Archives of Hepatitis Research
Vitamin K: A potential Liver Cancer treatment way
Sofia Dahlberg1 and Ulf Schött1,2*
2Department of Anesthesiar and Intensive Care, Skane University Rrsity Hospital Lund, Lund,
Cite this as
Dahlberg S, Schött U (2017) Vitamin K: A potential Liver Cancer treatment way. Archives of Hepatitis Research 3(2): 049-052. DOI: 10.17352/ahr.000017A review on different vitamin K1-3 effects on hepatocellular cancer and their tumour cell biology mechanism indicate possible synergistic treatment strategies. Monitoring of dysfunctional carboxylation of the vitamin K dependent coagulation factor II, with the commercial ELISA test PIVKA-II has been used as a hepatocellular cancer marker. Its relevance is also reviewed. Currently the PIVKA-II test has been withdrawn due to marketing reasons by Stago.
Introduction
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and roughly 75% of cases are attributed to viral infections with hepatitis B or C [1]. Furthermore, HCC is the second leading cause of cancer death surpassed only by lung malignancies [2], and is often unresectable at diagnosis. The most commonly used tumour marker for HCC is alpha-fetoprotein (AFP) but its diagnostic efficiency is poor, especially in hepatitis infected individuals [3]. Hence, better diagnostic and prognostic markers would aid in early detection and treatment of HCC.
Vitamin K is best known for its involvement in haemostasis, where it functions as a cofactor in γ-carboxylation of hepatic clotting factors II, VII, IX and X as well as protein C, S, Z and M. This post-translational modification induces a conformational change which allows the protein to bind calcium ions, a crucial step for their involvement in haemostasis [4]. In addition to aforementioned clotting factors several vitamin K dependent proteins originating from extra hepatic tissues have been identified and are collectively referred to as Gla proteins. Accumulating evidence from pre-clinical studies suggest that vitamin K and the Gla proteins are involved in a wide range of diseases, such as cardiovascular disease, osteoporosis and cancer [5].
Proteins induced by vitamin K absence for factor II (PIVKA-II, or des-gamma-carboxy prothrombin, DCP) is a measure of hypocarboxylated prothrombin and has been suggested to function as a tumour marker for HCC. In the following sections, the efficacy of PIVKA-II as a tumour marker and the role of vitamin K in HCC treatment are discussed.
Performance of PIVKA-II as a HCC tumour marker
Multiple studies have shown increased PIVKA-II in HCC patients, and PIVKA-II is considered a valuable complement to AFP. Whereas the AFP measure reflects intrahepatic tumour burden, PIVKA-II correlates with vascular invasion and extra hepatic disease [6]. A suggested mechanism of action of PIVKA-II is that it facilitates the secretion of matrix metalloproteinase (MMP) 9 and MMP-2 through the ERK1/2MAPK pathway [7]. These proteolytic enzymes have the ability to degrade extracellular matrix and facilitate metastasis [8]. PIVKA-II has also been suggested to function as an autocrine/paracrine mitogen for HCC cells by stimulating the Met-JAK-STAT pathway [9]. The mechanism underlying the PIVKA-II increase is not completely understood, but several explanations have been proposed. For instance, animal experiments have shown decreased gamma-carboxylase activity in hepatic tumour tissue [10]. Other suggestions are vitamin K insufficiency in the tumour cells [11], defective vitamin K uptake [12] and excessive synthesis of prothrombin precursors [13].
Several studies comparing the diagnostic capability of different biomarkers for HCC exist. Using PIVKA-II in combination with AFP has been reported to improve accuracy [14]. Other studies looking exclusively at hepatitis B infected individuals suggest PIVKA-II is superior to AFP, and that the combination of both measures may enhance early detection [15]. A recent study comprising roughly 2 000 participants concluded that 230 HCC cases would have been neglected if using AFP alone, and that PIVKA-II levels increased over a year before image discovery [16]. Also, high PIVKA-II levels where associated with greater risk of developing HCC within a 2-year period in high risk populations with chronic hepatitis B. Furthermore, PIVKA-II has also been associated with portal vein thrombosis [17] and microvascular invasion, which is a major prognostic factor in HCC [18]. In addition to PIVKA-II and AFP, several other HCC biomarkers have been proposed. Due to the heterogeneity of HCC it seems that a combination of biomarkers is favorable, but currently no large scale studies investigating the optimal biomarker combination have been performed [19].
