Archives of Hepatitis Research
Seroprevalence and associated risk factors for Hepatitis B Virus infections among apparently healthy pregnant mothers attending Anc in Rubkona primary health care center in Rubkona County, Unity State, South Sudan
Michael Pou Machar1* and Dube Jara2
2MPH, Assistant Professor, PhD Candidate, College of Health Sciences, Rift Valley University, Ethiopia
Cite this as
Machar MP, Jara D (2021) Seroprevalence and associated risk factors for Hepatitis B Virus infections among apparently healthy pregnant mothers attending Anc in Rubkona primary health care center in Rubkona County, Unity State, South Sudan. Arch Hepat Res 7(1): 004-013. DOI: 10.17352/ahr.000029Background: Background: Hepatitis B is an infection caused by the Hepatitis B Virus (HBV), which is enveloped DNA virus that infects the liver, and the common complications are; cirrhosis, liver cancer and rest of organs failure.
Method: A cross sectional study was conducted among 234 pregnant women attending antenatal Clinic in Rubkona primary Health care center in Rubkona County, South Sudan from March 1 to July 29. Data were collected using pretested interviewer administered questionnaire. Blood was withdrawn from each study participants and used to detect hepatitis B surface Antigen using an enzyme linked immune-sorbent assay test kit. Bivariate logistic regression was carried out to identify the predictors associated with HBV infection. All variables with p-value of ≤ 0.25 in bivariate logistic regression were taken into multivariable model. Variables having p value ≤ 0.05 in the multivariate analysis were taken as significant predictors.
Objectives: To assess the sero-prevalence Hepatitis B surface antigen and its associated factors among pregnant women attending Rubkona Primary Health Care Centre (South Sudan).
Result: The overall Sero-prevalence of HBV infection was, 16 (6.8%), 95% CI; 3.8-10.3). Having history jaundice AOR= 10.91:95%CI (2.6-45.2)], abortion history, [AOR= 5.5: 95%CI (1.5-23.5)] and history multiple sexual partner [(AOR= 9.5:95%CI (2.3-39.7)]. Were found to be associated factors of sero-prevalence of HBV infection.
Conclusion: Hepatitis B is identified to be a major health problem in this community. According to WHO classification, the prevalence of HBV infection in this study area can be categorized as moderate prevalence (2–7%). Abortion and history multiple sexual partners found to be the risk factor associated with HBV infection.
Abbreviations
AOR: Adjusted Odds Ratio; CDC: Center for Disease Control and Prevention; CHB: Chronic Hepatitis B virus; CIs: Confidence Intervals; COR: Crude Odds Ratio; DNA: Deoxy Ribonucleic Acid; ELISA: Enzyme Linked Immune-sorbent Assay; HbeAg; Hepatitis envelope Antigen; HbsAg: Hepatitis B surface Antigen; HBV: Hepatitis B Virus; HCC: Hepatocellular Carcinoma; HIV: Human Immunodeficiency Virus; MSM: Men who have Sex with Men; Ml: Millilitre; PLHIV: People who Live with HIV; PWID: People Who Inject Drugs; WHO: World Health Organization
Introduction
Background
Hepatitis B is an infection caused by the Hepatitis B Virus (HBV), which is enveloped DNA virus that infects the liver, and the common complications are; cirrhosis and Hepatocellular Carcinoma (HCC) [1,2]. Its incubation period ranges from 45 days to 6 months’ days. About 10% of children and 30-50% of adults with acute infection are clinically diagnosed, with anorexia, vague abdominal discomfort, nausea and vomiting, sometimes arthralgia and rash, often progressing to jaundice [1-4].
HBV has been found in almost in all body secretions and excretions. Though, it is found in all body secretions and excretions, the risk of transmission is only blood, body fluids containing noticeable blood, semen and vaginal secretions. Major modes of HBV transmission include sexual contact with infected person or close household contact with an infected person, perinatal mother to infant transmission, injecting drug used and nosocomial exposure [1-5].
Parenteral exposures that have contributed to HBV transmission include transfusion of unscreened blood or blood products, sharing unsterilized injection needles for IV drug use, haemodialysis, acupuncture, tattooing and injuries from contaminated sharp instruments sustained by hospital personnel [2,3].
The major complications of chronic hepatitis B are cirrhosis and Hepatocellular Carcinoma (HCC). Between 20% and 30% of those who become chronically infected will develop these complications, and an estimated 650, 000 people will die annually due to CHB [3]. The majority of people are unaware of their HBV infection, and therefore often present with advanced disease [1,3,5]. Universal hepatitis B immunization programmes that target infants, with the first dose at birth, have been highly effective in reducing the incidence and prevalence of hepatitis B in many endemic countries, however these programmes will not have an impact on HBV-related deaths until several decades after their introduction [1-4].
Statement of the problem
Infection with Hepatitis B Virus (HBV) is a major global public health problem with significant morbidity and mortality [1-4].
Globally, in 2015, an estimated 257 million people were living with chronic HBV predominantly in low- and middle-income countries. The leading region infected for HBV are African and Western Pacific regions accounted for 68% of those globally infected [4].
