Annals of Limnology and Oceanography
Using the Amphipod Hyale nigra to Assess the Quality of Marine Sediments
Passarelli MC1*, Cesar A2,3 and Abessa DMS4
2Department of Ocean Sciences. Federal University of São Paulo (UNIFESP), Santos, São Paulo, Brazil
3Department of Ecotoxicology. Santa Cecília University (UNISANTA), Santos, São Paulo, Brazil
4Department of Marine Science, State Universiry of São Paulo (UNESP), São Vicente, São Paulo, Brazil
Cite this as
Passarelli MC, Cesar A, Abessa DMS (2017) Using the Amphipod Hyale nigra to Assess the Quality of Marine Sediments. Ann Limnol Oceanogr 2(1): 025-030. DOI: 10.17352/alo.000006Few species are standardized and have been used as test organisms around the world in ecotoxicological assays. In the case of sediment assessment, there are only two amphipod species (Tiburonella viscana and Grandidierella bonnieroides) standardized protocols for toxicity test in South Atlantic region.In order to evaluate the possibility of using the epibenthic marine amphipod Hyale nigra in toxicity tests, a sensitivity test using spiked sediment method was applied, with cadmium chloride as a reference toxicant (CdCl2). Tests with environmental samples were performed to validate the test organisms. The samples were taken from Santos Estuary and Bay in state of Sao Paulo, Brazil. The historical toxicity and contamination are mentioned in the literature. The results obtained in the spiked sediment tests showed little variation in the sensitivity of Hyale nigra amphipod with a mean LC50 value of 5.73 mg.L-1 (1.51 mg.L-1 – 8.75 mg.L-1). In the tests with environmental samples, the results obtained were similar to those found in the literature regarding this sampling area. This study demonstrates the potential of Hyale nigra amphipod as a test organism in toxicity tests, once that the specie is sensitive and its results were similar to those reported in the literature on other species of amphipods that are already used in ecotoxicological studies.
Introduction
Toxicity tests have been used worldwide with different objectives, such as to determine environmental health, establish maximum limits for the discharge of chemicals and effluents, define critical areas, and provide a biological understanding of the chemicals found in the environment and among others goals [1-4]. Most of the purposes cited above require environmental studies (monitoring and assessment) or the establishment of maximum limits for chemical discharge into the environment. Thus, in order to provide proper estimations of the environmental effects or risks resulting from contamination, toxicity tests, preferably, with local species must be conducted.
The use of native species is important, because these species represent the local biota that may be directly exposed to the environmental contamination, and their use thus allows for the extrapolation of the results to the field with more reliability than no-indigenous organisms can provide [5]. However, despite the fact that ecotoxicological tests have been incorporated into environmental studies since the 1990s, few species have been used as test organisms around the world [6-8]. The limited number of species that present standardized protocols for toxicity tests is especially worrisome in Brazil, since the country has an extensive coastline that comprises many different ecosystems and environments, from the equatorial Amazon areas in the North to the temperate and subtropical regions in the South. Therefore, there is a crucial need for new local species to be found — ones that can serve as test organisms in ecotoxicological studies and thus provide reliable information for the protection of marine and coastal ecosystems. The search of new species for ecotoxicological purposes requires knowledge for varied aspects of the organisms, including their biology, reproductive cycles, growth patterns, feeding habits, seasonality, interactions with the environment, and sensitivity to contaminants or mixtures. According to [9], the sensitivity to toxicants plays a key role, since the use of extremely sensitive or resistant species may influence the results and reliability of toxicity tests.
In the case of sediments, the sensitivity of the test organisms can be estimated either through spiked sediment toxicity tests that use known concentrations of reference substances or by exposing the organisms to field-collected sediments that containa range of single or multiple contaminants [10].
Spiked sediment toxicity tests involve the addition of one or more chemicals to clean sediments at different concentrations, followed by the evaluation of the effect that the concentrations have on the exposed organisms [11-13]. This approach has been used to evaluate the sensitivity of test organisms to contaminants [5,14,15], particularly their sensitivity to metals such as cadmium, copper, and zinc [16-18].
