Archives of Hepatitis Research
Accidentally discovered Portal Vein Thrombosis before Splenectomy due to Hypersplenism - The role of Thrombogenic Genes Polymorphisms
Amr Shaaban Hanafy*
Cite this as
Hanafy AS (2017) Accidentally discovered Portal Vein Thrombosis before Splenectomy due to Hypersplenism - The role of Thrombogenic Genes Polymorphisms. Archives of Hepatitis Research 3(2): 037-040 DOI: 10.17352/ahr.000015Portal vein thrombosis in patients with liver cirrhosis may be due neoplastic growth invading portal vein or due to non-neoplastic causes. The indications for treating PVT in cirrhotic individuals are difficult to be established but at least are of great benefit in acute PVT. Investigating the cause of portal vein thrombosis in cirrhotic patient prepared for splenectomy including virology and immune markers, AFP, triphasic CT. Factor V Leiden mutation and prothrombin gene 20210. The case showed non-neoplastic portal vein thrombosis due to portal pyemia with underlying prothrombin gene 20210 polymorphism. The early initiation of anticoagulation after the diagnosis of acute PVT may be the only predictive factor for complete PV recanalization with preservation of liver function.
Abbreviations
APC: Activated Protein C; Anti d DNA: Anti double Stranded DNA; AFP: Alfafetoprotein; ANA: Anti-Nuclear Antibodies; AMA: Anti Mitochondrial Antibodies; AT: Antithrombin III; CT: Computed Tomography; EVL: Esophageal Varices Banding; INR: International Normalized Ratio, LMWH: Low Molecular Weight Heparin; PVT: Portal Vein Thrombosis; TM: Thrombomodulin.
Introduction
Portal vein thrombosis (PVT) in patients with liver cirrhosis may be due to neoplastic growth invading portal vein or non-neoplastic causes that may include local causes as cirrhosis, hepatobiliary malignancy, portal pyemia, inflammatory diseases as pancreatitis, cholecystitis, cholangitis, appendicitis, ulcerative colitis or diverticulitis. Systemic causes as factor V Leiden mutation, prothrombin gene 20210 mutation, myeloproliferative disorders, hyperhomocysteinemia, antiphospholipid syndrome, recent use of contraceptive pills or early months of gestation [1].
It appears that prevalence increases with progression of liver disease being 1% in patients with compensated cirrhosis up to 25% in patients with urgent need for liver transplantation [2].
PVT has been associated with increased risk of hepatic decompensation, worse outcome including post-liver transplantation outcome. Predisposing factors for PVT are reduced blood flow velocity [3], severity of thrombocytopenia which reflects the severity of portal hypertension, previous variceal hemorrhage, post-splenectomy and endoscopic treatment of esophageal varices which may be complicated by thrombosis [4].
Cirrhotic coagulopathy
Cirrhosis can be associated with a hypercoagulable state despite the events that favor bleeding tendency and thrombocytopenia due to portal hypertension. Normally coagulation process is regulated through inhibition of thrombin generation via factors V and VIII inactivation mediated by activated protein C (APC) together with thrombomodulin (TM), protein S and negatively charged phospholipids. In cirrhosis, there is impaired production of coagulation factors except endothelium derived factor VIII, low levels of hepatic-derived protein C, S which augment thrombin production that converts fibrinogen to fibrin clot. Fibrinogen is often preserved or only mildly decreased in cirrhosis [5,6].
Also the decreased plasma levels of plasminogen and increased levels of plasminogen activator inhibitor may add to the procoagulant imbalance found in cirrhosis [7].
The clinical sequel of PVT include variceal bleeding or inability to control it, intestinal infarction when thrombosis extends to the superior mesenteric vein, PVT is a well-known risk factor of early mortality after liver transplantation and can also contraindicate liver transplantation in cases where thrombosis extends to the splenic or inferior mesenteric veins.
The aim of anticoagulation is recanalization of portal vein, complete recanalization occurred in up to 45% of patients while partial PV recanalization is observed in up to 35% of cases [5] which are similar to that occurred in noncirrhotic patients [8].
The indications for treating PVT in cirrhotic individuals are difficult to be established but at least are of great benefit in acute PVT with extension to the superior mesenteric vein, or PVT in cirrhotic individuals with a documented prothrombotic disorder.
