Archives of Hepatitis Research
Occult Hepatitis B Virus infection in a cohort of patients with chronic Hepatitis C
MI Naga1, MA Amin1*, DA Algendy1, AI El Badry1, MM Fawzi1, AR Foda1, SM Esmat1, SM Gabal2, D Sabry3, M Kamal4, LA Rashed3
2Pathology, Cairo University, Egypt
3Biochemistry, Cairo University, Egypt
4Clinical & Chemical Pathology, Cairo University, Egypt
Cite this as
Naga MI, Amin MA, Algendy DA, El Badry AI, Fawzi MM, et al. (2019) Occult Hepatitis B Virus infection in a cohort of patients with chronic Hepatitis C. Arch Hepat Res 5(1): 017-021. DOI: 10.17352/ahr.000022Introduction: The prevalence of occult hepatitis B, defined by absence of HBsAg and HBV DNA, ranges widely in patients with hepatitis C. This may influence the treatment of hepatitis C and the severity of liver disease
Aim of work and methods: Was to determine the prevalence of occult hepatitis B virus infection and liver of HBsAg negative patients with chronic hepatitis C and to evaluate its clinical consequences on liver pathology and its impact on the response to treatment with peg-IFNa and Ribavirin. Immunohistochemistry staining for hepatitis B surface antigen and HBV DNA detection was assessed retrospectively on liver biopsies of HCV positive/ HBsAg negative patients before treatment.
Results: A 11.7 % (13/111) prevalence of occult hepatitis B was recorded in liver samples. There was no significant statistical difference between those with occult B and those without regarding liver AST, ALT, bilirubin level, fibrosis stage or the degree of liver inflammation on liver biopsy. 101 patients from the whole cohort received interferon-based therapy 13 with occult B and 88 without occult B with no significant difference in the response rate between both groups.
Conclusion: The frequency of occult HBV infection was not affected by the presence of hepatitis C and occult HBV infection did not have a significant effect on the disease severity of hepatitis C.
Introduction
Occult hepatitis B virus infection (OBI) is defined as the presence of hepatitis B virus (HBV) DNA in the liver of patients with negative results of hepatitis B s antigen (HBsAg) test with or without serological markers of previous viral exposure [1,2]. The molecular basis of occult HBV infection is related to the long-lasting persistence in the nuclei of hepatocytes of the viral covalently-closed-circular DNA (cccDNA) [3]. Almost all OBI cases are infected with replication-competent HBV showing strong suppression of replication and gene expression, probably due to host immune-surveillance and epigenetic factors [4]. Although OBI status is significantly associated with the presence of antibodies to HBV [2], the analysis of liver DNA extracts represents the gold standard for occult HBV evaluation [4]. Hence, serum analysis must be taken into account only in the absence of liver specimens. In any case, it is strongly recommended to use a highly sensitive nested polymerase chain reaction (PCR) or real time PCR with oligonucleotide primers specific for different HBV genomic regions and complementary to highly conserved nucleotide sequences [5]. Occult HBV infection has been found with a high prevalence in patients with chronic hepatitis C (CHC), probably because both HBV and hepatitis C virus (HCV) share the same parenteral way of transmission. In particular, HBV DNA is detectable in about one-third of CHC patients with negative results of HBsAg test in the Mediterranean basin [2].
Methods
The study was carried out after receiving the approval of the Ethics Committee of faculty of medicine, Cairo University, and obtaining informed written consent from patients. One hundred and eleven patients with chronic hepatitis C patients attending the outpatient clinic of internal medicine and hepatology clinic of Cairo University Hospital from January 2004 to December 2005 and who were assessed before starting treatment course of peg- IFNa and Ribavirin (the available option at that time).
Full clinical evaluation, abdominal ultrasonography, and a battery of laboratory investigations were done to all patients (complete liver and kidney function tests, complete blood picture, TSH, ANA, HBsAg, HCV antibodies, quantitative PCR for HCV –RNA). Liver biopsies were done for all patients.