In Brazil, the National Environment Council (CONAMA) has requested that ecotoxicological assays be part of studies in order to evaluate the quality of waters, sediments and effluents [19], and also to provide information on the management and disposal of dredged sediments [20]. However, when it comes to sediments, there are ecotoxicological protocols available for only two species of amphipods, two species of benthic copepods, one species of shrimp and one species of tanaid, and certainly this reduced amount is not nearly enough to cover the country’s coastline or its diversity of environments. [21] highlights the need for tests that are ecologically more realistic and which rely on native species. Results obtained from the use of local species may be also important for the development oflocal/regional sediment quality guidelines (SQGs) [13].
This way, the objective of the current study was to evaluate the sensitivity of the native amphipod Hyale nigra to contaminated sediments, as a first step in determining its suitability as a standard test species. The tests considered the species’ exposure to sediments spiked with reference toxicants, and also to field-collected samples with varied levels of contamination.
Materials and Methods
Test-organism
The Hyale nigra specie is a crustacea, amphipod from Hyalidae Family Bulycheva, (1957). These crustaceans are representatives of vagile epifauna and stand out from the other groups for its abundance and wealth specie in macrophyte communities around the world [22-24]. Moreover, these organisms are important source feed to some fish and crustaceans species [25, 26], thus enabling the transfer to contaminants throughout the food chain, for instance, by the biomagnification process.
Collection, selection and acclimation of test organisms
Hyale nigra specimens were collected from stalks of the macroalgae Ulva sp obtained from rocky reefs in Palmas Island (24º0.511’S – 46º19.448’W) Santos Bay - São Paulo, Brazil. The algae stalks were collected at the intertidal zone, conditioned in cool boxes filled with seawater, and transported to the laboratory. The organisms collected were sorted and separated by species. Juveniles and egg-bearing females were excluded. H. nigra specimens were identified using an identification keys [27-28], transferred to aquariums of 1L with seawater, and acclimated for two days to the test conditions (salinity of 35, > 80% O.D and 25°C). Small pieces of nylon mesh (600µm) were used as artificial substrate, following the recommendations set by the Brazilian protocol for toxicity testing with Hyalella spp [29]. During the acclimatization period, the organisms were fed both Ulva lactuca and 1.5ml of concentrated fish food daily (the concentrated fish food was 2g of fish food Tetra-Min® diluted into 80 ml of pond seawater, and stirred until small flakes formed).
Experiment set-up
The sensitivity of H. nigra amphipod was tested on different steps. At first, its sensitivity to different sediment textures (very coarse sand, coarse sand, medium sand, fine sand and very fine sand) was assessed. For it, the control sample was washed, sieved and separated according to the Wentworth scale. The experiments were based on preliminary studies and followed the recommendations established by the ABNT NBR 15470/2007 standard [29], which describes the test conditions for freshwater species. The tests were conducted in 1-L chambers containing 2 cm layer (≈150g) of sediment and 600 ml of seawater. Next, the pieces of nylon mesh were added to all replicates. Three replicates with 10 organisms were used. The experiment lasted 10 days with renewal of the overlying water (2/3) in each 3 days, and gentle aeration and continuous light.
Secondly, the control sediment was spiked with cadmium [30-31].The sediment spiking was prepared via the suspension method, in which 600 ml of saturated solutions of CdCl2 were mixed with 150 g of control sediment; the mixtures were stirred for 4 hours at 130 rpm (rotations per minute) with the help of a magnetic stirrer. After 24 h, the liquid solution was withdrawn from the test bottles. Then the sediments were left to rest for 14 days in order to allow for the stabilization between the sediment and the added metal. Aliquots of the sediments were then separated and sent to a commercial analytical laboratory (Ecolabor – São Paulo; ISO/IEC 17025accredited) in order to measure the concentrations of Cd in the sediments. Five tests were performed by exposing H. nigra to spiked sediment using 4 concentrations of spiked sediment (1 mg, 2 mg, 4 mg and 8 mg Cd/Kg of wet sediment) and four replicates. The tests were carried out with the same conditions described above in the first step experiment. At the beginning and the end of each experiment, the overlying physical-chemical parameters of the water (temperature, salinity, dissolved oxygen, and pH) were measured in order to ensure the acceptability of the tests; the measurements followed standard methods (APHA 1998).