The early initiation of anticoagulation within 14 days after the diagnosis of acute PVT may be the only predictive factor for complete PV recanalization [9].
In situation where cirrhotic patients develop PVT with preexisting bleeding esophageal varices; Amitrano et al noted no benefit in terms of variceal re-bleeding but a surprising improvement in survival in the treated group [10].
Clinically, patients may develop abdominal pain (22%) resistant to conventional analgesics especially when acute or extending to the mesenteric veins to cause intestinal ischemia, 45% is asymptomatic and 30% may present with upper gastrointestinal tract bleeding.
Choice of anticoagulants in cirrhotic patients with PVT
Choice of anticoagulants is difficult in cirrhotic patients due to problems in monitoring and the existing coagulopathy.Vitamin K antagonists have a rate of complete PV recanalization in cirrhotics between 40% and 45% after a mean duration 8.1 months of anticoagulation therapy [11]. The problem encountered is monitoring INR value which is altered in cirrhosis [5].
Low molecular weight heparin (LMWH) as enoxaparin has also been used to treat PVT in cirrhotic individuals, with complete PV recanalization in 33% after 6 months of treatment and in 75% of the cases when therapy was extended to another 6 months (median 6.5 months) [12].
The problems encountered are difficult dose calculation due to increased volume of distribution because of ascites and edema [13] and monitoring of anti-Xa cannot be used to guide therapy as it is dependent on antithrombin-III (AT) level, which is decreased in cirrhotic patients [13], also renal function is often impaired in cirrhotic patients and as LMWH is eliminated by the kidneys, their half-life will be increased.
The use of direct thrombin inhibitors as Dabigatran is an interesting subject; the potential advantage is that their mechanism of action is independent of AT. The rates of intracranial bleeding were significantly lower than in warfarin, but significantly higher with regard to gastrointestinal bleed [14] and the lack of an antidote , however a promising agent such as Andexanet Alfa is currently being evaluated in two randomized, double-blind, placebo-controlled Phase 3 studies.
The optimal duration of anticoagulation therapy was not determined but partial response to anticoagulation at 6 months of therapy can get benefit from prolonged therapy up to 12 months [15], continuation of anticoagulation after 12 months in nonresponders was associated with a decreased risk of thrombosis progression [16].
As regards complications under anticoagulation therapy in cirrhotic patients; A platelet count <50,000 was the only factor more frequently associated with bleeding. It was shown that bleeding secondary to portal hypertension was more frequent in cirrhotic individuals with PVT without previous anticoagulation therapy [16].
It is preferable to screen for varices before starting anticoagulation. Patients with previous variceal bleeding, grade II or more esophageal varices with mucosal risky signs should be treated by esophageal varices banding (EVL) before anticoagulation together with nonselective beta-blockers. Anticoagulation therapy then will be initiated within 15 days after the procedure, some authors prefer to postpone the initiation of anticoagulation till eradication of esophageal varices to avoid post EVL bleeding but this will affect the rate of portal vein recanalization.
Case Report
46 year old female patient presented for medical evaluation before splenectomy due to pancytopenia. The family history was noncontributory, specifically for bowel disease or bowel cancer, and negative for any history of bleeding or clotting disorders.