All patients were anti- HCV-positive, had detectable serum HCV RNA using PCR, had ALT serum levels higher than the upper limit of normal on at least one occasion in the 6 months before the initiation of treatment, and had a histological diagnosis of chronic hepatitis by liver biopsy. Those under 18 years old, or the presence of serum HBsAg or anti-HIV antibodies, as well as those with causes of chronic liver disease, severe cirrhosis, severe systemic illness, and pregnancy were excluded from the study. Patients were treated with peg-IFNa 2a (Pegasys 180 mg/week) or peg-IFNa 2b (peginteron 1.5 mg/kg/week) or Reiferon (160 mg /week ) and Ribavirin (800–1,400 mg according to weight) for 48 weeks and the virological response to treatment at weeks 12 and 24. Sustained virological response to treatment was defined by the absence of detectable serum HCV RNA using PCR, 24 weeks after the end of treatment.
Histological examination of liver lesions was carried out by a single pathologist without knowledge of any clinical or biochemical information. The results were expressed according to the METAVIR classification. Activity was graded according to the intensity of necro inflammatory lesions: A0 = no histological activity, A1= mild activity, A2= moderate activity, A3= severe activity. The stage of fibrosis was assessed on a five-point scale: F0= no fibrosis, F1= portal fibrosis without septa, F2= few septa, F3= numerous septa without cirrhosis, F4= cirrhosis
Retrospectively the liver paraffin sections were examined after being deparaffinized for both immunohistochemistry detection of HBsAg and HBV-DNA detection by PCR.
Immunohistochemistry for hepatitis B virus
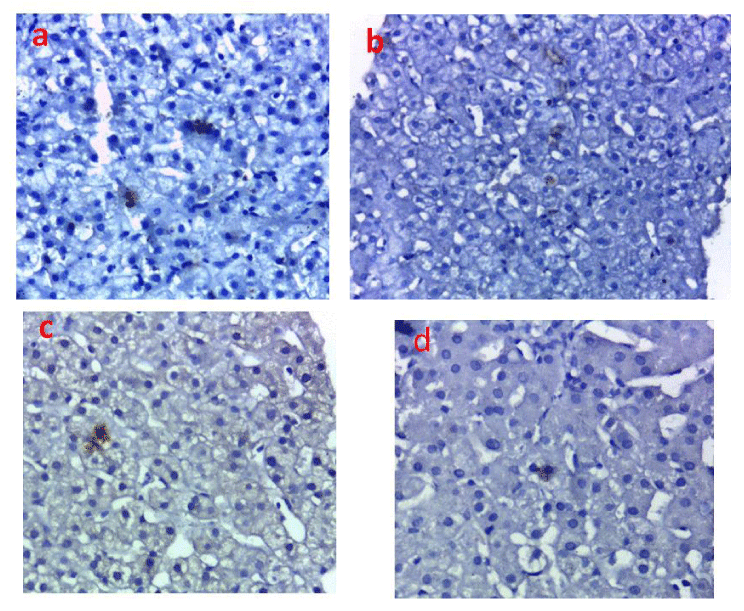
Each paraffin section was first deparaffinized the sections were heated in 10 mM sodium citrate buffer (pH 6.0) at 100°C for 10 min in microwave and then treated with 0.3% hydrogen peroxide in methanol for 15 min. After that the sections were washed three times with phosphate-buffered saline (PBS), they were incubated with Protein Block for 1 h at room temperature and sequentially reacted with mouse monoclonal antibody against HBs Ag antibody (3E7) (1:50 dilution; Novus Biologicals, USA) overnight at room temperature. Then the sections were rinsed in PBS three times. For the detection of the staining, the power stain 1.0 poly HRP DAB kit (Genemed, Biotechnologies Inc. USA) was used with DAB as the chromagen. This antibody stained the cytoplasm of the liver cells infected with the HB virus (Figure 1).