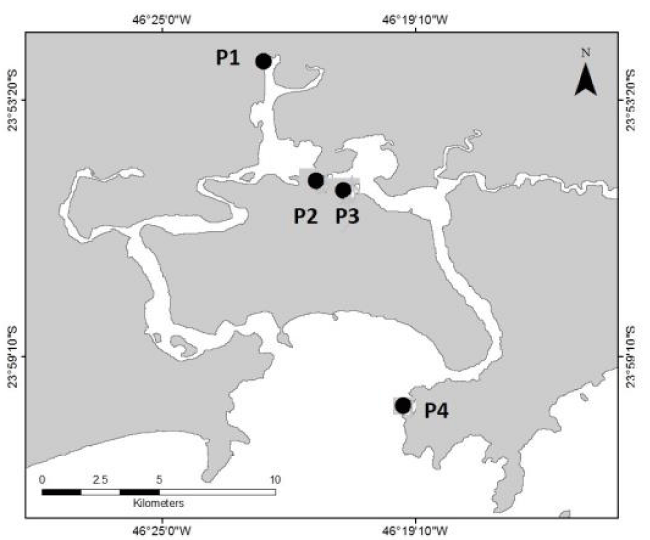
The third experiment step was performed with environmental samples from Santos Estuary and Bay. The establishment of the sampling sites (Figure 1) was based on previous studies [32-36], which showed contamination gradients from the inner portions of the estuary to the external areas. Four sampling sites were collected following [11] recommendations. Two kind of experiment were performed with the environmental samples. The whole sediment toxicity tests were conducted following the international and Brazilian standards for sediment testing [28-30]. The basic description of the tests was previously presented in the description. The sediment samples were also evaluated using liquid phase assays employing sediment elutriate. For it, the sediment samples and seawater (ratio 1:4 v/v) were mixed for 30 minutes. The samples remained stabilized for 1 hour, and the supernatant was separated and used in the ecotoxicological tests [37]. The tests were conducted using 4 replicates per treatment. Each replicated containing 400ml of elutriates. The elutriate tests lasted for 96h. At the end of all experiment step, the organisms were transferred to Petri dishes and examined under a stereomicroscope. The numbers of dead and living organisms were counted. When the final sum did not reach 10, the organisms that were not found were considered dead.
Data Analyses
The results of the toxicity tests were analyzed statistically using TOXSTAT Software, version 3.5. The results of both sediment elutriation and whole sediment were analyzed using ANOVA, followed by Dunnett’s test in order to compare the survival rates to that reference sample (P4). Student’s t-test for independent samples was used to compare the survival rates of the specimens in each sample to their specific control (p=0.05). Prior to the statistical analysis, the data were checked for normality using the chisquare method and for homogeneity of variances using Bartlett’s test. For the tests with cadmium-spiked sediments, the lethal concentrations after 10 days of exposure (10-d LC50) and their respective 95% confidence limits were calculated using the Trimmed Spearman-Karber method [38].
Results and Discussion
The tests with different sediment textures showed survival rates of H. nigra in the sediment control above 80%. The control sample presented a predominance of medium sands. There are no significant differences in the results with different sediment textures when compared to the control (p < 0.05) to H. nigra amphipod. According to [39], sediment grain size distribution has been found to directly influence to test results with amphipods and it may influence their survival rates. In the current study, H. nigra was not presented a survival significantly different from the control (>60%) when exposed to different particle sizes tested being the best result from medium sand.
In relation to cadmium-spiked sediment tests the amphipod H. nigra showed little variation in the results (CV % = 26) and the mean LC50 estimated after 10 days was 5.73 mg Cd/kg (1.51 – 8.75 mg Cd/kg). The data are shown in table 1.
The results showed that H. nigra is highly sensitive to Cd in sediments. This value is close to the LC50value (5.6 mg Cd/kg) obtained by [16] in their study on the freshwater amphipod Hyalella curvispina. When the LC50 values (LC50 10-d value of 5.73 mg Cd/kg, n=5) are compared to the Brazilian sediment quality guidelines for dredged sediments [20], which are 1.2 and 7.2 mg Cd/kg for the threshold and probable effect levels, respectively, it becomes clear that these levels may not completely protect the native biota [40]. Reported that these sediment guidelines did not provide effective protection for marine and estuarine ecosystems, since many toxic samples exhibited concentrations of chemicals below the theoretical threshold levels. These authors also stated these SQGs were established by adopting the values proposed by the North American and Canadian protocols [41, 42] and without performing studies with native species and at the conditions expected to be found in the Brazilian sediments [43]. Showed that the interim sediment quality guidelines (ISQGs) were conservative, since the amphipod Melita plumulosa was not affected in concentrations below the ISQGs. In this sense, both cadmium-spiked sediment tests and tests using other chemicals may provide information that can improve the federal legislation and achieve the effective protection of native biota. Further tests with other aquatic species should be run in order to provide information on the Cd sensitivity of a broad range of organisms.