There is no specific treatment for acute hepatitis B, and only general supportive care is used in symptomatic cases. Chronic HBV infection can be treated with drugs used for treating HIV infection such as ternofovir or entecavir. These drugs do not completely eradicate the virus from the patient’s blood but can slow the progression of chronic disease and improve long-term survival, however may require life-long treatment [2].
Worldwide, 21 countries accounted for more than 80% of the total number of HBsAg-positive infections in the general population. More than half of all HBsAg-positive infections are from China, India, Nigeria, Indonesia, and the Philippines [6]. Only 16 countries accounted for more than 80% of the estimated number of infections in children aged 5 years with HBsAg [6]. Nigeria, India, Indonesia, and the Democratic Republic of the Congo accounting for almost 57% of all infections [6].
Among the 36.7 million persons living with HIV in 2015, an estimated 2.7 million had chronic HBV infection [4]. Liver diseases are a major cause of morbidity and mortality among those living with HIV and co-infected with viral hepatitis [7]. These people should be diagnosed and provided with appropriate and effective treatment for both HIV and hepatitis as a priority [3,4,7].
In sub-Saharan Africa, vertical transmission HBV increases co-infection with HIV [8]. A co-infected pregnant woman is twice as likely to test positive for HBeAg, three times more likely to have detectable HBV DNA [9]. And have higher HBV DNA serum concentrations than those who are not co-infected by HIV, hence the increased HBV DNA serum concentrations greatly increased the risk of vertical transmission [8,9].
South Sudan is also one of the countries with high prevalence HBV carrier, which is about 6.3 % of the pregnant women are Hepatitis B Virus carriers [10]. Perinatal transmission is the major route of HBV transmission in many parts of the world. It is the main mode of transmission in high-prevalence in Africa. Perinatal infection increases the risk of developing chronic infection of an infant bout 90% of the cases, therefore; the risk of progression to chronic infection decreases to 20–60% between the ages of 6 months to 5 years [11]. The ideal time of preventing HBV infection is through immunization at birth, which offers over 95% protection against the development of chronic infection [12].
Like many other developing countries, South Sudan also includes hepatitis B vaccine at 6, 10 and 14 weeks after birth as national immunization program [10]. Delay in 6 weeks for vaccination will decrease the efficacy of the vaccine in the prevention of vertical transmission [13].
Significance of the study
Viral hepatitis is responsible for the death of 1.34 million people in 2015 and this number is comparable to deaths caused by tuberculosis and higher than those caused by HIV. However, the number of deaths due to viral hepatitis is increasing over time, while mortality caused by tuberculosis and HIV is decreasing. The epidemic caused by HBV affects mostly the WHO African Region and the Western Pacific.
Since Perinatal transmission is the major route of HBV transmission in many parts of the world. It is the main mode of transmission in high-prevalence in Africa, particularly in endemic areas where up to 20% of women of childbearing age may have HBV. Hence, prevention of prenatal transmission remains an important target in the struggle for global eradication of HBV infection.
Therefore, the finding of this study will help Ministry of Health, and other stakeholders working on the health sector to develop strategies for promoting community awareness and improving HBV vaccine coverage, and other preventive strategies.
Literature review
Global HBsAg endemicity: Globally, in 2015, an estimated 257 million people were living with chronic HBV predominantly in low- and middle-income countries. The leading region infected for HBV are African and Western Pacific regions accounted for 68% of those globally infected. It was estimated that prevalence of HBV infection in the general population was 3.5%. Among those born before the hepatitis B vaccine became available, the proportion of persons living with chronic HBV infection remains high. Prevalence was the highest in the African (6.1%) and Western Pacific regions (6.2%). Assuming that women of reproductive age constitute 25.3% of the world’s population (United Nations data), adults chronically infected may include 65 million women of childbearing age who can potentially transmit HBV to their babies [4]. Prevalence of HBV varies from region to region. WHO categorized into three regions; high prevalence, moderate prevalence and low prevalence. The high prevalence (≥8%) includes sub-Saharan Africa, South-East Asia, the Eastern Mediterranean countries, south and western Pacific islands, the interior of the Amazon basin and certain parts of the Caribbean. The moderate prevalence (2–7%) includes south-central and south-west Asia, eastern and southern Europe, the Russian Federation and most of central and South America. Low prevalence (<2%) region includes Australia, New Zealand, northern and western Europe, and North America [14].
The European Centre for Disease Prevention and Control, showed that highest risk population for HBV or have a high disease burden in EU/EEA are dialysis/haemodialysis patients, People Living with HIV (PLHIV), PLHIV with multiple risks such as Men who have sex with Men (MSM) living with HIV, People Who Injected Drugs (PWID) living with HIV, PLHIV in prison [15].