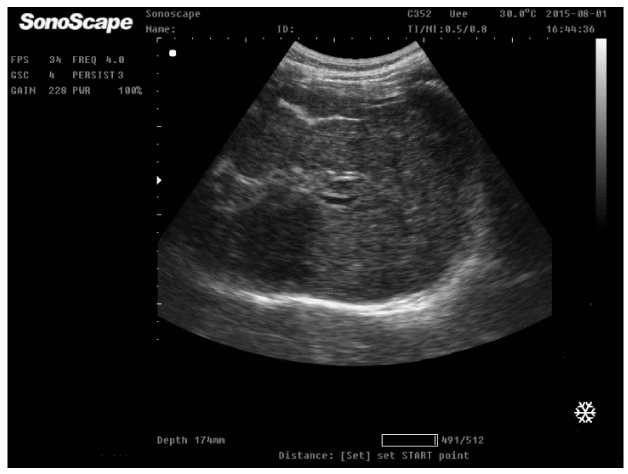
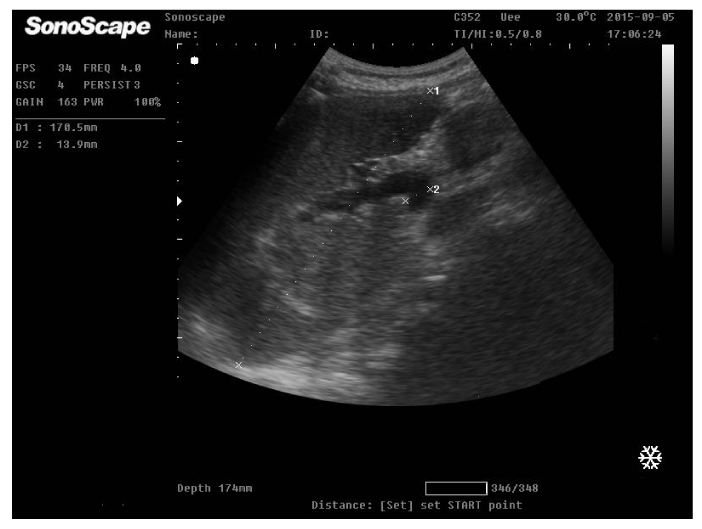
On physical examination, the patient was afebrile with normal blood pressure 110/60 mmHg; heart rate 68 b/min, Specific examination findings was unrevealing except for palpable spleen. She did not demonstrate signs of anaemia or parenchymatous liver cell failure as jaundice, astrexis, ascites or lower limb oedema. Laboratory studies included complete blood count; WBCs 2300 cell/ul, platelets 34000/ul, prothrombin concentration 42%, INR 1.9. Virology as HBsAg, HBcAb, and HCV Ab were negative. Markers of immune liver diseases as ANA, anti dDNA, AMA were negative, hemoglobin electrophoresis was normal, ferritin, serum cupper and ceruloplasmin was normal. The patient was positive for antibilharzial antibodies and circulating bilharzial antigen. AFP was 5.6ug/dl, Bone marrow aspiration showed normal blood elements indicating peripheral destruction. Reticulocytic count was normal. Abdominal ultrasonography revealed cirrhotic liver with complete portal vein thrombosis, no focal lesions and splenomegally as shown (Figures 1,2).
Triphasic CT was performed and revealed complete PVT with intact mesenteric circulation and no hepatic focal lesion. At the time of examination, the patient was asymptomatic without abdominal pain, however the patient mentioned that she developed 6 months ago an attack of severe periumblical abdominal pain 1 week after the development of thrombosed internal piles which lasted for 2 weeks but not investigated. Factor V Leiden and prothrombin gene 20210 were performed and the patient was positive for prothrombin gene 20210 polymorphism.
The diagnosis suggested is portal vein thrombosis induced by portal pyemia due to infected and thrombosed internal piles augmented by the thrombotic tendency due to positivity of prothrombin gene 20210.
Liver biopsy and the intention to treat by oral anticoagulation were deferred due to severe thrombocytopenia and INR prolongation. Upper GIT endoscopy was performed and revealed grade II-III esophageal varices which were banded in 2 sessions. 2 months later, the patient developed decompensation with ascites that responded will to torsamide 10 mg and spironolactone 100mg. the patient now on liver support and is preparing for liver transplantation.
Discussion
The false fixed belief that prolonged prothrombin time may enhance the tendency of bleeding in cirrhosis is now changing, complex and multifactorial processes make this hemostatic dysequilibrium; both bleeding and coagulation tendency can occur in liver cirrhosis. Parenchymatous decompensation, severity of portal hypertension, sepsis and renal impairment increase the risk for bleeding [17].
The low protein C and S, the increase in the endothelium derived factors as factor VIII and von Willebrand factor (vWf) together with the sluggish portal blood flow in cirrhosis may enhance clotting [18].
A Platelet count more than 50,000/m3 in liver cirrhosis is adequate for a normal hemostasis as the high level of VWF can replace the platelet defect for thrombin generation [19].
Among the most common hereditary causes of venous thrombosis are Factor V Leiden (FVL) G1691A, prothrombin gene G20210A [20]. The gene that codes the Factor V Leiden protein is (F5) and is due to a mutation in exon 10 as a missense substitution of base A to base G, and this will change the protein’s amino acid from arginine to glutamine, the mutation prevents efficient inactivation of factor V [20].
