Annals of Molecular and Genetic Medicine
Influence of Concentration on Surface Tension & Viscosity of Tamarind (Tamarindus Indica) Seed Gum
Sunita Thakur*, Pramod Kumar Sharma and Rishabha Malviya
Cite this as
Sunita T, Sharma PK, Malviya R (2017) Influence of Concentration on Surface Tension & Viscosity of Tamarind (Tamarindus Indica) Seed Gum. Ann Mol Genet Med 1(1): 008-012. DOI: 10.17352/amgm.000002The influence of concentration (0.125-1%w/v) on viscosity and surface tension of tamarind gum has been studied. This research establishes a direct relationship between concentration and viscosity. The intrinsic viscosity was found out to be 0.696dl/g. The value of the Huggins constant was about 0.32 indicates the presence of molecular association. Huggins parameter ‘b’ (5.042) indicates that tamarind gum has random coil confirmation. A Kraemer constant of 0.180 gives information about the molecular association between gum molecules. Power law model has been used for predicting the molecular confirmation. A coil overlaps parameter of 0.4484 is the indication of presence of molecular entanglement. Their Values indicates polymer chain structure which are coiled & interpenetrating & overlapping with one another. The present investigation shows that surface tension decreases with an increase in the concentration of gum in solution. From these findings, it can be concluded that the gum molecules have a large interaction with more liquid than present in the air.
Introduction
Viscosity is a quantitative property of a fluid. Resistance to the flow of a fluid is termed as viscosity. The S.I unit of viscosity is the poise (P); dyne-sec/cm2or g/cm-s [1]. The tendency of fluid to acquire the least surface area is termed as surface tension. The S.I unit of surface tension is force/length or energy/area. Importance of surface tension of liquids is to tell about the requirement of free energy per unit area for forming interface of liquid-air at constant composition, pressure and temperature. On the other hand, viscosity determination of liquids mainly characterizes its resistance to flow. In the present work, natural polysaccharides have been used for developing controlled as well as sustained release formulations. The naturally obtained polymers are in the form of macromolecules. The natural gum has a great utilization in reducing environmentally related pollution as they are easily disposed. The main features possessed by natural gums are their recycling capacity, nontoxicity, biodegradability and biocompatibility. There is a tendency of natural gums to swell when placed in an aqueous medium [2].
The Tamarindus Indica L, (Tamarind) a member of the family Leguminosae (Fabaceae), is native to dry savanna of tropical Africa [3]. A rich source of tamarind seed powder is tamarind. Commonly tamarind is known as imli. Tamarid gum was obtained from the seed of Tamarind indica [4]. Xyloglucan is the major structure found in tamarind seeds. Broad pH tolerance, gelling capacity, high viscosity and great adhesion are all properties possessed by tamarind gum [5]. These properties help in understanding solution preparations, fundamental and structural organization. These properties have key role in predicting the heat transfer phenomenon in fluid products. These products are easily getting affected by the dispersion state, its concentration, and temperature [6]. Information regarding processing effects, effects of aging dealing with stability and changes in formulation can be predicted by measuring rheological parameters. For tamarind gum, the rheological properties serve as applicability factor in the formulation of various dosage forms in the pharmaceutical industry [7].
Material and Methods
Tamarind seed was procured from a local market in New Delhi. Distilled water has been taken as a reference to compare the effect of mixing in the estimation of viscosity. It is given that at 25˚C, 0.809 centipoise is the viscosity of water [1]. Solution of TSP prepared by dissolving required amount of TSP powder in a specified volume of distilled water.
Experimentation
Seeds of tamarind were taken. The outer coverings of seed were peeled off in such a way that the only white portion of the tamarind seeds observed. These should be crushed properly in order to get a fine powder. Crushed seeds were placed in water (24 hr) for soaking purposes. Soaked seeds were filtered through a muclin cloth to get gum. Marc was discarded and subject for multiple extraction with increasing volume of water. The formed gum was washed with ethyl alcohol (in equal amount) to precipitate the same. Further filtration was performed with ethyl alcohol. Extraction was continued in repeated manner untill the material gets free from gum. The obtained gum was subjected to drying process. Drying was done at temperature 400C. After drying, the gum was powdered and passed through sieve. Dried gum was stored at room temperature in air tight containers [8].