In Europe there is steady decrease of report in the acute cases of HBV and the likely reason for decreased report is the impact of vaccination campaigns [16]. However, the total percentage of people infected with HBV varies between different countries with in Europe. For HBV, the prevalence in the general population ranged from 0.1% in Ireland to 4.4% in Romania. Highest prevalence of HBV have been reported in Greece and Romania, 3.3% and 4.4% respectively, while the vast majority of other European countries have HBV prevalence around or below 1% [17].
European Centre for Disease Prevention and Control, in 2013, reported that data on transmission of hepatitis B were complete for only 21.3% of cases. Among cases with complete information, heterosexual transmission (30.5%), nosocomial transmission (18.9%), injecting drug use (13.2%) and transmission among men who have sex with men (9.4%) were most commonly reported for acute infections. Perinatal transmission was the most common route (43.5%) for chronic cases [18].
Prevalence of hepatitis B
Here are some of the literatures on the sero-prevalence of HBsAg in different countries especially African countries and some other part of the world was summarized in the following as follows:
The finding of the studies conducted in different part of Africa showed that the prevalence of HBsAg range from 0.8% to 11.8%. For instance, the study conducted in South Sudan, Juba [6.3%] [10], Democratic Republic of the Congo in Lubumbashi [6.69%] [19], South Africa[0.8%] [20], Ethiopia, Bishoftu [5.4%] [21], Ethiopia, Hawassa [7.8%] [22], Eritrea, Asmara [3.2 %] [23], Cameroon, in the rural milieu [10.2%] [24], Sudan, Khartoum [7.5%] [25], Uganda, Kampala [11.8%] [26], Kenya, Nairobi and 8 other regions [9.3%] [27], South Sudan, Juba [11%] [28]. Ethiopia, Gambella 7.9% [29], Ethiopia Harrar City [6.3%] [30], which indicates intermediate endemicity. The finding of similar study conducted in Brazil, São Luís indicated that the prevalence of HBsAg was 7.4% [31].
On the contrary, the prevalence of HBsAg western countries is low, that is < 2%. Here are some of the countries with low prevalence of HBsAg. Namely a study conducted across four hospitals in London over 2 years (2009-2010) attending ANC showed that only 1.05% were positive for HBsAg [32], Italy ,in the region of Apulia the prevalence of HBsAg were 0.5%, in Ireland, the prevalence in the general population were 0.1% [17].
Associated risk factor for HBsAg among pregnant woman
In most African countries most pregnant woman were not aware of their serologic status with HBV. The finding of research conducted on seroprevalence and risk factors for hepatitis B infection in pregnant women in Lubumbashi, Democratic Republic of the Congo showed that all pregnant women were previously unaware of their serologic status with HBV. In this study the highest prevalence of hepatitis B was observed in the age group 31-40(10.53%), though it was not statistically significant. However, HBV was significantly higher in HIV-positive pregnant women, who presented a risk of nearly 9 times higher compared to HIV-negative pregnant women [19].
A retrospective study conducted at the antenatal clinic of one Military Hospital, Tshwane, South Africa for the period January 2008-December 2013. A total of 2,368 patients were enrolled as both their HBV and HIV serology results were available. The finding revealed that an overall HBV prevalence was 0.8%. However hepatitis B surface antigen (HBsAg) prevalence was significantly higher (2.1%) among HIV co-infected compared with HIV-uninfected patients (0.4%) [20].
A study conducted in Ethiopia, on Hepatitis B Virus infection and risk factors among pregnant women public hospital, revealed that none of the study participants were aware of their HBV sero-status. Risk factors such as history of abortion, surgery and family history for hepatitis were significantly associated with Sero-positivity of HBsAg [21].
The finding of the study conducted in the Southern Ethiopia on prevalence and associated risk factor of HBV infection among pregnant woman in 2015, showed that educational status of the study participant has a significant association with HBV infection, in which those with no formal education were more likely to be infected than those who had completed secondary school. Despite HBsAg was detected more often in pregnant women with multiple exposure factors (8.8%) than in pregnant women who had not experienced possible risk factors (4%), this difference was not statistically significant [22].
The finding of another study conducted in Eritrea, showed that the overall HBsAg prevalence among pregnant women was 3.2%. In this research, the researchers tried to see whether there were significant association with socio-demographic characteristics and HBV infection. No significant association observed in relation to marital status, occupation, spouse occupation or religion. However, a significant relationship between the rate of HBV infection and the level of education were observed. It showed that illiterate were 2 times prone to HBV infection compared to those who had attained secondary or higher education [23].
The finding of a study conducted in rural district of the Far North Region of Cameroon revealed that Only 4 women (1.2%) had been vaccinated against HBV. Thirty-three women (10.2%) were HBsAg-positive. Five (1.5%) women were co-infected with HIV and HBV. This research identified risk factors such as concurrent infection with HIV and blood transfusion is highly associated with HBV infection [24].
A study conducted in Sudan, neighbouring country, among a pregnant woman attending Khartoum teaching hospital showed that prevalence of HBV infection was 7.5 %. This study indicated that there were significant relationship between some risk factors and HBV infection. These are history of surgery with positive HBsAg, history of jaundice with positive HBsAg and histories of jaundice in their husbands, and with positive HBsAg. Others risk factors such as parity and age group have no significant association [25].