DNA extraction
Viral DNA extracted from Paraffin sections using GF-1 total DNA extraction kit (Vivantis, California, USA) according to manufactures instructions.
Conventional PCR
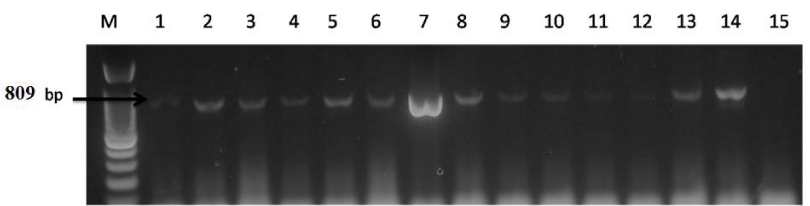
The primers sequence used for conventional PCR for amplification of the polymerase region (P) amplification (809 bp), 10 µL of extracted purified DNA from paraffin block of liver biopsy were mixed in a 50 µL reaction volume containing GoTaq Flexi buffer 1X, 1.5mM MgCl2, 200 µM dNTP, 2.5 U GoTaq Flexi DNA polymerase (Thermo scientific) and 200 nM of each primer POL3 (forward, 5’-GAC TCG TGG TGG ACT TCT CTC A-3’) and POL4 (reverse, 5’-GGC ATT AAA GCA GGA TAT CCA CAT TG-3’) (?). PCR products of polymerase gene were visualized and analyzed by gel documentation system (Biometra Germany) (Figure 2).
Results
The 111 anti-HCV positive patients included in this study included 77 (70%) males and 33 (30%) females, with a male to female ratio of 2.3/1. Their mean age was 45.2 ± 10.2 years. All of the 111 HCV RNA positive patients are serum HBsAg negative.
The HBsAg was detected in the liver tissue of 13/111 (11.7%) HCV-infected patients, and HBV DNA was detected in these same cases. Their characteristics are presented in Table 1.
There was no significant statistical difference between those infected with HCV alone and those with combined HCV/HBV as regard AST, ALT, bilirubin, albumin, CBC, HCV RNA levels as well as the hepatic fibrosis score (Tables 2,3).
One hundred and one patients (90%) received optimized peg-IFNa and Ribavirin therapy. Overall, a sustained virological response was observed in 59.4 % of those patients. All patients with HCV and occult HBV received therapy; only 6 (46.2%) responded to treatment while among the patients with HCV infection alone, only 54 patient (55.1 %) responded to treatment and there was no significant difference in response rate between those with and those without occult B (P =0.27).
We compared the hepatic histological activities of the 13 cases of chronic hepatitis C who had detectable intrahepatic HBV DNA. They were divided into mild 10 (76.9%) moderate 3 (23.1%) and severe activity 0 (0%) and as regards the fibrosis stage it was mild fibrosis in 7 (53.8%) moderate in 5 (38.5%) and severe in 1 (7.7%) while on the other hand those without occult HBV there was mild activity in 44 (44.9%) moderate in 32 (32.7%)and severe activity in 22 (22.4%) while as regards fibrosis staging it was mild in 38 (38.7%) and moderate in 28 (28.8%) and advanced fibrosis and cirrhosis in 32 (32.6%) but the difference in activity and fibrosis between those with and without concomitant occult HBV was not statistically significant (P = 0.17 for activity stage and P=0 .42 for fibrosis score).
Discussion
Occult HBV infection has frequently been identified in patients with chronic HCV infection [6,7]. This occult infection may be associated with more severe liver damage and even the development of hepatocellular carcinoma (HCC) [8,9].
In this study, we have investigated the prevalence of occult HBV infection in a population of HCV-infected patients at different stages of the disease, from chronic hepatitis to liver cirrhosis.
The study included 111 patients with HCV viral infection presenting for liver biopsy before interferon-based treatment with negative viral markers for hepatitis B surface antigen in serum samples. Retrospectively, we examined the liver tissues for the presence of hepatitis B surface antigen by immune-histochemistry as well as PCR detection of HBV DNA in liver specimens. We found 13 patients (11.7%) had evidence of hepatitis B viral infection by both methods used.
