function determines the form of the diet. Moisture contents in the feed were determined by the following oven method [41].
The percentage of the moisture content in the sample was calculated by the following formulae:
% of moisture = {(Weight of original sample–Weight of dried sample) /Weight of original sample}×100.
Carbohydrate determination: The percentage of carbohydrate was calculated as follows:
% of Carbohydrate = 100-% of (protein+lipid+ash+moisture)
Calcium determination: The evaluation of calcium in feed is very important since imbalance with phosphorous or other minerals will lead to reducing growth.
% of Ca = V×N× use of diquot from mother solution/wt. of sample ×250
=0.5×7.4×100/2×250
=0.74
% of Ca (mg/100g) =740
Iron (Fe) determination: Fe (mg/100g) =Abs×0.49×100/wt. of sample
Phosphorus (P) determination: The percentage of Phosphorus =1.435 a/p
Where, a = The weight in gm of the ammonium phosphate molybdate precipitate
p = Weight in gm of the sample taken for analysis
Statistical analysis
One-Way Analysis of Variance (ANOVA) was executed to show the significant variations and relations among the concentrations (SPSS v.22). MS-Excel was used to produce graphs.
Results and Discussion
Protein is the major biochemical constituent of wet mussel meat forming 12.184% to 14.291%. The average protein content of Meretrix meretrix is 13.26% (Table 1). In the case of clam, it was noticed that as temperature increases protein content also increases. Bivalves use reserve protein during harsh condition. This scenario was observed in Turbo sarmaticus and in Paphia malabarica [28,42]. During monsoon season gradual fall in protein content can be seen. While in the case of mussel two peaks can be seen during post-monsoon (14.291%) and pre-monsoon (13.321%). In the present study, seasonal changes of protein and lipid in Meretrix meretrix were found to follow a similar pattern. Kamble and Muley [26], reported that the highest protein content in Meretrix meretrix in winter and monsoon season than the summer season. Nagabhushanam and Dhamane [43], recorded relatively high amount of protein in Papiha laterisulca all the year round. Durve and Bal [44], also reported higher concentrations of protein in C. gryphoides. Different Protein concentrations were found in A. ligamertina and A. plictata [45]. High protein concentration was found in adult stage since bivalves use protein to form oocytes [46-48].
Hence, a significant positive correlation was found between the percentage of protein and lipid. Similar results were reported by Fatima et al. [49], in the green mussel, Perna viridis.
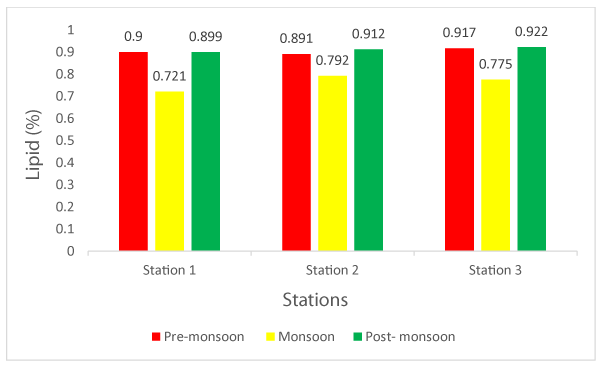
Lipid is very important for energy metabolism of bivalves such as Patinopecten vessoensis [50]. Lipids are found basically in gonads of adult bivalves [51,52]. In the present study, the lipid content ranged between 0.721% to 0.922% in wet mussel (Figure 2). The average lipid content of mussel 0.86%. Like carbohydrate content lipids also exhibited large fluctuations in all months of the year. Lipid content in mussel was very high in post-monsoon.
During monsoon, temperature and salinity decreases sharply lipids also decreases sharply. Kamble and Muley [26], stated that the lipid concentration was high during summer and monsoon seasons and low during the winter season. In the case of mussel, lipids showed an inverse relation with carbohydrates. As lipid increases carbohydrate decreases.