The finding of the study conducted in Uganda revealed that history of scarification, number of sexual partners, history of blood transfusion or polygamy had no statistically significant relationship with HBsAg positivity, however women 20 years of age or younger were 2.5-fold more likely to test positive for HBsAg than those aged above 20 years [26]. On the contrary the finding of the study conducted in Kenya showed that scarification’s, blood transfusion, alcohol and STD were statistically significant association with HBV transmission [27].
Very Few studies have been conducted in South Sudan on prevalence and associated factors of HBV infection among pregnant woman. Among these studies [10,28], a study conducted by Akway M. Cham et.al revealed that, age range between 15-34 years has shown possible link to HBV infection than other ranges. However surgical procedure, blood transfusion, cultural background and marital status did not show any significant association with HBV infection [10]. Similarly, another study conducted in Juba teaching hospital revealed loss of marital partner and history of jaundice was statistically significant. Other risk factors such as occupation, education, multiple sexual partners, blood transfusion and history of surgery were not significantly associated with positive HBsAg [28].
A study conducted in Gambella, south western Ethiopia, History of abortion occupation and multiple sexual partner had statistical significant association with HBsAg sero-positivity [29]. Another study conducted in Harrar city, Ethiopia, revealed that blood transfusion history of surgical procedure history of sexually transmitted infection and history of tooth extraction were independent predictors of HBV infection [30].
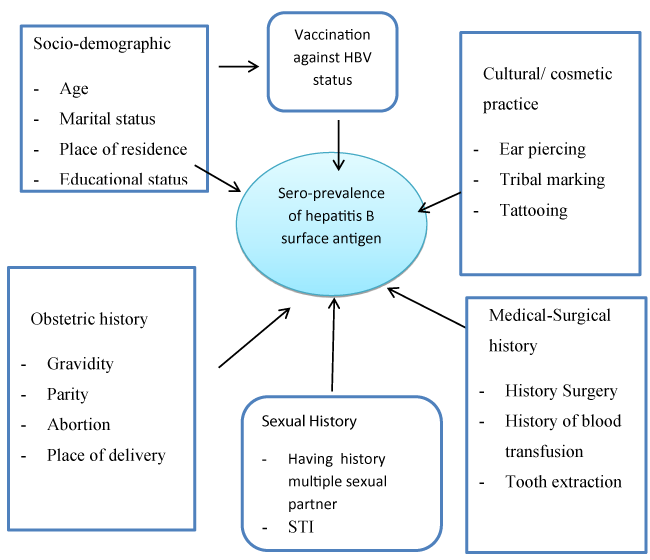
Conceptual framework
This is a set of interactions between socio-demographic factors and risk factors such as cultural practice, obstetric history and medical surgical history which contribute for increased seroprevalence of HBsAg. This conceptual frame work was adopted from different literature and modified accordingly.
Objectives
General objective: To assess the sero-prevalence Hepatitis B surface antigen and its associated factors among pregnant women attending Rubkona Primary Health Care Centre (South Sudan).
Specific objectives: To determine the prevalence of Hepatitis B surface antigen among pregnant women attending Rubkona primary health care Centre.
To identify factors associated with Hepatitis B surface antigen among pregnant women attending Rubkona primary health care Centre.
Methods and materials
Study area and period
Rubkona is the most important county in Unity State, since it had been the capital of a comparatively larger state; it remained the center for trade, communication and programme intervention. This county has the largest internally displaced persons’ camp in the country, accommodating 120,000 people. It borders Panrieng County to the north, Koch County to the south, Mayom County to the west and Guit County to the south-east. The Bhar El Arab River, which is part of the Nile River system, flows through it from east to west towards Mayom County. This river is partly navigable. The county is composed of floodplain, and becomes inundated or inaccessible during the rainy season. It has one health facility which serves the entire population, called Rubkona Primary Health Care Center (PHCC) [33]. Study period was from 1st July to July 29, 2020.
Study design
A cross sectional study was conducted among pregnant women attending antenatal Clinic in Rubkona primary Health care center in Rubkona County, South Sudan.
Population
Source of population: All pregnant women who are living in Rubkona County were the source of population.
Study population: All Pregnant women who are attending antenatal care at Rubkona Primary Health Care Center during the study period were the study Population.
Eligibility
Inclusion criteria: All Pregnant women who are attending antenatal clinic in Rubkona Primary Health Care Center during the study period were included.
Exclusion criteria: Those women who are unable to communicate or seriously ill at the time of data collection.
Sample Size determination
The single population proportion formula was used to determine the sample size by considering previous prevalence in Juba which was 6.3% [10]. Since the prevalence of the previous study is 6.3% which was less than 10% the recommended d (tolerable margin of error) should be half of it. That is 3.15% level of significance/margin of error. If the prevalence of the previous study is below 10% and above 90% we should take half of the Prevalence to be d (margin of error).
The following assumptions were made during sample size calculation.