Influence of concentration on viscosity
The intrinsic viscosity is a measure of the hydrodynamic volume which is occupied by the macromolecule related closely to the confirmation and size of chains of macromolecule in its specific solvent system. It facilitates measurement of size as well as confirmation of chains of macromolecules [9]. When c approaches 0, reduced viscosity ɳsp/c becomes ‘ɳ ‘. Then expression can be represented as Huggins equation [10].
ɳred = [ɳ] +kH[ɳ]2 C ……Equation 1
Where, ɳred is the reduced viscosity; [ɳ] is the intrinsic viscosity and C is the intercept and kH is the intrinsic viscosity
Huggins equation can be determined through following equation:
kH [ɳ]2 = C ……Equation 2
Graphical relationship between ln relative viscosity and concentration estimated on extrapolation of line to zero concentration [11]. It gives ln intrinsic viscosity, determined through the following equation-
Ln ɳrel/c = [ɳ] +kk [ɳ]2 C ……Equation 3
Where, Ln ɳrel is the log value of relative viscosity; kk is the Kraemer constant; [ɳ]is the intrinsic viscosity; C is the intercept and c is the concentration.
Other viscosities can also be estimated such as the relative viscosity; the specific viscosity; the inherent viscosity; and the reduced viscosity
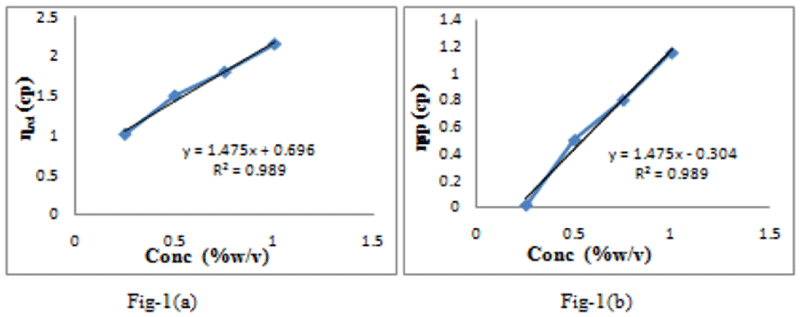
a. The relative viscosity is the ratio of the viscosity of a solution to the viscosity of solvent used .It can be determined by using the eqn 4. Figure 2a shows the
ɳrel=ɳ solution/ ɳ solvent … Equation 4
b. Specific viscosity can be expressed as the ratio of absolute viscosity of fluid that of a reference. It can also be determined by:
ɳsp =ɳrel -1 ..…Equation 5
c. The inherent viscosity is the ratio of the natural logarithm of the relative viscosity to the mass concentration of gum.
ɳinh= (lnɳr)/c ….Equation 6
d. The reduced viscosity is equal to the ratio of relative viscosity increment to the mass concentration of gum. The S.I unit of reduced viscosity is kg/(s.m).
ɳred =ɳrel-1 ..….Equation 7
A power law equation can be used to explain the polymer interactions as shown by equation 8
ɳsp= aCb .….Equation 8
This equation can be used to estimate the ‘b’ value from the slope of a double logarithm plot of ɳsp against concentrations. It provides information regarding polysaccharide confirmation [10]. The Huggins equation expresses ɳsp as a function of concentration of polymer in the following equation-
ɳsp/c= [ɳ]+Bc …Equation 9
b=kH[ɳ]2 .…Equation 10
Where, b and k are Huggins parameters and [ɳ] is intrinsic viscosity respectively.
The molecular conformation and the coil overlap can be estimated via the power law model equation shown in the following equation has greater utility in studying molecular interactions are in the solution of polymer and its confirmation
lnɳrel/c = [ɳ] +kk [ɳ]2 c …Equation 11
On simplification, equation 4, can be represented as -
ɳsp=aCb …Equation 12
Where ‘a’ and ‘b’ are the power law constants; kk is the Kreamer constant; lnɳrel is the logarithm form of relative viscosity and ‘c’ is the concentration of sample respectively.
lnɳsp =lna+blnc ....Equation 13
The surface tension is another important parameter to be considered during formulation of better products. The chemistry which deals with surface properties, interface mechanism between two phases. The chemistry deals with chemical processes at the interface between two phases [12]. The surface tension of gum solution determined using a Stalagmometer and formula:
ɳ1/ɳ2=n1d1/n2d2 …...Equation 14
Where, ɳ1is the surface tension of water; ɳ2 is the surface tension of sample; n1 is the no.of drops of water; n2=no.of drops of sample solution; d1 and d2 are density of water and sample respectively.