In contrary to protein and lipid, seasonal changes in carbohydrate followed a different course. i.e. when carbohydrate content decreased, protein and lipid fractions were found to increase (Table 1). Thus, a reciprocal relationship between the percentage of protein and carbohydrate was found in Meretrix meretrix. A similar relationship was reported in case of Medulis by Dare and Edwards [53], and Drzycimski [54], and in the case of Meretrix meretrix by Fatima et al. [49]. But for all the oyster species, a positive correlation was reported between the carbohydrate and the lipid level [55]. This difference between mussel and oyster may be related to the form of the reproductive cycle [55].
Comparison of functional ingredients of M. meretrix with other studies presented in Table 2.
The ash content varied from 2.435% to 3.136% in wet mussel (Table 1). The average lipid content of clam is 2.844%. Ash content in clam was very high in monsoon whereas in summer months it was low (Table 1). Eswar et al. [25], found 5.56% ash content in G. divaricatum collected from Mumbai, West Coast of India. Ash content showed an inverse relation with protein content. On the basis of yearly average, mature mussels contained higher ash content than the immature ones which was just an opposite trend found in the case of carbohydrate and lipid content but similar to the protein content.
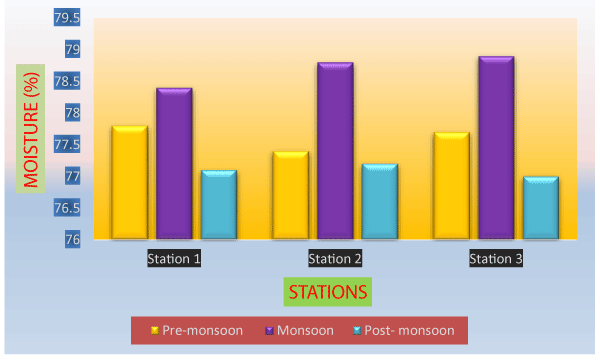
The moisture content showed a range of 77.00% to 78.9% in wet mussel (Table 1). The average moisture content of 77.81%. The highest percentage of moisture was recorded in the monsoon while the lowest concentration was found in post-monsoon (Figure 3). Eswar et al. [25], recorded 6.11% ash content in G. divaricatum collected from Mumbai, West Coast of India. Moisture had an inverse relationship with salinity. Moisture contents in Meretrix meretrix, Katelysia opima, Marcia opima, Lima trains, Nucula sulcate, Donax cuneatus, Donax incarnates increased with the decreased salinity during monsoon season [32,56-62]. Similar results were found in the present study.
Another major biochemical content was carbohydrate found in Meretrix meretrix. Carbohydrate exhibited large fluctuations in all months of the year. The concentrations varied from 4.914% to 5.907% in wet mussel (Table 1). The average carbohydrate content of clam was recorded 5.229%. Eswar et al. [25], found 11.23% ash content in G. divaricatum collected from Mumbai, West Coast of India. The trend of the percentage of carbohydrate was just opposite to that of protein. The higher percentage values of carbohydrate were observed during the monsoon period and lower during the post-monsoon and pre-monsoon period for both the mature and immature mussels (Table 1). Arun [24], recorded comparatively low carbohydrate concentration during pre-monsoon. Tivela stultorum, Meretrix meretrix, Donax cuneatus, Villorita cyprinoides var. cochinensis and Sunetta scripta converted carbohydrate to lipid and protein and stored in the gametes [32,43,63-65]. They used these reserve lipid and protein during stress environment [39,66].