Z = Standard deviation of the normal distribution = 1.96 (confidence level at 95%)
P = prevalence 6.3 %( prevalence of HBsAg in serological survey in Juba [24]
d = Tolerable error / level of significance = 3.15%.
X= 5% non-response rate
N= Minimum sample size
Sample size= n (Minimum sample size) + X (non-respondent)
n = Zα/22 P (1-P) /d2
n = 1.962 0.063(1-0.063)/0.03152
n = 3.8416 x 0.063(0.937)/0.00099
N= n+x
N = 229 + 5% Non-response rate
N= 229+11.45
N= 241
Sample size (N)= 241
Sampling technique
Systematic random sampling method was used to recruit study participants. The sampling frame will include all pregnant women attending ANC Rubkona PHCC. Then, sampling interval (K unit) was calculated based on ANC records of the last month. After calculating K unit, a random starting point was then selected by lottery method. Finally, select every Kth unit after that first number.
Study variables
Dependent variable: Hepatitis B Surface Antigen Prevalence
Independent variables
Socio demographic characteristics
➢ Age
➢ Marital status
➢ Place of residence
➢ Educational status
Obstetric history
➢ Gravidity
➢ Parity
➢ Abortion
➢ Place of delivery
Cultural/cosmetic practice
➢ Ear piercing
➢ Ethnic scar
➢ Tattooing
Medical-Surgical history
➢ History Surgery
➢ History of blood transfusion
➢ Tooth extraction
Sexual history
➢ Having history multiple sexual partner
➢ STI
Vaccination
➢ Vaccination against HBV status
Measurement and data collection
Data collection: The data for the study were derived from serological testing and questionnaires. Both the questionnaire and blood test were done after having received a clear explanation of the objective of the study and written consent from the participants. Socio demographic and pertinent data on risk of acquiring HBsAg were collected using a standard structured questionnaire by health professional.
Specimen collection and processing: After obtaining written consent, 5 ml of venous blood was collected in plane tubes under aseptic conditions from peripheral vein by experienced laboratory personnel from all consenting pregnant women. Proper handling and labelling of the specimen will follow.
Quality assurance
Trained data collector was collect Information on socio-demographic and other pertinent data using structured questionnaire. This questionnaire was prepared first in English and translated to Arabic language, and was finally back translated to English by linguistic professionals in order to ensure its consistency. Pre-testing of 5% the questionnaire was done prior to the study. The questions were standardized during the pre-test to ensure they provided desired answers. Data completeness and consistence were checked daily by principal investigator while in the field. All the data were double entered to ensure the data quality.
Quality control of serological test: Known positive and negative controls were run in parallel with test samples. All laboratory procedures were carried out following Standard Operating Procedures (SOPs). The quality assurances of pre-analytical, analytical and post-analytical stages were applied.
Pre-analytical stage
First the specimens were collected by trained lab technician from women and labelled by the patient unique identification number. Then samples were centrifuged; the serum was evaluated and separated; appropriately and stored until transported to the laboratory. The transported samples were stored at the optimum temperature until they were processed.
Analytical stage
The blood test for HBsAg was performed by trained laboratory technologist. All samples were tested, using Enzyme Linked Immunosorbent Assay (ELISA) for HBsAg. The standard laboratory procedures were also followed and the results were checked by the supervisors.
Post-analytical stage
The results were recorded with the patients’ unique identification number; the results were reported to the principal investigator.
Data processing and analysis
After the data collection, each questionnaire was checked for its completeness. Data entry, cleaning and coding done by Epi-Info version 3.5.4 statistical software package and was exported to SPSS window version 25 for analysis. Bivariate logistic regression was carried out to identify the predictors associated with HBV infection. All variables with p-value of ≤ 0.25 in bivariate logistic regression were taken into multivariable model. Variables having p value ≤ 0.05 in the multivariate analysis were taken as significant predictors. Crude Odd Ratios (COR) and Adjusted Odds Ratios (AOR) with their 95% Confidence Intervals (CI) were calculated. Tables, cross tabulations was used to present the data.
Ethical considerations
Ethical clearance was obtained from the Research and Publication Committee of rift valley University Abichu campus postgraduate studies coordinating office. Permission was obtained from Rubkona Primary Health Center. The respondent’s written consent, privacy and confidentiality was the primary concern of the study. Written consent was obtained from each participant. Confidentiality of the information was kept anonymously.
Result
Socio-demographic characteristics
In this study a total of 234/241 pregnant women attending Antenatal care were participated with a response rate of 97.09%. The mean (±SD) age of the participants was 25.5(±5.64) years. The majority 101(43.2%) of the participants were found in 21-25 age groups, followed by 26-30; 50(21.4%) and age range between 17-43 years.
Majority of the respondent were primary school, 94(40.2%), followed by can’t read and write, 89(38%). Regarding occupational status of respondents, 132(56.4%) of them were House wife at the time of data collection, followed by private employed 50(21.4%). Pertaining to the marital status of the participants, 232(99.1%) were married Table 1.