Prothrombin gene mutation is caused by G→A transition at nucleotide 20210 leading to elevation of plasma prothrombin levels [21].
Portal vein thrombosis is a quite uncommon situation and is associated with liver cirrhosis or prothrombotic disorder. Patients with acute PVT may present clinically acute abdominal pain unresolved with conventional analgesics, acute ascites or life threatening mesenteric ischemia with nausea and vomiting [22].
If hypercoagulable states are documented, then and patients are subjected to a long-term anticoagulation with the goal of complete recanalization of the portal vein otherwise, anticoagulation should be stopped within 3–6 months.
In conclusion, Portal vein thrombosis represents a life threatening clinical problem and should be treated urgently depending on its onset, associated vascular and parenchymatous complications.
We thank the many physicians who participated in this study. We also thank Ms. Yasuko Motodate and Ms. Tokuko Komagamine for excellent technical assistance.
- Omana VN, Guoliang X, Gilberto V, Harold SM (2006) Diagnosis of hepatitis A virus infection: a molecular approach. Clin Microbiol Rev 19: 63-79. Link: https://goo.gl/XumraZ
- Jacobsen KH, Koopman JS (2004) Declining hepatitis A seroprevalence: a global review. Epidemiol Infect 132: 1005-1022. Link: https://goo.gl/tau7PK
- Jeorg SH, Lee HS (2010) Hepatitis A: Clinical manifestations and manegement. Intervirology 53: 15-19. Link : https://goo.gl/fCF5UY
- Okamoto H, Takahashi M, Nishizawa T (2003) Features of hepatitis E virus infection in Japan. Intern Med 42: 1065-1071. Link: https://goo.gl/AsfKDv
- Purcell RH, Emerson SU (2008) Hepatitis E: an emerging awareness of an old disease. J Hepatol 48: 494-503. Link: https://goo.gl/6LY3Q5
- Hoofnagle JH, Nelson KE, Purcell RH (2012) Hepatitis E. N Engl J Med 27; 367: 1237-1244. Link: https://goo.gl/9cZF8p
- Wedemeyer H, Pischke S, Manns MP (2012) Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology 142: 1388-1397. Link: https://goo.gl/JXRWR6
- Takahashi M, Okamoto H (2014) Features of hepatitis E virus infection in humans and animals in Japan. Hepatol Res 44: 43-58. Link: https://goo.gl/p6uCDk
- Khuroo MS, Kamili S (2003) Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat 10: 61-69. Link: https://goo.gl/Af1kS6
- Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK (2007) Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 147: 28-33. Link: https://goo.gl/NJnXvK
- Ruggeri FM, Bartolo ID, Ponterio E, Angelomi G, Trevisani M, et al. (2013) Zoonotic transmission of hepatitis E in industrialized countries. New Microbiol 36: 331-344. Link: https://goo.gl/nKpC3w
- Mizuo H, Suzuki K, Takikawa Y, Sugai Y, Tokita H, et al. (2002) Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J Clin Microbiol 40: 3209-3218. Link: https://goo.gl/YS7uJe
- Krishna YR, Saraswat VA, Das K, Himanshu G, Yachha SK, et al. (2009) Clinical features and predictors of putcome in acute hepatitis A and hepatitis E virus on cirrhosis. Liver Int 29: 392-398. Link: https://goo.gl/4RNncC
- Manka P, Bechmann LP, Coombes JD, Thodou V, Schlattjan M, et al. (2015) Hepatitis E virus infection as a possible cause of acute liver failure in Europe. Clin Gastroenterol and Hepatol 13: 1836-1842. Link: https://goo.gl/d2SWoU
- Fujiwara K, Yokosuka O, Ehata T, Saisho H, Saotome N, et al. (2002) Association between severity of type A hepatitis and nucleotide variations in the 5' non-translated region of hepatitis A virus RNA: strains from fulminant hepatitis have fewer nucleotide substitutions. Gut 51: 82-88. Link: https://goo.gl/nQ1QvH