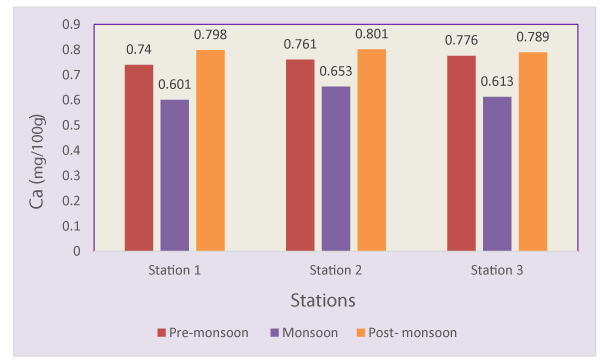
Calcium concentrations ranged from 0.601-0.801mg/100g (Table 1). The calcium content an observed Maximum in the season of post-monsoon (0.801mg/100g) and minimum in monsoon (0.601mg/100g) in wet mussel and average 0.726 mg/100g (Figure 4). Ranjan and Babu [67], recorded 51.03±1.65gm and 909±45.3mg CaCO3/gm in Meretrix meretrix collected from Bhavanapadu mangroves, North East coastal Andhra Pradesh. High concentrations of Ca was found in the shells of Achatinaa chatina, Turritella sp., Cardium edule, Ampullela sp., and Spondylus spinosus [68]. Darwin and Padmavathi [69], recorded 912±31.21mg CaCO3/g in the shells of Meretrix meretrix collected from Tamilnadu coast, India.
The amount of Fe was found between 0.07-0.99mg/100g (Table 1). The Iron (Fe) content was found to be highest in post-monsoon (0.99mg/100g) and the lowest in monsoon season (0.070mg/100g). The average value was estimated 0.083 mg/100g for Fe (Table 1). Zakaria et al. [70], recorded a small amount of Iron in the hard clam shells collected from several stalls in the wet market located in Selangor and Peninsular Malaysia. Böhlmark [71], reported elevated levels of Fe in Meretrix meretrix at Villa do Pescadores, Maputo Bay.
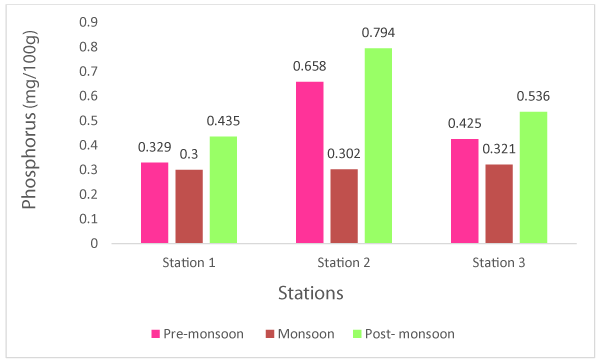
Phosphorus values fluctuated between 0.300-0.794 mg/100g (Table 1). The highest amount (0.794mg/100g) was found in the post-monsoon season while the minimum concentration (0.300mg/100g) was recorded in monsoon in wet mussel (Figure 5). The average 0.455mg/100g of phosphorus was documented in Meretrix meretrix. Zakaria et al. [70], reported 0.05% phosphorus in hard clam shells collected from several stalls in the wet market located in, Selangor, Peninsular Malaysia.
Conclusion
Meretrix meretrix known as Asiatic hard clam used mainly for marine food and a valuable source of traditional medicine. It is widely distributed in the estuarine and coastal ecosystems of South and Southeast Asia, including China, Korea, Japan, and India. In the present study, Meretrix meretrix was recorded in the Bakkhali river estuary. Biochemical compositions were found in good concentrations in Meretrix meretrix. The compositions were varied spatiotemporally in the present study. It is important to study further since it is traditionally used as food by the coastal community.
- Babu A, Venkatesan V, Rajagopal S (2012) Biochemical composition of different body parts of Gafrarium tumidum (Roding, 1798) from Mandapam, South East Coast of India. Afr J Biotechnol 11: 1700-1704. Link: http://bit.ly/2m4HGdL
- Jagadis I (2005) Biology of an intertidal clam Gafrarium tumidum (Roding) from south east coast of India. Ph.D, thesis, Annamalai University 155.
- Jia W, Peng Q, Su L, Yu X, Ma CW (2018) Novel Bioactive Peptides from Meretrix meretrix Protect Caenorhabditis elegans against Free Radical-Induced Oxidative Stress through the Stress Response Factor DAF-16/FOXO. Mar Drugs 16: 444. Link: http://bit.ly/2k7dwWN
- Dincer T (2006) Differences of Turkish clam (Ruditapes decussates) and Manila clam (R. philippinarum) according to their proximate composition and heavy metal contents. J Shellfish Res 25: 455-459. Link: http://bit.ly/2lZkvBw
- George C, Gopakumar K (1995) Biochemical and microbiological studies on Clam Villorita cyprinoides. J Mar Biol Assoc India 37: 27-30.