Sero-prevalence of HBV infection
The overall sero-prevalence of HBV infection was, 16 (6.8%), 95% CI; 3.8-10.3). Out 0f these, 7(14%) were belong to age group 26- 30 years. Pertaining to educational status versus positivity 9 (10.2%) of respondents can’t read and write followed by primary education, 6(6.3%), and 1(2.3%) of respondents with secondary education were positive for HBsAg. Based on their occupation, 10(7.5%) of pregnant Women who were house wives and 3(6%) of those who were private employee were positive for HBsAg. Other risk factors such as abortion, history of jaundice and multiple sexual partner were 6(25%), 6(30%) and 10(4.67%) were positive for HBsAg respectively Table 2.
Factors associated with Hepatitis B Virus infection
Bivariate and multivariate logistic regressions were done to assess the predictors of Hepatitis infection. All variables in the bivariate analysis at p-value < 0.2 were entered to the multivariate analysis. Among the variables that were included in the multivariate analysis model abortion, History of jaundice and multiple sexual partners were significantly associated with HBsAg sero-status.
Pregnant women who had history jaundice (Yellowing of the skin and the whites of the eyes ) were almost eleven times more likely to have sero-positive for HBV infection compare to those with no history of jaundice [AOR= 10.91: 95%CI (2.6-45.2)]. Pertaining abortion history, pregnant women who had history of abortion were five times more likely to have sero-positive for HBV infection as their counterpart [AOR= 5.5:95%CI (1.5-23.5)]. Similarly the odds of being sero-positive for HBV infection among pregnant women who had history multiple sexual partner, were nine times greater than those pregnant women who had single partner [(AOR= 9.5:95%CI(2.3-39.7)] Table 3.
Discussion
The finding of this study showed that the overall Seroprevalence of HBV infection among pregnant women who attend antenatal care at Rubkona County, Primary Health Care Center, was 16(6.8%). According to W.H.O classification, the prevalence of HBV infection in this study area can be categorized as moderate prevalence (2–7%) [14]. The overall prevalence of this study was similar with the finding of study conducted in South Sudan, Juba [6.3%] [10], Democratic Republic of the Congo in Lubumbashi [6.69%] [19] and Ethiopia, Harrar City [6.3%] [30].
In contrast, this finding is higher than that reports from Ethiopia, Bishoftu [5.4%] [21], Eritrea, Asmara [3.2 %] [23] and South Africa [0.8%] [20]. However the finding of this study is lower than that finding reported in Ethiopia (Hawassa) [7.8%] [22], Uganda, Kampala [11.8%] [26], Cameroon in the rural milieu [10.2%] [24], Sudan (Khartoum) [7.5%] [25], Kenya (Nairobi) and 8 other regions [9.3%] [27], South Sudan (Juba) [11%] [28]. Ethiopia (Gambella)[7.9%] [29].The likely reason for such discrepancy might be due to the differences in study design, sample size difference, socio-cultural environment, traditional practices, sexual practices, and period of study difference and supportive health policy in prevention.
In this study, it revealed that history of multiple sexual partners, history of jaundice, and history of abortion are highly associated with Sero-prevalence of HBV infection, however; social demographic factors and rest of associated factors such as medical surgical, culture practise and obstetric history are not significant to this study. The odds of having HBV among those who had multiple partners was greater than those pregnant women who had single partner.
This finding is in line with the finding which were studied in ( Asmara), Eritrea, (Juba) South Sudan, (Gambella), South western of Ethiopia, (Harrar) Ethiopia (23,28-30), in which age, occupation, marital status have no significant.
This finding is also congruent or corresponding with the study conducted in (Bishoftu General Hospita), Ethiopia, (Gambella), South western Ethiopia [21,29], in term of abortion. On the other hands, a study conducted in Uganda [10] and in South Sudan [10,28] revealed that, number of sexual partners, or polygamy had no statistically significant.
The likely explanation for higher odds in abortion, is when a woman has experienced abortion in the rural area, might have higher chance of having unsafe abortion which might predispose to HBV infection. However, a research conducted by Anthony Laku Stephen Kirbak et al., contradicted this finding. The likely reason for the discrepancy might be the study area, where Juba is a capital city whereas Rubkona is a rural area. Hence education, peace stability and ANC coverage in Juba is better than Rubkona.
Among the risk factors that were identified to have relationship with prevalence of HBV, history of jaundice is also found to be statistically significant with HBV positivity. This finding agrees with a study in neighbouring country (Khartoum), Sudan, and (Juba) South Sudan [25,28].
Unlike the current finding study, an others studies were conducted in Southern Ethiopia, Eritrea, South Sudan which had had supported the educational associated risk factor on HBV infection among pregnant woman [22,23,28] respectively. The difference could be due to: in this study, majority are primary and less than but in those studies majority of, were high school and colleges and above than my study.
Limitations of the study
The study was conducted in antenatal clinic (institution based) and therefore results of the research may not be representative of the entire pregnant women in the community of Rubkona County.