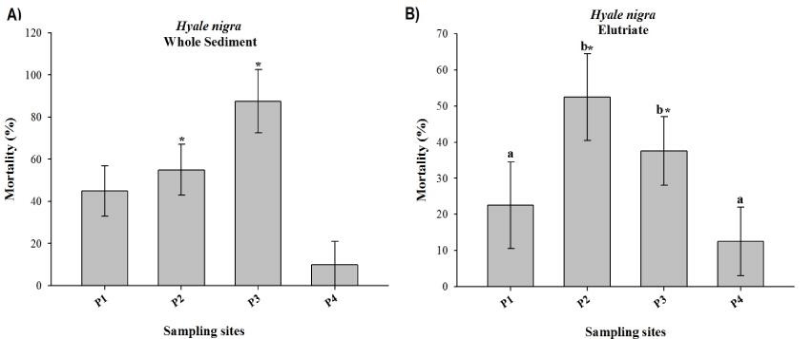
The results obtained in whole sediment and elutriate toxicity tests with H. nigra are shown in figure 2. The sediment grain size distribution presented higher values of silt at P1, P2, and P3; at P4, the highest values were found in medium sand. Out of all of the samples tested, the total concentration of ammonia was highest at P2 (1.47 mgL-1). Organisms exposed to the sediment from P4 (control) presented the highest survival rate, while sediments from P2 and P3 displayed significantly lower survival relative to the reference control.
The Santos Estuarine System is considered a strategic region in Brazil, since it comprises the biggest port of Latin America, an industrial complex, and a large urban area. The SES is also of high ecological importance because it is surrounded by mangroves and a set of protected areas. The region constitutes a refuge and shelter for many species of vertebrates and invertebrates alike.
Although is this study the concentrations of contaminant did not have be quantified, a number of past and present studies have shown that the SES region is ecologically damaged due to the presence of multiple contaminants in the area, some of which have increased in quantity [3,31-35,44]. As previously mentioned in this article, many contamination sources are located within the estuarine portion or in its surroundings. Thus, sediments from the region have been considered contaminated and toxic and present moderate to high levels of chlorine, detergent, ammonia, sulfur, and mercury, among other pollutants.
Sediments from P1, P2 and P3 were considered contaminated and toxic to marine invertebrates (results which were consistent with [32,45], and especially [44]), whereas sediment from P4 was considered only slightly degraded. The aforementioned authors attributed toxicities to metals, PAHs, and detergents. In the current study, sediments from P2 and P3 were toxic relative to the reference sediment (P4). These results are in accordance with these previous studies since the sediment sample from P4 is usually used as a reference site (control site) in the environment studies in this area. Additionally, it is important to highlight that the sediment sample from P4 is located in Santos Bay and receive less influence from the estuary degradation than the others. Moreover, the absence of toxicity in the sediment from P1 may be explained by the fact that the region was dredged a few months before the sampling.
Conslusion
In conclusion, the amphipod Hyale nigra presents a good tolerance to different grain size of sediment and high sensitivity to contaminated sediments. Our result proves that H. nigra is a suitable potential to test-specie in ecotoxicological studies with sediment. Furthermore, because H. nigra presents epibenthic habits, it can also be used to evaluate different means of exposure, as demonstrated in the elutriate tests and whole-sediment tests.
Therefore, H. nigra represents a suitable species for use in toxicity tests with sediments. This species is also advantageous in that it can be used to evaluate different means of exposure. In studies on local issues, this species represents a new option to be considered when toxicity tests are demanded by the Brazilian legislation.
This study was funded by the São Paulo Research Foundation – FAPESP (grant #2011/159248). The first author would like to thank the Erasmus Mundus Program for the doctoral fellowship (2014-0693/001-EMJD). A. Cesar and D. Abessa thank the National Council known as CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, MEC - Brazil) for their respective research productivity fellowships (PQ#305869/2013-2 and PQ#308649/2011-7) and also to the Research Project ECO2Mar (CAPES 126/2012-6).