- Wenne R, Styczyska-Jurewicz E (1987) Gross biochemical composition of the bivalve Macoma balthica from the Gulf of Gdarisk (Southern Baltic). Mar Biol 96: 73-78.
- Lakshmanan PT, Nambisan PNK (1981) Biochemical composition of the bivalve molluscs Villorita cyprinoides var. cochinensis (Hanley) and Meretrix casta (Chemnitz). Indian J Mar Sci 9: 63-67. Link: http://bit.ly/2lDN4EG
- Dong FM (2001) The nutritional value of shellfish. 1-4. Link: http://bit.ly/2k74CbH
- Krzynowek J, D'entremont DL, Murphy J (1989) Proximate composition and fatty acid and cholesterol content of squid Loligo pealei and Illex illecebrosus. J Food Sci 54: 45-48. Link: http://bit.ly/2m7gFXf
- Ramakrishnan S, Venkat Rao S (1995) Nutritional Biochemistry. TR Publication, Chennai, India.
- Nagabhushanam R, Mane UH (1978) Seasonal variations in the biochemical composition of Mytilus viridis at Ratnagiri, on west coast of India. Hydrobiologia 57: 69-72. Link: http://bit.ly/2m89Rc1
- Sawant PP, Mohite SA (2013) Study of proximate composition of Meretrix meretrix (Linnaeus, 1758) of the Ratnagiri coast, Maharashtra, India. Biosci Biotech Res Asia 10: 311-317. Link: http://bit.ly/2kCatWD
- Ngo TTT, Kang S, Kang D, Sorgeloos P, Choi K (2006) Effect of culture depth on the proximate composition and reproduction of the Pacific oyster, Crassostrea gigas from Gosung Bay, Korea. Aquaculture 253: 712-720. Link: http://bit.ly/2lCk8g9
- Litaay M, De Silva SS (2003) Spawning season, fecundity and proximate composition of the gonads of wild-caught blacklip abalone (Haliotis rubra) from Port Fairy waters, south-eastern Australia. Aquatic Living Resource 16: 353-361. Link: http://bit.ly/2kAb3nW
- Rivonker CU, Parulekar AH (1995) Proximate biochemical composition and calorific potential in the raft grown green mussel Perna viridis. J Mar Biol Assoc India 37: 231-236. Link: http://bit.ly/2kzpRTP
- Qasim SZ, Parulekar AH, Harikantra SN, Ansari ZA, Nair A (1977) Aquaculture of green mussel Mytilus viridis L. cultivation on ropes from floating rafts. Indian J Marine Sci 6: 18-25. Link: http://bit.ly/2kaw3S4
- Durve VS (1964) Preliminary observation on the seasonal gonadal changes and spawning in the clam Meretrix casta (Chemnitz) from the marine fish farm. J Mar Bio Assoc India 6: 241-248. Link: http://bit.ly/2kCxuJd
- Tanaka S, Hatano H (1952) Studies on the seasonal changes in the chemical constituents of the pearl oyster. Publications of the Seto Marine Biological Laboratory 2: 341-355. Link: http://bit.ly/2lJrXko
- Joshi MC, Bal DV (1965) Observations on the chemical composition of the clam Katelysia mermorata Lamark. J Zool Soc India 17: 108-113.
- Durve VS, Bal DV (1961) Studies on the chemical composition of the oyster, Crassostrea gryphoides. J Zool Soc India 13: 70-77.
- Venkataraman R, Chari SDT (1951) Studies on oysters and clams-biochemical variations. Indian J Med Res 39: 533-541. Link: http://bit.ly/2lDQo2C
- Pease HD (1932) The oyster-Modern science comes to the support of an ancient food. J Chem Educ 9: 1674. Link: http://bit.ly/2lZoKwW
- Giese AC (1969) A new approach to the biochemical composition of the molluscs body. Oceanogr Mar Biol Ann Rev 7: 175-229.