Conclusion and recommendation
Conclusion
Hepatitis B is identified to be a major health problem in this community. According to W.H.O classification, the prevalence of HBV infection in this study area can be categorized as moderate prevalence (2–7%). In this study, Abortion, history of multiple sexual partners and jaundice are found to be the risk factors associated with HBV infection. The associated risk factors prevalence of Hepatitis B Virus were found to be highly between ages of 26-30 7 (14.0%) years old, and most of them were house wives 10 (7.5%).
Recommendation
Based on the findings of this study the following recommendations shall be forwarded to Ministry of Health, and other stakeholders working on the health sector to develop strategies of:
- Vaccination of all neonates against Hepatitis B Virus at birth if possible will reduce the virus.
- Increasing the screening on both female and male will let the declination of the virus.
- Increase awareness on screening, vaccination must be introduced through health education during antenatal care follow up by the concerned bodies
- Since this study is the first in its type the study area , further study should be done to identify the risk factors related to the HBV
- Extensive health education campaign should be provided to general population and especially to pregnant to increase their awareness towards hepatitis B infection.
Declaration
I declare and confirm that the thesis is my own work, in which I have followed all ethical principles of academic during preparation, data collection, analysis, and completion of this thesis. All grant matter needed in the thesis have been recognized through quotation.
The thesis is submitted in fractional fulfillment of the requirement for graduate degree in Master degree of public health. The thesis is submitted to Rift valley university library for borrowers under the rules of library.
This thesis would not have been possible without the generous help and encouragement I had have received from various source, of varied character, and over a period of some months. Of the debt owed by Dube Jara, the thesis itself speaks with sufficient eloquence. But for the interest evinced by him, I perhaps would have abandoned the project at a very early stage, and its completion is a product of his inspiration and guidance. Am further indebted to the head department of the public Health for he has dedicated to me Dube Jara for the thesis to be tight. Am grateful to all the officers of Rift Valley university: president, vice president, dean, head of different departments, and the vital elements of the university (instructors) who in various ways have contributed to the task and were suffering teaching us here and there from the year 2018 and mark the end of 2020 and aimed to bring desirable change on us, irrespective to our religious, believe, nationalities, gender and colours.
On the material side, I indebted to my families as they were my Dad, Moms, sisters, and brothers grate us in 2018 that enable me to go to Rift valley university and financing my state of being and their standing firm and unlimited encouragement and support on me without that, this thesis would have impossible to be achieved.
My happiness go to God for he had stood with, and taking care of me from the beginning of my study till the time of day without being tried for so long.
- American Public Health Association (2005) Control of communicable diseases manual. 18th ed. Washington.
- WHO (2017) Fact sheet – Hepatitis B in the WHO European Region. Link: http://bit.ly/3uTAgcd
- WHO (2015) Guidelines for the prevention, care and treatment of persons with chronic hepatitis b infection. 166. Link: http://bit.ly/388nRHG
- Global Hepatitis Report (2017) Geneva: World Health Organization 2017. Link: http://bit.ly/3dh4iMY
- Abdi F, GhaffariNovin M, Afrakhteh M, Khorvash F (2015) Hepatitis B and pregnancy: An update review article. World J Obstet Gynecol 4: 1-8 Link: https://bit.ly/2OtAYMQ
- Polaris Observatory C (2018) Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet GastroenterolHepatol. 26: 26. Link: https://bit.ly/3e7LUdL
- Matthews PC, Geretti AM, Goulder PJ, Klenerman P (2014) Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in sub-Saharan Africa. J Clin Virol 61: 20-33. Link: http://bit.ly/3uWz6Nm
- Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ (2012) HIV–HBVco-infection—a global challenge. N Engl J Med 366: 1749-1752. Link: http://bit.ly/3riNgpz
- Hoffmann CJ, Thio CL (2007) Clinical implications of HIV and hepatitis Bco-infection in Asia and Africa. Lancet Infect Dis 7: 402-409. Link: http://bit.ly/2MOH2Pr
- Cham AM, Clever SS, Celestino RJ, Thomas EM (2017) The Burden Of Hepatitis B Among Pregnant Women Attending Antenatal Care (Anc) Services In Juba Teaching Hospital (Jth)-Juba South Sudan. IJRDO Journal of Health Sciences and Nursing 2. Link: http://bit.ly/38cssZm
- Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, et al. (1983) Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 2: 1099-1102. Link: http://bit.ly/3rj12ZB
- Lavanchy D (2004) Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatol 11: 97-107. Link: http://bit.ly/3c2vz7h
- Kirbak AL, Ng’ang’a Z, Omolo J, Idris H, Usman A, et al. (2017) Sero-prevalence for Hepatitis B virus among pregnant women attending antenatal clinic in Juba Teaching Hospital, Republic of South Sudan. Pan African Medical Journal 26: 72. Link: http://bit.ly/3eclgQH
- World Health Organization (2004) Hepatitis B vaccines. WHO position paper. Weekly Epidemiological Record. 79: 253-264.