- Martin-Díaz ML, Franzellitti S, Burati S, Valbonesi P, Capuzzo A, et al. (2009). Effects of environmental concentrations of the antiepilectic drug carbamazepine on biomarkers and cAMP-mediated cell signaling in the mussel Mytilus galloprovincialis. Link: https://goo.gl/H8FmcZ
- Cortez FS, Pereira CDS, Santos AR, Cesar, A, Choueri RB, et al. (2012). Biological effects of environmentally relevant concentrations of the pharmaceutical Triclosan in the marine mussel Perna perna (Linnaeus, 1758). Environmental Pollution 168, 145-150. Link: https://goo.gl/voNPJz
- Cesar A, Lia LRB, Pereira CDS, Santos AR, Cortez FS, Choueri RB, et al. (2014). Environmental assessment of dredged sediment in the major Latin American seaport (Santos, São Paulo - Brazil): An integrated approach. Science of the Total Environment. 497-498, 679-687. Link: https://goo.gl/7JLvhy
- Basallote MD, DelValls TÁ, Riba I (2014) Studying the effect of CO2-Induced acidification on sediment toxicity using acute amphipod toxicity test. Environmental Science Technology 48: 8864-8872. Link: https://goo.gl/oypEh2
- (1992) ASTM American Society for Testing and Materials. Guide for conducting 10-day static sediment toxicity tests with marine and estuarine amphipods. ASTM 1992 Annual Book of Standards Vol. 13.04, E1367 -92. Philadelphia PA. Link: https://goo.gl/hdX1be
- Krull M, Barros F (2012) Key Issues in Aquatic Ecotoxicology in Brazil: A Critical Review. Journal of the Brazilian Society of Ecotoxicology 7: 57-66. Link: https://goo.gl/S8ZoAH
- Martins SE, Bianchini A (2011) Toxicity tests aiming to protect Brazilian aquatic systems: current status and implications for management. Journal of Environmental Monitoring 13: 1866-1875. Link: https://goo.gl/eVDNXC
- VanDam RA, Harford AJ, Houston MA, Hogan AC, Negri AP (2008) Tropical marine toxicity testing in Australia: a review and recommendations. Australasian Journal of Ecotoxicology 14: 55-88. Link: https://goo.gl/y2bPFZ
- Abessa DMS, Sousa ECPM (2003) Sensitivity of the amphipod Tiburonella viscana to K2Cr2O7. Brazilian Archives of Biology and Technology 46: 53-55. Link: https://goo.gl/nSLSFZ
- Lamberson JO, Redmond MS, Jones JKP. 1992. Development of an amphipod sediment toxicity test for Hawaii. US EPA, ERL - N ASCI. Link:
- (2001) USEPA - United States Environmental Protection Agency Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analysis: Technical Manual. EPA-823-B-01-002 US Environmental Protection Agency 208p. Link: https://goo.gl/pQtR94
- (1984) OECD Organization for Economic Co- operation and Development Earthworm acute toxicity tests. Guidelines for the testing of chemicals N° 207. Link: https://goo.gl/fk2YEj
- Simpson SL, King CK (2005) Exposure-pathway models explain causality in whole-sediment toxicity tests. Environmental Science and Technoology 39: 837 - 841. Link: https://goo.gl/BpW5TF
- (1990) Environment Canada Guidance document on control of toxicity test precision using reference toxicants. Report EPS 1/RM/12, Conservation and Protection. Ottawa ON. Link: https://goo.gl/WvG149
- (1995) Environment Canada Guidance document on measurenment of toxicity test precision using control spiked sediment with a reference toxicant. Report EPS 1/RM/5. Environmental Technology Centre. Ottawa ON. Link:
- Giutso A, Somma AL, Ferrari L (2012) Cadmium toxicity assessment in juveniles of the Austral South America amphipod Hyaella curvispina. Ecotoxicology and Environmental Safety 79: 163 -169. Link: https://goo.gl/Nq8AmG
- King CK, Gale SA, Hyne RV, Stauber JL, Simpson SL, et al. (2006) Sensitivities of Australian and New Zealand amphipods to copper and zinc in waters and metal-spiked sediments. Chemosphere 63: 1466 - 1476. Link: https://goo.gl/epnMy5