In addition to viral hepatitis, alcohol-induced liver damage is a major risk factor for developing HCC [20]. In a study investigating PIVKA-II levels in patients with benign alcoholic liver disease (ALD), 21% of cases had PIVKA-II levels above the cut-off value commonly used for the tumour marker [21]. Similar results were demonstrated in a more recent study [22]. The causative mechanism of the PIVKA-II increase is unknown. Vitamin K deficiency has been suggested [23], but no correlation between plasma vitamin K levels and PIVKA-II has been demonstrated [21, 24]. The confounding effect of ALD on PIVKA-II levels should be taken into account when interpreting PIVKA-II levels, and a different cut-off value might be needed to avoid false-positive results in ALD patients.
HCC treatment with vitamin K
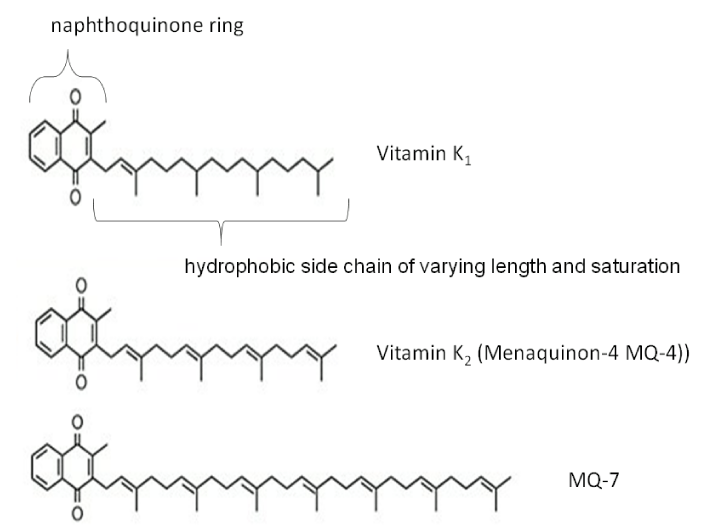
Due to the aforementioned effects of PIVKA-II on HCC tumour cells, and that PIVKA-II seems to predispose for more aggressive disease [25], studies have investigated whether vitamin K administration could affect the disease course. Vitamin K occurs naturally in two forms, vitamin K1 and vitamin K2, which is further subdivided depending on the length and saturation of its side chain [26]. In addition, synthetic forms vitamin K3–K5 exist. Vitamin K2 subspecies MK-4 is a ligand to the steroid and xenobiotics receptor (SXR) which regulates cell growth. In vitro studies have demonstrated vitamin K2-dependent suppression of proliferation and motility of HCC cells [27]. Furthermore, administration of vitamin K to patients with HCC subsequently decreased plasma PIVKA-II levels [28]. This study also demonstrated that PIVKA-II levels were not correlated with plasma levels of vitamin K derivatives, suggesting that the increase is not due to vitamin K deficiency but rather related to tumour metabolism.
In 2004 vitamin K2 supplementation was reported to prevent HCC in women with viral cirrhosis [29]. Since then, several supplementation studies with MK-4 to HCC patients have been performed with mixed results. Although a few smaller studies have demonstrated a reduction of HCC recurrence when administrating MK-4 [30], this effect was not confirmed in larger study populations using MK-4 [31]. In a recent meta-analysis of five randomized control trials evaluating the effect of vitamin K2 on HCC recurrence after resection, survival was not improved [32]. However, synergistic effects were observed when MK-4 and the multikinase inhibitor sorafenib were administered together [33]. The vitamin K2 derivative MK-7 has a longer half-life and better bioavailability for both hepatic and extra hepatic Gla proteins [34]. Several clinical trials investigating the effects of MK-7 on vascular disease are currently ongoing [35], but its effects on HCC has not been investigated.
Vitamin K3 has been investigated in several studies due to its reported ability to generate ROS. In a clinical trial where vitamin K3 was administered to patients with advanced HCC reduction of tumour size was demonstrated in 17% of the patient population. These patients also demonstrated increased mean survival time, but overall mortality was not affected [36]. Vitamin K3 has also been used as a radio-sensitizer, and older studies have demonstrates increased survival time compared to radiotherapy alone in bronchial carcinoma patients [37]. Similar potentiation have been demonstrated in animal experiments [38, 39], but whether this can be used as adjuvant therapy in humans have not been studied in recent years. Vitamin K3 has not been extensively studied in humans due to its hematological toxicities related to erythrocyte glutathione metabolism [40]. However, if administered concomitant with vitamin C, the necessary dose decreases due to synergistic effects. A small clinical trial investigating the effect of vitamin K3 and vitamin C on therapy-resistant prostate cancer demonstrated a decrease in the rate of prostate specific antigen (PSA) increase after 12 weeks [41], but the combination has not been studied on HCC patients.
- Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127: S5-s16. Link: https://goo.gl/8iXk1z
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-386. Link: https://goo.gl/o57bkY
- Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, et al. (2001) Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 34: 570-575. Link: https://goo.gl/ueDuJc
- Weston BW, Monahan PE (2008) Familial deficiency of vitamin K-dependent clotting factors. Haemophilia 14: 1209-1213. Link: https://goo.gl/DqriTq
- Cranenburg EC, Schurgers LJ, Vermeer C (2007) Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost 98: 120-125. Link: https://goo.gl/DYP4ru
- Park H, Park JY (2013) Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed Res Int 2013: 310427. Link: https://goo.gl/mmHMBb
- Yue P, Gao ZH, Xue X, Cui SX, Zhao CR, et al. (2011) Des-gamma-carboxyl prothrombin induces matrix metalloproteinase activity in hepatocellular carcinoma cells by involving the ERK1/2 MAPK signalling pathway. Eur J Cancer 47: 1115-1124. Link: https://goo.gl/UuDEJV
- Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M, et al. (2002) Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer 97: 425-431. Link: https://goo.gl/9x5DrA
- Suzuki M, Shiraha H, Fujikawa T, Takaoka N, Ueda N, et al. (2005) Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J Biol Chem 280: 6409-6415. Link: https://goo.gl/6tRcSj
- Shah DV, Engelke JA, Suttie JW (1987) Abnormal prothrombin in the plasma of rats carrying hepatic tumors. Blood 69: 850-854. Link: https://goo.gl/8i5Xe5
- Okuda H, Obata H, Nakanishi T, Furukawa R, Hashimoto E (1987) Production of abnormal prothrombin (des-gamma-carboxy prothrombin) by hepatocellular carcinoma. A clinical and experimental study. J Hepatol 4: 357-363. Link: https://goo.gl/EU6JL5
- Murata K, Sakamoto A (2008) Impairment of clathrin-mediated endocytosis via cytoskeletal change by epithelial to fibroblastoid conversion in HepG2 cells: a possible mechanism of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol 33: 1149-1155. Link: https://goo.gl/T8tQVe
- Yamagata H, Nakanishi T, Furukawa M, Okuda H, Obata H (1995) Levels of vitamin K, immunoreactive prothrombin, des-gamma-carboxy prothrombin and gamma-glutamyl carboxylase activity in hepatocellular carcinoma tissue. J Gastroenterol Hepatol 10: 8-13. Link: https://goo.gl/uoNy2N
- Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, et al. (2008) AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer 8: 200. Link: https://goo.gl/UBTWmW
- Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, et al. (2015) Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 21: 3928-3935. Link: https://goo.gl/C1wsYJ
- Yu R, Tan Z, Xiang X, Dan Y, Deng G (2017) Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer 17: 608. Link: https://goo.gl/mkWrRo
- Truong BX, Yano Y, Van VT, Seo Y, Nam NH, et al. (2013) Clinical utility of protein induced by vitamin K absence in patients with chronic hepatitis B virus infection. Biomed Rep 1: 122-128. Link: https://goo.gl/cBFWY3
- Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, et al. (2014) Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion 2014. Link: https://goo.gl/m2JR7u
- Van Hees S, Michielsen P, Vanwolleghem T (2016) Circulating predictive and diagnostic biomarkers for hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 22: 8271-8282. Link: https://goo.gl/FDyMQm
- Testino G, Leone S, Borro P (2014) Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol 20: 15943-15954. Link: https://goo.gl/W4f92A
- Ohhira M, Ohtake T, Saito H, Ikuta K, Tanaka K, et al. (1999) Increase of serum des-gamma-carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma. Alcohol Clin Exp Res 23: 67s-70s. Link: https://goo.gl/FWN9GX
- Kang KH, Kim JH, Kang SH, Lee BJ, Seo YS, et al. (2015) The influence of alcoholic liver disease on serum PIVKA-II levels in patients without hepatocellular carcinoma. Gut Liver 9: 224-230. Link: https://goo.gl/nGc5y9