- Arun AU (2017) Seasonal Variation in Biochemical Composition of Black Clam (Villorita cyprinoides) in Cochin Estuary with Special Emphasis on the Impact of Thanneermukkom Bund. Int J Sci Res 6: 1496-1508. Link: http://bit.ly/2kzwXaM
- Eswar A, Nanda RK, Ramamoorthy K, Isha Z, Gokulakrishnan S (2016) Biochemical Composition and Preliminary Qualitative Analysis of Marine Clam Gafrarium divaricatum (Gmelin) From Mumbai, West Coast of India. Asian J Biomed Pharm Sci 6: 01-06. Link: http://bit.ly/2m4M9x3
- Kamble SP, Muley DV (2015) Studies on some Biochemical Composition of Estuarine Clam, Meretrix Meretrix from Ratnagiri Coast, Maharashtra. Appl Zool 84: 33536-33538.
- Laxmilatha (2009) Proximate composition of the surf clam Mactra violacea (Gmelin 1791). Indian J Fish 56: 147-150. Link: http://bit.ly/2m1D8op
- Appukuttan KK, Arvindan CM (1995) Studies on the biochemical composition of the short neck clam, Paphia malabarica from Ashtamudi estuary, southwest coast of India. Seafood Export J 26: 17-21. Link: http://bit.ly/2lEdWV2
- Balasubramanyan K, Natarajan R (1988) Seasonal variations in the biochemical composition of Meretrix casta (chemnitz) in velar estuary. CMFRI Bulletin 42: 184 -188. Link: http://bit.ly/2lGhwOi
- Jayabal R, Kalyani M (1986) Age and growth of the estuarine clam Meretrix meretrix (L) inhabiting the Vellar estuary. Mahasagar 19: 141-146.
- Salih KYM (1979) Studies on biochemical composition of the clam Meretrix casta (Chemnitz) off Cochin bar mouth. Cochin University Bulletin of Marine Science 47-73.
- Nagabhushanam R, Deshmukh RS (1974) Seasonal changes in body components indices and chemical composition in the estuarine clam Meretrix meretrix (L). Indian J Fish 21: 531-542.
- Nagarkar S, Williams GA (1999) Spatial and temporal variation of cyanobacteria-dominated epilithic communities on a tropical shore in Hong Kong. Phycologia 38: 385-393. Link: http://bit.ly/2lLjsF7
- Hawk PB, Oser BL, Summerson WH (1954) Practical physiological chemistry. J Am Pharm Assoc (Scientific ed.). The Blakiston Co., Inc. New York, 13th ed 1439.
- Kemp PA, Heijningen AJMVK (1954) A colorimetric micro-method for the determination of glycogen in tissue. Biochem J 56: 646-648. Link: http://bit.ly/2m2MYq8
- Crampton EW, Harris LE (1969) Applied Animal Nutrition (2nd Edn). San Francisco: WH Freeman 753.
- Mitchell HL (1972) Microdetermination of nitrogen in plant tissues. J Assoc Official Agricult Chem 55: l-3.
- Pearson D (1976) The Chemical Analysis of Foods. 7th Edn. Churchill Livingstone, Edinburgh, London, UK 575. Link: http://bit.ly/2lDSM9A
- Maynard AJ (1970) Method in Food Analysis. Academic Press, New York, USA 176.
- Jacobs MB (1973) The Chemical Analysis of Foods and Food Products. 3rd Edn Krieger Publ, New York, USA 970.
- Lovell RT (1975) Laboratory manual for fish feed analysis and fish nutrition studies. Department of Fisheries and Allied Aquacultures, International Center for Aquaculture, Auburn University, Alabama, USA 1-63.
- McLachlan A, Lombard HW (1980) Seasonal variation in energy and biochemical components of an edible gastropod, Turbo sarmaticus (turbinidae). Aquaculture 19: 117-125. Link: http://bit.ly/2lM1kej
- Nagabhushnam R, Dhamane KP (1977) Seasonal variations in biochemical constituents of the clam, Paphia laterisulca. Hydrobiologia 54: 209. Link: http://bit.ly/2m2OzME
- Durve VS, Bal DV (1986) Studies on the chemical composition of the oyster Crassostrea gryphoides. J Zool Soc India 13: 70-73.