Result and Discussion
Rheology involves Concentration -dependent viscosity determination. To predict material’s quality, viscosity determination is necessary. It serves as main tool to establish rheological behavior of products [9]. Viscometers are used to determine viscosity. Viscosity is determined through Ostwald viscometer. It has been shown in following figure 1a that increase in relative viscosity of gum is observed, as we increase the concentration of the gum. It is due to increment at internal resistance flow of gum and an exertion of intermolecular friction between fluids layers which are slide over one another. Observed values gives direct relationship between viscosity with concentration while inverse relationship between concentration and the surface tension. The intrinsic viscosity can be estimated from intercept Figure 1a and was found to be 0.696dl/g. The value of Huggins constant is 0.32.Kraemer constant estimated from equation-3 shown in figure 1b. The value observed is about 0.180 and gives information about molecular association between gum molecules. In a theta solvent, the value of ‘k’ is between 0.5 to 0.7 ranges [13]. The intrinsic viscosity can also be determined from the Kraemer equation, gives the line between ln relative viscosity and concentration and fitting to kraemer equation. This equation provides the information regarding molecular association between gum molecules [14]. A Figure 1a shown below for gum reveals the extent degree of linearity in graph (R2=0.989). The intrinsic viscosity can also be estimated from intercept. The intercept from figure 1a is found to be 0.696dl/g. The KH value can be calculated from equation 10. The value of kH observed is 0.32 in a theta solvent, kH value should lies in between the range of 0.3-0.8 [15]. On putting values in above equation we get kk[ɳ]2=0.17,On calculating ,the Kraemer constant is found to be 0.180. The sum of Huggins and Kraemer constant should be below 0.5%+10% and signifies an absence of molecular association. The Kraemer equation gives the information regarding molecular association between gums molecules. Hence tamarind gum has molecular association between gum molecules.
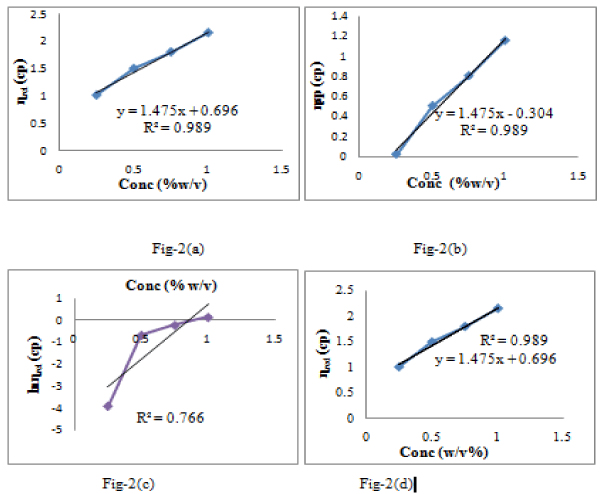
The relative viscosity; specific viscosity; inherent viscosity; and reduced viscosity were determined by using equation 4,5,6,7 and their plots are shown via figure 2a-d. Intrinsic viscosity also involves Huggins parameters such as ‘b’ value which is found to be 5.042. This value indicates a random coil confirmation for tamarind gum. Calculated value of critical overlap concentration is found to be 0.4484 and represents the presence of molecular entalgment in gum.
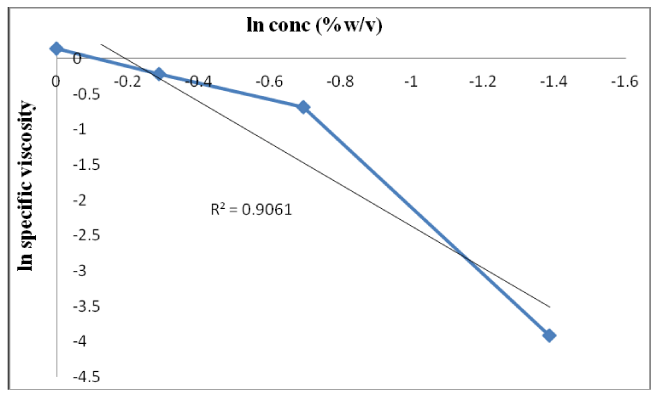
After getting the equation 13, a graph has been plotted in between ln(ɳsp) vs concentration which gives intercept equals to ln(a) as well as slope b. Equation for above graph is given by equation 13 and shown in figure 3. A good correlation is exhibited ie.R2=0.766 and the value of ‘b’ is found to be 5.042. The value ‘b’ designate index for polysaccharides conformation [16]. Tamarind gum contains polymers which get coiled & started interpenetrating and overlapping to one another. This phenomenon is observed when we increase the concentration [17]. This confirms that tamarind gum contains molecular confirmation. The coil overlap property is also exhibited by the gum. If ‘b’ >1= Random coil confirmation; b’ <1 is in rod like confirmation. The observed value of ‘b’ is found to be greater than unity, i.e.1 hence, the gum have random coil confirmation.