- Barjhoux I, Baudrimont M, Bénédicte M, Lande L, Gonzalez P, et al. (2012) Effects of copper and cadmium-spikedsediments on embryonic development of Japanese medaka (Oryziaslatipes). Ecotoxicologyand Environmental Safety 79: 272 - 282. Link: https://goo.gl/aR8DHE
- Brasil. Resolução CONAMA No 357.2005. Dispõe sobre a classificação dos corpos de águas e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providencias. Conselho Nacional de Meio Ambiente (CONAMA), Brasília, DF. Link: https://goo.gl/Lh5Tib
- Brasil Resolução CONAMA No 454. 2012. Estabelece as diretrizes gerais e os procedimentos referenciais para o gerenciamento do material a ser dragado em águas sob jurisdição nacional. Conselho Nacional de Meio Ambiente (CONAMA), Brasília, DF. Link: https://goo.gl/e6RmhE
- Nipper M (1998) The development and application of sediment toxicity tests for regulatory purposes. In: Wells PG, Lee K, Blaise C. Microscale Aquatic Toxicology - Advances, Techniques and Pratices. CRC Press Inc Boca Rotan FL 631 - 643. Link: https://goo.gl/StquHP
- Mukai H (1971) The phytal animals on the thalli of Sargassum serratifolium in the Sargassum region, with reference to their seasonal fluctuations. Marine Biology 8: 170-182. Link: https://goo.gl/Jej8kD
- Edgar GJ (1983) The ecology of south-east Tasmanian phytal animal communities II Seasonal change in plant and animal populations. Journal of Experimental Marine Biology and Ecology 70: 159-179. Link: https://goo.gl/3coean
- Gunnill Growth FC (1985) morphology and microherbivore faunas of Pelvetia fastigiata (Phaeophyta, Fucaceae) at La Jolla California USA. Botanica Marina 28: 187-199. Link: https://goo.gl/6ctx6P
- Zamprogno C (1989) Distribuição e hábitos alimentares dos peixes da zona entre - marés de recifes rochosos da praia de Manguinhos, Espírito Santo. Dissertação de Mestrado. Instituto de Biologia. Universidade Estadual de Campinas Campinas SP. Link: https://goo.gl/kZeqRY
- Dubiaski-Silva JO, fital de Sargassum cymosum C (1999) Agardh 1820 (Phaeophyta - Fucales) e seu papel na dieta de peixes e braquiúros na ponta das Garoupas, Bombinhas, Santa Catarina. Curitiba: UFPR Tese de Doutorado. Universidade Federal do Paraná. Curitiba, PR. 1999. Link: https://goo.gl/6kgdNu
- Barnard JL, Karaman GS (1991) The families and genera of marine amphipoda (except marine gammaroids). Record of the Australian Museum Supplement part 1-2: 1-866. Link: https://goo.gl/xzHjNK
- Serejo CS (2001) A new species of amphipod from the Brazilian coast, with redescription of Hyales pinidactyla Chevreux, 1925 (Crustacea, Amphipoda, Hyalidae). Zoosystema 23: 479-492. Link: https://goo.gl/T2Q2pz
- (2007) ABNT NBR 15470 Associação Brasileira de Normas Técnicas. Ecotoxicologia Aquática - Toxicidade em sedimento - Método de ensaio com Hyalellaspp (Amphipoda) São Paulo. Link: https://goo.gl/ZPBECm
- (2008) ABNT NBR 15638 Associação Brasileira de Normas Técnicas Qualidade da água - Determinação da toxicidade aguda de sedimentos marinhos ou estuarino com anfípodes. São Paulo. Link: https://goo.gl/UyyPNQ
- (1994) USEPA - United States Environmental Protection Agency Methods for assessing the toxicity of sediment - associated contaminants with estuarine and marine amphipods. Technical Manual, DC.EPA/600/R-94/025 US Environmental Protection Agency Washington DC. Link: https://goo.gl/qRKSdE
- Lamparelli MC, Costa MP, Prósperi VA, Bevilacqua JE, Araújo RPA, et al. (2001) Sistema estuarino de Santos e São Vicente Relatório Técnico CETESB São Paulo 183p. Link:
- Abessa DMS, Carr RS, Sousa ECPM, Rachid BR, Zaroni LP (2008) Integrative ecotoxicological assessment of a complex tropical estuarine system In Hoffer TN Nova Science Publishers New York 125-159. Link: https://goo.gl/Skg5TU