- Endo K, Inoue J, Takahashi M, Mitsui T, Masuko K, et al. (2007) Analysis of the full-length genome of a subgenotype IIIB hepatitis A virus isolate: primers fro broadly reactive PCR and genotype analysis. J Med Viol 79: 8-17. Link: https://goo.gl/nLKh26
- Yano K, Tamada Y, Yatsuhashi H, Komori A, Abiru S, et al. (2010) Dynamic epidemiology of acute viral hepatitis in Japan. Interviology 53: 70-75. Link: https://goo.gl/mdGi33
- Takahashi H, Yotsuyanagi H, Yasuda K, Koibuchi T, Suzuki M, et al. (2006) Molecular epidemiology of hepatitis A virus in metropolitan areas in Japan. J Gastroenterol 41: 981-986. Link: https://goo.gl/8XNzS5
- Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, et al. (2009) Clinical and molecular characteristics of hepatitis A virus infections during the years 1992-2003 in Ogaki, a centrally located city of Japan. J Clin Virol 44: 145-148. Link: https://goo.gl/uMHTPr
- Tei S, Kitajima N, Takahashi K, Mishiro S. (2003) Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet2 362: 371-373. Link: https://goo.gl/wpBkK7
- Matsuda H, Okada K, Takahashi K, Mishiro S. (2003) Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis 188: 944. Link: https://goo.gl/PfJzS3
- Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, et al. (2005) Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis 11: 1958-1960. Link: https://goo.gl/KHc1TT
- Tamada Y, Yano K, Yatsuhashi H, Inoue O, Mawatari F, et al. (2004) Consumption of wild boar linked to cases of hepatitis E. J Hepatol 40: 869-870.
- Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, et al. (2010) Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 202: 825-834. Link: https://goo.gl/RyCJkN
- Sainokami S, Abe K, Kumagai I, Miyasaka A, Endo R, et al. (2004) Epidemiological and clinical study of sporadic acute hepatitis E caused by indigenous strains of hepatitis E virus in Japan compared with acute hepatitis A. J Gastroenterol 39: 640-648. Link: https://goo.gl/uuy3mY
- Takahashi M, Tamura K, Hoshino Y, Nagashima S, Yazaki Y, et al. (2010) A nationwide survey of hepatitis E virus infection in the general population of Japan. J Med Virol 82: 271-281. Link: https://goo.gl/g3fU9h
- Suzuki K, Kataoka K, Miyamoto Y, Miyasaka A, Kumagai I, et al. (2015) Clinical and molecular analysis of sporadic acute hepatitis A and E and the specific viral genotypes isolated in Iwate anf three neighboring prefectures in the northern part of Honshu, Japan, between 2004 and 2013. Hepatol Res 45: 714-729. Link: https://goo.gl/4nhVSL
- Su CW, Wu JC, Huang YS, Huo TI, Lin CC, et al. (2002) Comparison of clinical manifestations and epidemiology between acute hepatitis A and acute hepatitis E in Taiwan. J Gastroenterol Hepatol 17: 1187-1191. Link: https://goo.gl/YQYEz3
- Chau TN, Lai ST, Tse C, Ng TK, Leung VK, et al. (2006) Epidemiology and clinical features of of sporadic hepatitis E as compared with hepatitis A. Am J Gastroenterol 101: 292-296. Link: https://goo.gl/5nU8yY
- Peron JM, Danjoux M, Kamar N, Missoury R, Poirson H, et al. (2007) Liver histology in patients with sporadic acute hepatitis E: a study of 11 patients from South-west France. Virchows Arch 450: 405-410. Link: https://goo.gl/gBPb7k
- Malcolm P, Dalton H, Hussaini HS and Mathew J (2007) The histology of acute antochonous hepatitis E virus infection. Histopathol 51: 190-194.