- Iber FL, Shamszad M, Miller PA, Jacob R (1986) Vitamin K deficiency in chronic alcoholic males. Alcohol Clin Exp Res 10: 679-681. Link: https://goo.gl/irBjek
- Sakizono K, Oita T, Eto M, Bito S, Takegawa H, et al. (2002) [Studies on the mechanism of elevation of serum PIVKA-II levels in alcoholic liver cirrhosis]. Rinsho Byori 50: 289-295. Link: https://goo.gl/NCnVWX
- Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, et al. (2001) Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer 91: 561-569. Link: https://goo.gl/zkVRJ7
- Shearer MJ, Newman P (2008) Metabolism and cell biology of vitamin K. Thromb Haemost 100: 530-547. Link: https://goo.gl/2Qunx4
- Azuma K, Urano T, Ouchi Y, Inoue S (2009) Vitamin K2 suppresses proliferation and motility of hepatocellular carcinoma cells by activating steroid and xenobiotic receptor. Endocr J 56: 843-849. Link: https://goo.gl/pQEd2f
- Sakon M, Monden M, Gotoh M, Kobayashi K, Kanai T, et al. (1991) The effects of vitamin K on the generation of des-gamma-carboxy prothrombin (PIVKA-II) in patients with hepatocellular carcinoma. Am J Gastroenterol 86: 339-345. Link: https://goo.gl/icSQ6z
- Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, et al. (2004) Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. Jama 292: 358-361. Link: https://goo.gl/DSpZG2
- Chu KJ, Lai EC, Yao XP, Zhang HW, Lau WY, et al. (2010) Vitamin analogues in chemoprevention of hepatocellular carcinoma after resection or ablation--a systematic review and meta-analysis. Asian J Surg 33: 120-126. Link: https://goo.gl/HU3VHS
- Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, et al. (2011) Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 54: 532-540. Link: https://goo.gl/M3DNJv
- Riaz IB, Riaz H, Riaz T, Rahman S, Amir M, et al. (2012) Role of vitamin K2 in preventing the recurrence of hepatocellular carcinoma after curative treatment: a meta-analysis of randomized controlled trials. BMC Gastroenterol 12: 170. Link: https://goo.gl/h3Rc2N
- Jung DH, Hwang S, Song GW, Ryoo BY, Kim N, et al. (2015) An interim safety analysis of hepatocellular carcinoma patients administrating oral vitamin K with or without sorafenib. Korean J Hepatobiliary Pancreat Surg 19: 1-5. Link: https://goo.gl/oTgc2y
- Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, et al. (2007) Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 109: 3279-3283. Link: https://goo.gl/E37472
- Vossen LM, Schurgers LJ, van Varik BJ, Kietselaer BL, Vermeer C, et al. (2015) Menaquinone-7 Supplementation to Reduce Vascular Calcification in Patients with Coronary Artery Disease: Rationale and Study Protocol (VitaK-CAC Trial). Nutrients 7: 8905-8915. Link: https://goo.gl/AHJHeG
- Sarin SK, Kumar M, Garg S, Hissar S, Pandey C, et al. (2006) High dose vitamin K3 infusion in advanced hepatocellular carcinoma. J Gastroenterol Hepatol 21: 1478-1482. Link: https://goo.gl/3RcGaX
- Mitchell JS, Brinkley D, Haybittle JL (1965) Clinical trial of radiosensitizers, including synkavit and oxygen inhaled at atmospheric pressure. Acta Radiol Ther Phys Biol 3: 329-341. Link: https://goo.gl/j4TvRg
- Taper HS, Keyeux A, Roberfroid M (1996) Potentiation of radiotherapy by nontoxic pretreatment with combined vitamins C and K3 in mice bearing solid transplantable tumor. Anticancer Res 16: 499-503. Link: https://goo.gl/UoExX1
- Taper HS (2008) Altered deoxyribonuclease activity in cancer cells and its role in nontoxic adjuvant cancer therapy with mixed vitamins C and K3. Anticancer Res 28: 2727-2732. Link: https://goo.gl/j33znd
- Meyer TC, Angus J (1956) The effect of large doses of synkavit in the newborn. Arch Dis Child 31: 212-215. Link: https://goo.gl/uCCX9J
- Tareen B, Summers JL, Jamison JM, Neal DR, McGuire K, et al. (2008) A 12 week, open label, phase I/IIa study using apatone for the treatment of prostate cancer patients who have failed standard therapy. Int J Med Sci 5: 62-67. Link: https://goo.gl/yCHKUV
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