Effect of concentration dependent viscosity on molecular confirmation
A marked alteration in the concentration dependent viscosity occurs due to a transition from dilute to concentrated polymeric solution. The corresponding concentration is known as C* i.e. the critical or coil overlap concentration. The S.I unit of C* is g/dl. Concentration which corresponds to change in confirmation of polymer termed as critical concentration [C*]. Critical concentration for coil can be determined through interpretation from figure 3 [18]. At zero shear rate, the concentration dependence of specific viscosity on parameter C*.For tamarind, C[ɳ] is of 0.4484 indicating the presence of molecular entanglement. From this value, it can be predicted that gum has polymer which is coiled and started interpenetrating & overlapping to one another. Hence, it confirms that gum contains molecular confirmation.
Influence of concentration on surface tension
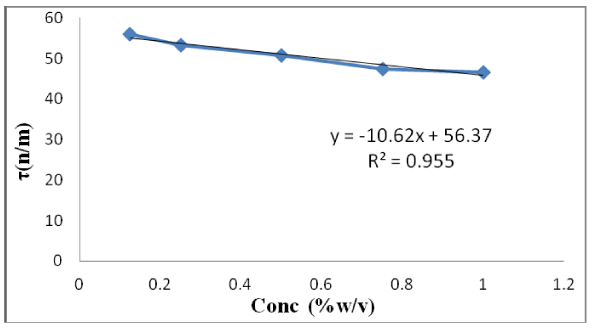
Stalagmometer filled with distilled water up to the mark. The number of bubble drops counted flowing between marked volumes of stalagmometer. Three readings were taken. The average is also calculated to minimize error. Stalagmometer washed with sample gum solution. Same procedure has been repeated for all sample concentration. Using equation -14, the surface tension can be estimated. Density was determined by R.D bottle and is expressed in g/ml unit. It was found that on increasing the concentration, the surface tension gets a decrease which is shown in figure 4. It has been observed that as we increase the concentration of the gum solution from 0.125, 0.25% ,0.5%, 0.75%to 1%,the surface tension decreases from 55.91,53.22,50.75,47.47,46.62. The surface tension (τ) of 1% solution is comparatively lower than 0.75%, 0.5%, 0.25% and 0.125%.
The surface tension can be determined from equation 14. The graph is plotted between surface tension (τ) and concentration (conc.) as shown in figure 4. The observed values shows that surface tension is inversely proportional to the concentration as shown in figure 4. Hence, study shows that surface tension decreases as we increase the concentration of gum solution. This occurs due to larger interaction between molecules of liquid than molecules present in air or in non-polar solvents. The cohesion forces of liquid molecule are greater than adhesion force of air molecules and leads to inward force at the surface. Tension exerted on the surface due to presence of the imbalanced force. Hence surface tension phenomena occurs [19].
Conclusions
The present investigation has shown that tamarind gum is precipitated in ethyl alcohol and extracted with water efficiently. Rheological properties have been determined by using Huggins model, Arrhenius model, Kraemer model and Power law models. The result obtained from the present study lead one to the conclusion that the gum has random coil confirmation and molecular entanglement. Values computed from these studies predict the influence of concentration establishes a direct relationship with viscosity. The observed value shows that surface tension gets decreases with increase in concentration of gum solution.
The Authors are immensely thankful to the faculty’s members and Department of pharmacy, School of Medical and Allied Sciences, Galgotias University, Greater Noida for providing support, library and computer facilities and inspiring me for research work.