- Baker SM, Hornbach DJ (2001) Seasonal metabolism and biochemical composition of two unionid mussels, Actinonaias ligamentina and Amblema plicata. J Molluscan Stud 67: 407-416. Link: http://bit.ly/2m0eXXt
- Holland DL (1978) Lipid reserves and energy metabolism in the larva of benthic marine invertebrates. Biochemical and Biophysical Perspectives in Mar Biol 4: 85-123.
- Yan HW, Li Q, Liu WG, Yu RH, Kong LF (2010a) Seasonal changes in reproductive activity and biochemical composition of the razor clam Sinonovacula constricta (Lamarck 1818). Mar Biol Res 6: 78-88. Link: http://bit.ly/2lEgXok
- Ke QZ, Li Q (2012) Annual dynamics of glycogen, lipids, and proteins during the reproductive cycle of the surf clam Mactra veneriformis from the north coast of Shandong Peninsular, China. Invertebr Reprod Dev 57: 49-60. Link: http://bit.ly/2m2Plcw
- Takashi K, Mori K (1971) Seasonal variations in the metabolism of lipids and glycogen in the scallop, Patinopecten yessoensis. Tohoku J agricult res 22: 114-133.
- Gabbott PA (1975) Storage cycles in marine bivalve molluscs: A hypothesis concerning the relationship between glycogen metabolism and gametogenesis. Proc. 9th Europ. Mar. Biol. Symp. H. Barnes Ed. Aberdeen University Press 191-211.
- Gabbott PA (1976) Energy metabolism. Inc. Marine mussels, their ecology and physiology. Ed. by Bayne, Cambridge: Cambridge University Press 94-355.
- Dare PJ (1976) Settlement growth and production of the mussel. Mytilus edulis L. in Vlorecambe Bay England. Fish Invest Land Ser II. 28: 1-25. Link: http://bit.ly/2kpVsYd
- Drzycimski I (1961) Nutrition, chemical composition and possibilities for utilization of the sea mussel, Mytilus edulis L., from the southern Baltic. Annals Biol 18: 209-210. Link: http://bit.ly/2lHlsyj
- Fatima M, Qasem R, Bahkatti S (1986) Variation in biochemical composition of the green mussel, Perna viridis Linnaeus from the northern Arabian Sea. Pak J Sci Med Res 29: 30-34.
- Walne PR (1972) The influence of current speed, body size and water temperature on the filtration rate of five species of bivalves. J Mar Biol Assoc United Kingdom 52: 345-374. Link: http://bit.ly/2k7qBzg
- Deshmukh RS (1972) Some aspects of the biology of Meretrix meretrix Ph.D. thesis, Marathwada University, Aurangabad, India.
- Nagabhushanam R, Mane UH (1975) Reproduction and breeding of the clam Katelysia opima in the Kalbadevi estuary at Ratnagiri, west coast of India. Indian J Mar Sci 4: 86-92. Link: http://bit.ly/2m6rBnZ
- Maqbool TK (1993) Studies on the biology of the clam Marcia opima (Gmelin) from Kayamkulam Lake. Ph.D. Thesis, Cochin Univ. Sci. Tech., Cochin, India.
- Ansell AD (1974) Seasonal changes in biochemical composition of the bivalve Nucula sulcata from the Clyde Sea Area. Marine Biol 25: 101-108. Link: http://bit.ly/2kCDIJ5
- Ansell AD (1974) Seasonal changes in biochemical composition of the bivalve Lima trians from the Clyde sea area. Mar Biol 27: 115-122.
- Nagabhushanam R, Talikhedkar PM (1977) Reproductive biology of the wedge clam Donax cuneatus. Indian J Mar Sci 6: 35-38. Link: http://bit.ly/2lK3efu
- Sunila G (1998) Studies on the Biology of the wedge clam Donax incarnates (Gmelin) from the Malippuram beach of Kerala. Ph.D. thesis submitted to Cochin University of Science and Technology.