Recent studies on occult HBV in hepatitis C patients reported highly variable prevalence, from 0% to 52% [7,10-15]. In addition to geographical variability [Lo et al., 1993], the prevalence of occult HBV infection varies depending on the hepatitis B risk factors [16]. Another reason for the heterogeneity of the results on the prevalence of occult hepatitis B is the great variability in methodological approaches to its detection. First, some studies have looked for HBV DNA in liver tissue and some in serum. If the strong suppression of HBV activity is responsible for HBsAg negativity, it will also create the very low or even undetectable levels of serum HBV DNA characterizing most cases with occult infection.
A number of studies where investigators evaluated the presence of occult HBV in both liver and serum samples have shown that a relevant percentage of patients who are negative for HBV DNA in the serum may have HBV DNA in the liver tissue [17,10,13]. Thus, the examination of liver DNA extracts seems to be the most reliable methodological approach for occult HBV detection [18,19], but is difficult to apply in clinical practice.
HCV patients co-infected with occult HBV presented biochemical parameters as well as degrees of liver fibrosis that were not statistically significantly different from those patients solely infected with HCV. The same findings were reported by Jae Young et al 2011 who concluded that the frequency of occult HBV infection was not affected by chronic hepatitis C infection, and the effect of occult HBV infection on the disease severity of hepatitis C was not significant [20].
This is in contrast to a previous study done by Fernanda B et al 2007 who found higher levels of biochemical parameters as well as a greater degree of liver fibrosis in HCV/occult HBV patients when compared with patients with HCV alone [11]. Moreover, several studies [7,10,21] emphasized the clinical impact of silent HBV in patients suffering from chronic liver disease as a result of HCV and reported that higher levels of disease severity were seen in the liver.
Although the response rate to interferon-based therapy for both HCV/Occult HBV co-infected patients is lower than the response rate in those with patients solely infected with HCV yet the difference did not attain statistical significance (46.2% Vs 55.1 % P=0.27).
In a study done by Marion Levast et al 2010 where one hundred and twenty-four patients (88.5%) received optimized peg-IFNa and Ribavirin therapy, a sustained virological response was observed in 39.2% of the patients. This rate was slightly higher in anti- HBc-positive patients compared to anti-HBc-negative patients (43.2% vs. 37.5%), but this difference was also not significant [22].
In conclusion, occult HBV infection is a fact in our community and intrahepatic HBV DNA is detectable in HBsAg-negative subjects. Although we have found that the effect of occult HBV infection on the disease severity of hepatitis C as well as the response to standard Interferon/ ribavirin therapy was not significant, the effect of this occult infection on the response to direct acting antiviral therapy is yet to be seen.
Fund: Supported by the Science and Technology Development Fund, Ministry of Scientific Research Egypt, Project No. 1587; and Cairo University.
- Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, et al. (2001) Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely "occult"? Hepatology 34: 194-203. Link: https://bit.ly/32Rq8CH
- Torbenson M, Thomas DL (2002) Occult hepatitis B. Lancet Infect Dis 2: 479-486. Link: https://bit.ly/2OeZjVZ
- Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, et al. (2009) Control of cccDNA function in hepatitis B virus infection. J Hepatol 51: 581-592. Link: https://bit.ly/2Ymbqk0
- Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, et al. (2008) Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 49: 652-657. Link: https://bit.ly/2K2smGT
- Raimondo G, Pollicino T, Romano L, Zanetti AR (2010) A 2010 update on occult hepatitis B infection. Pathol Biol (Paris) 58: 254-257. Link:
- Allain JP (2004) Occult hepatitis B virus. Transfus Clin Biol 11: 11–25. Link: https://bit.ly/2Z7vUOA
- Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, et al. (1999) Occult hepatitis B. Virus infection in patients with chronic hepatitis C liverdisease N Engl J Med 341: 22–26. Link: https://bit.ly/2K45arH
- Yotsuyanagi H, Shintani Y, Moriya K, Fujie H (2000) Virologic analysis of non-B, non-C hepatolcellular carcinoma in Japan: frequent involvement of hepatitis B virus. J Infect Dis 181: 1920–1928. Link: https://bit.ly/2SD6Uwf
- Ocana S, Casas ML, Buhigas I, Lledo JL (2011) Diagnostic strategy for occult hepatitis B virus infection. World J Gastroenterol 17: 1553–1537. Link:
- Fabris P, Brown D, Tositti G, Bozzola L, Giordani MT, et al. (2004) Occult hepatitis B virus infection does not affect liver histology or response to therapy with interferon alpha and ribavirin in intravenous drug users with chronic hepatitis C. J Clin Virol 29: 160-166. Link: https://bit.ly/32QOk8k
- Branco F, de Mattos AA, Perdomo G (2007) Occult hepatitis B virus infection in patients with chronic liver disease due to hepatitis c virus and hepatocellular carcinoma in brazil. Arq Gastroenterol 44: 58-63. Link: https://bit.ly/2y8mBC1
- Kao JH, Chen PJ, Lai MY, Chen DS (2002) Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol 40: 4068-4071. Link: https://bit.ly/2Y4eoOt
- Mariscal LF, Rodriguez-Inigo E, Bartolome J, Castillo I, Ortiz-Movilla N (2004) Hepatitis B infection of the liver in chronic hepatitis C without detectable hepatitis B virus DNA in serum. J Med Virol 73: 177-186. Link: https://bit.ly/30Td6my
- Sagnelli E, Coppola N, Scolastico C, Mogavero Ar, Filippini P, et al. (2001) HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol 64: 350-355. Link: http://bit.ly/2LFyacE
- Shintani Y, Yotsuyanagi H, Moriya K, Fujie H, Tsutsumi T, et al. (2000) The significance of hepatitis B virus DNA detected in hepatocellular carcinoma of patients with hepatitis C. Cancer 88: 2478-86. Link: https://bit.ly/2LF6jcF
- Lo YM, Lo ES, Mehal WZ, Sampietro M, Fiorelli G, et al. (1993) Geographical variation in prevalence of hepatitis B virus DNA in HBsAg negative patients. J Clin Pathol 46: 304–308. Link: https://bit.ly/32S2BBS
- De Maria N, Colantoni A, Friedlander L, Leandro G, Idilman R, et al. (2000) The impact of previous HBV infection on the course of chronic hepatitis C. Am J Gastroenterol 95: 3529–3536. Link:
- Raimondo G, PollicinoT, Cacciola I, Squadrito G (2007) Occult hepatitis B virus infection. J Hepatol 46: 160–170. Link: https://bit.ly/2YmXkmb
- Sagnelli E, Imparato M, Coppola N, Pisapia R, Sagnelli C, et al. (2008) Diagnosis and clinical impact of occult hepatitis B infection in patients with biopsy proven chronic hepatitis C: A multicenter study. J Med Virol 80: 1547–1553. Link: https://bit.ly/2OhXveY
- Jang JY, Jeong SW, Cheon SR, Lee SH (2011) Clinical significance of occult hepatitis B virus infection in chronic hepatitis C patients The Korean Journal of Hepatology 17: 206-212. Link: https://bit.ly/2Ol7H6g
- Miura Y, Shibuya A, Adachi S, Takeuchi A, Tsuchihashi T, et al. (2008) Occult hepatitis B virus infection as a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C in whom viral eradication fails. Hepatol Res 38: 546–556. Link:
- Levast M, Larrat S, Thelu MA, Nicod S (2010) Prevalence and Impact of Occult Hepatitis B Infection in Chronic Hepatitis C Patients Treated with Pegylated Interferon and Ribavirin. Journal of Medical Virology 82: 747–754. Link: https://bit.ly/30S59Os
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