- Drebber U, Odenthal M, Aberle SW, Winkel N, Wedemeyer I, et al. (2013) Hepatitis E in liver biopsies from patinets with acute hepatitis of clinically unexplained origin. Frontiers in Physiology 4: 1-5. Link: https://goo.gl/2p743A
- Takikawa Y, Endo R, Suzuki K, Fujiwara K, Omata M (2006) Prediction of hepatic encephalopathy development in patients with severe acute hepatitis. Dig Dis Sci 51: 359-364. Link: https://goo.gl/UQcK9L
- Takikawa Y, Endo R, Suzuki K, Tsubouchi H (2009) Early prediction of short-term development of hepatic encephalopathy in patients with acute liver disease unrelated to paracetamol. A prospective study in Japan. J Hepatol 51: 1021-1029. Link: https://goo.gl/uGphUv
- Sugawara K, Nakayama N, Mochida S (2012) Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol 47: 849-861. Link: https://goo.gl/1QjGk2
- Mochida S, Takikawa Y, Nakayama N, Oketani M, Naiki T, et al. (2014) Classification of the etiologies of acute liver failure in Japan: A report by the Intractable Hepato-Biliary Diseases Study Group of Japan. Hepatol Res 44: 365-367. Link: https://goo.gl/Aeo9Nm
- Nyfeler B, Pichler WJ (1997) The lymphocyte transforming test for the diagnosis of drug allergy sensitivity and specificity. Clin Exp Allergy 27: 175-181. Link: https://goo.gl/7Qrbts
- Takikawa H, Murata Y, Horike N, Fukui H, Onji M (2009) Drug-induced liver injury in Japan: an analysis of 1676 cases between 1997 and 2006. Hepatol Res 39: 427-431. Link: https://goo.gl/XTXS3C
- Takikawa H (2009) Recent status of drug-induced liver injury. Hepatol Res 39: 1-6. Link: https://goo.gl/bWxxVy
- P. Bedossa (1994) Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 20: 15-20. Link: https://goo.gl/ju23Tm
- Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR cooperative study group. Hepatology 24: 289-293. Link: https://goo.gl/KZvCHR
- Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, et al. (2111) Acute hepatitis E infection acoounts for some cases of suspected drug-induced liver injury. Gastroenterology 141: 1665-1672. Link: https://goo.gl/hGtW1p
- Devarbhavi H (2012) An update on drug-induced liver injury. J Clin Exp Hepatol 2: 247-259. Link: https://goo.gl/2vMTpW
- Khoury T, Rmeileh AA, Yosha L, Benson AA, Daher S, et al. (2015) Drug induced liver injury: Review with a focus on genetic factors, tissue diagnosis, and treatment options. J Clin Transl Hepatol 3: 99-108. Link: https://goo.gl/x29nrU
- Kleiner DF, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, et al. (2014) Hepatic histological findings in suspected drug-induced liver injury: systemic evaluation and clinical associations. Hepatology 59: 661-670. Link: https://goo.gl/6yrMKL
- Fisher K, Vuppalanchi R, Saxena R (2015) Drug-induced liver injury. Arch Pathol Lab Med 139: 876-887. Link: https://goo.gl/PLqvEr
- Björnsson ES (2015) Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol 89: 327-334. Link: https://goo.gl/ZoVxyv
- Mantani N, Kogure T, Tamura J, Shimada Y, Terasawa K. (2003) Lymphocyte transforming test for medical herbs yiels false-positive results for first-visit patients. Clin Diagn Lab Immunol 10: 479-480. Link: https://goo.gl/XxKJo4
- Takikawa Y, Yasumi Y, Sato A, Endo R, Suzuki K, et al. (2007) A case of acute hepatitis E associated with multidrug hypersensitivity and cytomegalovirus reactivation. Hepatol Res 37: 158-165. Link: https://goo.gl/oSFgwb
- Lee SST, Buters JTM, Pineau T, Fernandez-Salguero P, Gonzalez FJ (1996) Role of CYP2E1 in the hepatotoxicity of acetoaminophen. J Biol Chem 271: 12063-12067. Link: https://goo.gl/cvZ2tx
- Deka M, Bose M, Baruah B, Bose PD, Medhi S, et al. (2010) Role of CYP2E1 gene polymorphisms asdsocation with hepatitis risk in Norhgteast India. World J Gastroenterol 16: 4800-4808.
- Tripathy AS, DasR, Rathod SB, Gurav YK, Arankalle VA (2013) Peripheral T regulation cells and cytokines in hepatitis E infection. Eur J Clin Microbiol Infect Dis 31: 179-184. Link: https://goo.gl/xbj6SA
- Gupta P, Jagya N, Pabhu SB, Durgapal H, Acharya SK, et al. (2012) Immunohistochemistry for the diagnosis of hepatitis E virus infection. J Viral Hep 19:e177-183.
- Wedemeyer H, Rybczynska J, Picschke S, Krawczynski K (2013) Immunopathogenesis of hepatitis E virus infction. Semin Liver Dis 33: 71-78. Link: https://goo.gl/MUQP9e
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