- Cesar A, Pereira CDS, Santos AR, Abessa DMS, Fernández N, et al. (2006) Ecotoxicological assessment of sediments from the Santos and São Vicente estuarine system - Brazil. Brazilian Journal of Oceanography 54: 55-63. Link: https://goo.gl/1FqZfv
- Cesar A, Choueri RB, Riba I, Moralles-Caselles C, Pereira CDS, et al. (2007) Comparative sediment quality assessment in different littoral ecosystems from Spain (Gulf of Cadiz) and Brazil (Santos and São Vicente estuarine system). Environment International 33: 429-435. Link: https://goo.gl/6QsuXr
- Cesar A, Abessa DMS, Pereira CDS, Choueri RB, DelValls AT (2009) Simple approach to integrate ecotoxicological and chemical data for the establishment of environmental risk levels. Brazilian Archives of Biology and Technology 52: 233-240. Link: https://goo.gl/xHyBu8
- (2012) ABNT NBR 15350 Associação Brasileira de Normas Técnicas. Ecotoxicologia aquática - Toxicidade crônica de curta duração - Método de ensaio com ouriço-do-mar (Echinodermata; Echinoidea). São Paulo. Link: https://goo.gl/R8EHb3
- Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicological bioassays. Envir Sci Technol 11: 714-719. Link: https://goo.gl/vLt3T9
- Melo SLR, Nipper M (2007) Sediment toxicity tests using the burrowing amphipod Tiburonella viscana (Amphipoda: Platyischnopidae). Ecotoxicology and Environmental Safety 66: 412 - 420p. Link: https://goo.gl/hf6FiV
- Abessa DMS, Bicego MC, Sarkis JES, Hortellani MA, Sousa ECPM (2006) Poder predictivo de valores-guias da qualidade de sedimentos para o sistema estuarino de Santos. Society of environmental toxicology and chemistry. Link:
- (1999) Environment Canada Guidance document to understanding the Canadian Environmental Protection Act Ottawa ON. Link: https://goo.gl/gpML1d
- MacDonald DD, Carr RS, Calder FD, Long ER, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5: 253-278. Link: https://goo.gl/myDpLQ
- Gale SA, King CK, Hyne RV (2006) Chronic sublethal sediment toxicity testing using the estuarine amphipod, Melita plumulosa (Zeidler): evaluation using metal-spiked and field-contaminated sediments. Environmental Toxicology and Chemistry 25: 1887-1898. Link: https://goo.gl/4EEtvJ
- Torres RJ, Cesar A, Pastor VA, Pereira CDS, Choueri RB, et al. (2015) A Critical Comparison of Different Approaches to Sediment- Quality Assessments in the Santos Estuarine System in Brazil. Archives of Environmntal Contamination and Toxicology 68: 132-147. Link: https://goo.gl/ojFeK2
- Prósperi VA (2002) Comparação de métodos ecotoxicológicos na avaliação de sedimentos marinhos e estuarinos. Tese de Doutorado. Escola de Engenharia de São Carlos - USP. São Carlos. Link:
- Chapman PM, Long ER (1983) The use of bioassays as part of a comprehensive approach to marine pollution assessment. Marine Pollution Bulletin 14: 81-84. Link: https://goo.gl/Mn1R6j
- Choueri RB, Cesar A, Torres RJ, Abessa DMS, Morais RD, et al. (2009) Integrated sediment quality assessment in Paranaguá Estuarine system, M Southern Brazil. Ecotoxicology and Environmental Safety 72: 1824 - 1831. Link: https://goo.gl/AumKuv
- (1991) USEPA/USACE - United States Environmental Protection Agency /United States Army Corps of Engineers. Evaluation of Dredged Material Proposed for Ocean Disposal - Testing Manual, EPA-503-8-91/001, Environmental Protection Agency, Washington, DC. Link: https://goo.gl/x2D92i
- Walker CH, Hopkin SP, Sibly RM, Peakall DB (1996) Principles of ecotoxicology. Taylor & Francis, London, UK, 321p. Link: https://goo.gl/KrpwtE
- Werner I, Nagel R (1997) Stress proteins HSP60 and HSP70 in three species of amphipods exposed to cadmium, diazinon, dieldrin and fluoranthene. Environmental Toxicology and Chemistry 16: 2393-2403. Link: https://goo.gl/KHUNph
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