- Giese AC, Hart MA, Smith AM, Cheung MA (1967) Seasonal change in body component indices and chemical composition in the Pismo clam Tivela stultosum. Comp Biochem Physiol 22: 549-561.
- Lakshmanan PT, Nambisan PNK (1980) Biochemical composition of the bivalve molluscs, Villorita cyprinoides var. cochinensis (Hanley) and Meretrix casta (Chemnitz). Indian J MarSci 9: 65-67. Link: http://bit.ly/2lDN4EG
- Katticaran CM (1988) Studies on the biology of the clam Sunetta scripta (Linne), from the sub tidal waters of Cochin. PhD Thesis, Cochin University of science and technology, Cochin Link: http://bit.ly/2lK8G1U
- Ansell AD (1973) Oxygen Consumption by the Bivalve Donax vittatus (da Costa). J Experimental Marine Biol Ecol 11: 311-328. Link: http://bit.ly/2kAjn7a
- Ranjan TJU, Babu KR (2015) Evaluation of calcium in some commercially important Molluscan shells of Bhavanapadu mangroves, North East coastal Andhra Pradesh. Int J Sciand Appl Res 2: 27-32. Link: http://bit.ly/2m6sOf1
- Effiong GS, Ibia TO, Ogban PI, Inyang ND (2009) Evaluation of Locally-Sourced Liming Materials for Acid Soils in AkwaIbom State, Southeastern Nigeria. American Eurasian Journal of Agronomy 2: 144-151. Link: http://bit.ly/2kchb5K
- Darwin Ch, Padmavathi P (2018) Preliminary assessment of calcium in six molluscan shells of Tamilnadu coast, India. Ecology Environ Conserv 24: 302-305. Link: http://bit.ly/2m7DTfS
- Zakaria ZAB, Zakaria N, Kasim Z (2004) Mineral Composition of the Cockle (Anadara granosa) Shells, Hard Clamp (Meretrix meretrix) Shells and Corals (Pontes spp.): A Comparative Study. J Animal Vet Adv 3: 445-447. Link: http://bit.ly/2k5ecMc
- Böhlmark J (2003) Meretrix meretrix as an Indicator of Heavy Metal Contamination in Maputo Bay. A Theses Work at Uppsala University School of Engineering Program for Aquatic Environ Eng Link: http://bit.ly/2k7sVX0
- Kang JH, Zheng GX, Fan CH (2008) Analysis of Components in Meretrix meretrix Peptides. J Xiamen University (in Chinese) 47: 135-137.
- Li XY, Dong ZG, Yan BL (2010) Analysis and Evaluation of Nutritional Components in Cyclinasinensis and Meretrix meretrix. Food Sci (in Chinese) 31: 366-70.
- Yang J, Tao NP, Wang XC (2007) Nutritional composition of Meretrix meretrix and effect on Flavor. Food Nutr in China (in Chinese) 5: 43-45.
- Zhang B, Wu WT, Wu LJ (2006) Studies on Stability and Anticancer Activities of Meretrix meretrix Glycopeptide MGP0405. Pharm Biotechnol 13: 24-27.
- Yan HW, Li Q, Yu RH, Kong LF (2010b) Seasonal variations in biochemical composition and reproductive activity of venus clam Cyclina sinensis (Gmelin) from the Yellow River Delta in northern China in relation to environmental factors. J Shellfish Res 29: 91-99. Link: http://bit.ly/2knkib7
- Jayabal R, Kalyani M (1986) Biochemical studies in the hard clam Meretrix meretrix (L) from Vellar estuary, east coast of India. Indian J Mar Sci 5: 63-64. Link: http://bit.ly/2m4TkoZ
- Alagarrswami K, Narasimham KA (1973) Clam, Cockle and oyster resources of the Indian coasts. In: Proceedings of the Symposium on the Living Resources of the seas around India. Central Marine Fisheries Research Institute, Special Publication, Cochin, India: 648-658.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