- Halpern AM, Reeves JH (1999) Experimental Physical Chemistry, 1 st ed., Scottland Foresman 2:198-108. Link: https://goo.gl/XUhTXn
- Gwen MJ, Joseph RR, Rhodes CT (1996) Modern pharmaceutics, marcel Dekker, inc: New York 58-59. Link: https://goo.gl/GI2VqE
- Mohamad HA, Mohamad BE, Ahmed KE (2015) Physicochemical properties of tamarind seed polysaccharide. J Food Process Technol 6: 1-5. Link: https://goo.gl/4yOQTN
- Chandramauli Y, Firoz B, Vikram A, Mahitha B, Rubia yasmeen B, et al. (2012) Tamarind seed polysaccharide-an adaptable excipient for novel drug delivery system. International Journal of Pharmacy Practice & Drug Research 2: 57-63. Link: https://goo.gl/aqvS6Z
- Takahashi Y, Takeda C, Sate I, Marinda Y (2007) Formulation and evaluation of lacteferrin bioadhesive tablets. Int J Pharm 343: 220-227. Link: https://goo.gl/dTxt66
- Deveswaran R (2009) Design and characterization of Diclofenac sodium tablets containing tamarind seed polysaccharide as release retardant. International Journal of Pharm Tech Research 1: 191-195. Link: https://goo.gl/NamSHw
- Rolando M, Valente C (2007) Establishing the tolerability and performance of tamarind seed polysaccharide in treating dry eye syndrome: results of clinical study. BMC Ophthalmol 7: 5. Link: https://goo.gl/anrKux
- Malviya R, Srivastav P, Bansal M, Sharma PK, (2010) Formulation evaluation and comparison of sustained release matrix tablets of Diclofenac sodium using tamarind gum as release modifier. International Journal of Pharma and Bio Sciences 3: 238-241. Link: https://goo.gl/2bKuYY
- Rishabha Malviya, Pramod Kumar Sharma, Susheel Kumar Dubey Antioxidant potential and emulsifying properties of Kheri (Acacia chundra, Family: Mimosaceae) gum polysaccharide. Marma Pharmaceutical Journal (In Press).
- Bhattacharya BS, Mukherjee B S (1991) Kinetics of tamarind seed hydration. Journal of Food Engineering 13: 151-158. Link: https://goo.gl/Rjo6gq
- Sakai T (1968) Huggins constant ‘k’ for flexible chain polymer. Journal Of Polymer Science Part B: Polymer Physics 6: 1535-1549. Link: https://goo.gl/HvXn1K
- Ananita Fathi Azarbaviania, Abolghasem Jouyban, Sui Yang Chan (2009) Impact of surface tension in pharmaceutical sciences. J Pharm Pharm Sci 12: 218-228. Link: https://goo.gl/ltHx3M
- Kumar GKP, Battu G, Koth NS (2011) Isolation and evaluation of tamarind seed polysaccharide being used as a polymer in pharmaceutical dosage forms. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2: 274-290. Link: https://goo.gl/sfT6Ql
- You Q, Yin X, Zhang S, Jiang Z (2014) Extraction purification and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydrate polymers 99: 1-10. Link: https://goo.gl/IDcK4U
- Morris ER, Cutler AN, Ross-murphy SB, and Rees DA (1981) Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydr Polymers 1: 5-21. Link: https://goo.gl/5dfvVT
- Eddy NO, Ameh PO, Gimba PO (2013) Rheological modeling, surface morphology and physicochemical properties of Anogeissusleio carpus gum. Asian Journal of Chemistry 25: 1666-1672. Link: https://goo.gl/ctnLkV
- Ahmed N, Saeed A, Ahad K, Khan MS (1994) Physicochemical, Spectroscopic and Rheological Studies on Eucalyptus citriodora (EC) Gum. Journal of Chemistry Society Pakistan 16: 91. Link: https://goo.gl/lb7rld
- Eddy NO, Ja AM, Usman JNS (2016) Rheological modeling, physicochemical, spectroscopic and rheological characterizations of Trichilia roka (TR) gum exudates. Journal of Chemical, Biological and Physical Sciences 6: 1034-1055. Link: https://goo.gl/nH9p5p
- US Geological Survey, Surface Tension (Water properties)-USGS Water Science school.US Geological survey, Retrived 2 dec.2016.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