- European Centre for Disease Prevention and Control (2018) Hepatitis B and C epidemiology in selected population groups in the EU/EEA. Stockholm: ECDC. Link: http://bit.ly/3uVipS9
- European Centre for Disease Prevention and Control (2015) Hepatitis B surveillance in Europe – 2013. Stockholm: ECDC. Link: http://bit.ly/3uTywzH
- European Centre for Disease Prevention and Control (2016) Systematic review on hepatitis B and C prevalence in the EU/EEA. Stockholm: ECDC. Link: http://bit.ly/3c7wtiS
- European Centre for Disease Prevention and Control (2015) Hepatitis B surveillance in Europe – 2013. Stockholm: ECDC. Link: http://bit.ly/3uTywzH
- Tshanda NM, KitengeFwM, Kakoma JB (2018) Preliminary study of seroprevalence and risk factors for hepatitis B infection in pregnant women in Lubumbashi, Democratic Republic of the Congo. Afr J Health 2: 9. Link: https://bit.ly/3uUDzzW
- Diale Q, Pattinson R, Chokoe R, Masenyetse L, Mayaphi S (2015) Antenatal screening for hepatitis B virus in HIV-infected and uninfected pregnant women in the Tshwane district of South Africa. S Afr Med J 106: 97-100. Link: http://bit.ly/38cAmSG
- Desalegn Z, Mihret A, Beyene HB, Yilma M, Seid Y, et al. (2016) Survey of Hepatitis B virus infection and risk factors among pregnant women at public hospital in Ethiopia. International Journal of Biomedical Research 7: 450-456. Link: https://bit.ly/3c3HhyD
- Metaferia Y, Dessie W, Ali I, Amsalu A (2016) Seroprevalence and associated risk factors of hepatitis B virus among pregnant women in southern Ethiopia: a hospital-based cross-sectional study. Epidemiol Health 38: e2016027. Link: http://bit.ly/38bbril
- Fessehaye N, Berhane A, Ahmed H, Mohamed S, Tecle F, et al. (2018) Prevalence of Hepatitis B Virus Infection and Associated Seromarkers among Pregnant Women in Eritrea. J Hum VirolRetrovirol 6: 00191. Link: https://bit.ly/38bFeHv
- Noubiap JJ, Nansseu JR, Ndoula ST, Bigna JJ, Jingi AM, et al. (2015) Prevalence, infectivity 31 and correlates of hepatitis B virus infection among pregnant women in a rural district of the Far North Region of Cameroon. BMC Public Health 15: 454. Link: http://bit.ly/2PENd9X
- Mohammed Hammad A, Mohammed B, Koko B (2015) Prevalence of Hepatitis B Infection among Pregnant Women at Khartoum Teaching Hospital, Sudan. Journal of US-China Medical Science 12: 58-63. Link: https://bit.ly/2MOFjJX
- Bayo P, Ochola E, Oleo C, Mwaka AD (2014) High prevalence of hepatitis B virus infection among pregnant women attending antenatalcare: a cross-sectional study in two hospitals in northern Uganda. BMJ Open 4: e005889. Link: https://bit.ly/3kOdjmf
- Gatheru Z, Murila F, Mbuthia J, Okoth F, Kanyingi F, et al. (2018) Factors Associated with Hepatitis B Surface Antigen Seroprevalence amongst Pregnant Women in Kenya. Open Journal of Obstetrics and Gynecology 8: 456-467. Link: https://bit.ly/3kMAVHO
- Kirbak AL, Ng’ang’a Z, Omolo J, Idris H, Usman A, et al. (2017) Sero-prevalence for Hepatitis B virus among pregnant women attending antenatal clinic in Juba Teaching Hospital, Republic of South Sudan. Pan African Medical Journal 26: 72. Link: http://bit.ly/3eclgQH
- Abayneh T, Misanew T, Desta H, Chaltu F, Gemechu J (2019) Sero-prevalence of hepatitis B virus and associated factors among pregnant women in Gambella hospital, South Western Ethiopia: facility based cross-sectional study. BMC infectious disease 19: 602. Link: http://bit.ly/3sUtX6i
- Tiruye G, Shiferaw K, Tadesse F (2018) Seroprevalence of Hepatitis B Virus Infection and Associated Factors among Pregnant Women Attended Antenatal Care Services in Harar City, Eastern Ethiopia. J Women's Health 7: 436. Link: https://bit.ly/2O3Pbjx
- Marinilde TS, Tainá LR, Max DC, Santos Ad, Monteiro VL, et al. (2012) Prevalence of hepatitis B among pregnant women assisted at the public maternity hospitals of São Luís, Maranhão, Brazil. Braz J Infect Dis 16: 517-522 Link: http://bit.ly/3uWlsJV
- Godbole G, Irish D, Basarab M, Mahungu T, Fox-Lewis A, et al. (2013) Management of hepatitis B in pregnant women and infants: a multicentre audit from four London hospitals. BMC Pregnancy Childbirth 13: 222. Link: http://bit.ly/3kKVQem
- Polio global eradication initiative, published by UNICEF South Sudan 2018. Link: http://uni.cf/3c3FBVR
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
